ABSTRACT
This case describes a 57-year-old man with unrecognized cardiac sarcoidosis who presented with progressive heart failure leading to cardiogenic shock. He required extracorporeal membrane oxygenation (ECMO) as a bridge to orthotopic heart transplantation. The case highlights the potential acute and severe electrical and hemodynamic manifestations of cardiac sarcoidosis.
1. Case presentation
A 57-year-old man with a medical history significant only for hypercholesterolemia presented to a cardiology clinic with 6 months of progressive exercise intolerance manifest as a gradual increase in his 1-mile running time and a decline from running 30 miles to less than 3 miles each week. Days later, outpatient coronary angiography showed non-obstructive coronary atherosclerosis and ventriculogram left ventricular ejection fraction (LVEF) was 45%. The patient was started on carvedilol and aspirin.
One week later, he awoke with acute left-sided chest pain. Emergent electrocardiography demonstrated sinus tachycardia and ST-segment elevations and repolarization abnormalities (). Repeat coronary angiography revealed no change, but LVEF by ventriculography had declined to 20%. Post-procedurally, he developed hypotension requiring initiation of vasopressors and hemodynamically unstable wide-complex tachycardia requiring defibrillation. He was transferred to our hospital for further management.
Figure 1. Electrocardiograms
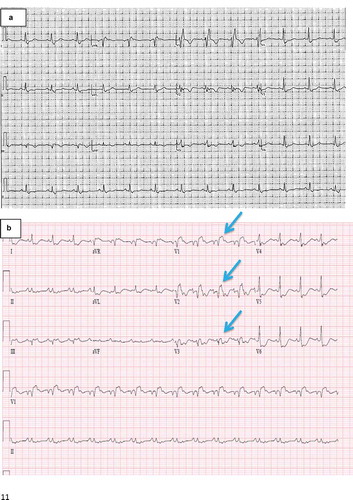
On arrival, the patient was comfortable but mean arterial pressure was 55 mmHg off vasopressors. Jugular venous pressure was elevated, breath sounds were decreased in the bilateral lung bases, and heart sounds were distant without evidence of a murmur or gallop. His extremities were cool. Arterial blood gas showed a pH of 7.4, PaCO2 of 29 mmHg, PaO2 of 63 mmHg on ambient air, bicarbonate of 20 mEq/L, lactate of 4.0 mmol/L, troponin I of 2.0 ng/mL, B-type natriuretic peptide of 2600 pg/mL, and a creatinine of 1.4 mg/dL increased from a baseline of 1.0 mg/dL. A Swan-Ganz catheter was placed revealing a right atrial pressure of 20 mmHg and pulmonary capillary wedge pressure of 22 mmHg. Pulmonary artery oxygen saturation was 40% and calculated cardiac output using the estimated Fick formula was 2.4 L/min, with cardiac index of 1.1 L/min/m2. Given the acuity of presentation and the rapidity of clinical decline, urgent laboratory testing and imaging studies were performed.
Transthoracic echocardiography showed biventricular dysfunction and dilation with global hypokinesis, without asymmetric myocardial thickening or structural valvular disease. There was biatrial dilation. Thyroid panel, cortisol, hemoglobin A1c, iron studies including ferritin, and autoimmune antibody levels were normal, while human immunodeficiency virus, Borrelia burgdorferi, respiratory viral panel, and blood culture testing were negative. Urinary toxicology was negative. C-reactive protein was elevated at 13.9 mg/dL. Cardiac myocardial resonance imaging (cMRI) demonstrated patchy gadolinium delayed enhancement within the right and left ventricular myocardia and pericardial thickening with enhancement, consistent with myocarditis or infiltrative disease though without T2-weighted evidence of edema (). Endomyocardial biopsy was not pursued due to the patient’s hemodynamic and electrical instability.
Figure 2. Cardiac magnetic resonance imaging
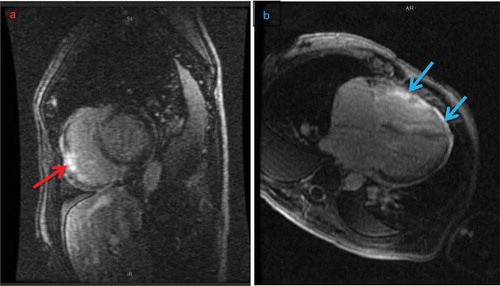
Figure 3. Photomicrographs of explanted cardiac myocardium
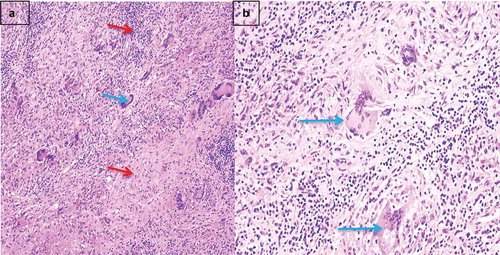
Milrinone and furosemide infusions were initiated, nitroprusside was added, and an intra-aortic balloon pump was eventually implanted for persistent cardiogenic shock (). He remained oliguric, and continued to have atrial and ventricular arrhythmias requiring defibrillation despite amiodarone infusion. Though the differential diagnoses of sarcoidosis and giant cell myocarditis were discussed, the patient was not initiated on corticosteroids. Due to continued hemodynamic and electrical instability he was femorally cannulated for veno-arterial extracorporeal membrane oxygenation (ECMO). Upon transplantation, pathological examination of the explanted heart revealed diffuse biventricular fibrosis, non-caseating granulomas, and multi-nucleated giant cells consistent with severe cardiac sarcoidosis ().
Table 1. Invasive hemodynamics
The patient was discharged home from the hospital two weeks after transplant and continues to do well. He has completed cardiac rehabilitation and started running again. His immunosuppression regimen consists of prednisone 5 mg daily, tacrolimus 2 mg twice daily, and mycophenolate 1000 mg twice daily. He has not had allograft rejection.
2. Discussion
Sarcoidosis is a multisystem granulomatous disease of unknown etiology that may affect any organ system. It has an estimated prevalence of about 200,000 people in the USA [Citation1]. Noncaseating granulomas are the pathologic hallmark of the disease, and it most commonly presents with pulmonary and lymphatic disease. Although autopsy studies have uncovered cardiac involvement in 25% of patients with sarcoidosis, the true prevalence may be underappreciated [Citation1]. The three principal sequelae of myocardial damage due to sarcoidosis (CS) are conduction abnormalities, ventricular arrhythmias, and heart failure, which can be insidious or fulminant [Citation1]. Diagnosis of CS can be challenging, as there are multiple proposed clinical criteria without a gold standard [Citation1]. Nevertheless, the mainstays of diagnosis include cardiac MRI with gadolinium for myocardial fibrosis and edema, and fluorodeoxyglucose positron emission tomography for myocardial inflammation. Endomyocardial biopsy has excellent specificity but an estimated sensitivity of less than 30%, and some severe cases are only diagnosed post-mortem.
Patients with CS generally have a poorer prognosis than patients with sarcoidosis but without cardiac involvement, and left ventricular function appears to predict survival. Of patients with CS and normal ventricular function 89% survive to 10 years compared to 27% of patients with systolic dysfunction [Citation1]. The diffuse myocardial fibrosis and granulomas with biventricular dilation and dysfunction in this case are consistent with longstanding unrecognized and life-threatening disease.
The mainstay of treatment for CS includes immunosuppression, typically with corticosteroids and variably with steroid-sparing agents such as methotrexate or infliximab [Citation2]. In combination with immunosuppression, antiarrhythmic medications including amiodarone or sotalol and ablation procedures can be used for CS-associated ventricular arrhythmias. However, ventricular tachycardia ablation in CS has had varied success and frequently is associated with recurrence [Citation3,Citation4] because of ongoing inflammation or progressive myocardial scarring. Given the propensity for ventricular arrhythmias and risk of sudden cardiac death, implantable cardioverter-defibrillator therapy may be considered in a broad array of situations according to expert consensus recommendations [Citation5]. Orthotopic heart transplant may be indicated in severe cases of CS unresponsive to drug and device therapy. CS recurrence after transplant is rare and may be prevented with long-term corticosteroid use. Nevertheless, patients with CS have similar rates of rejection and overall survival as patients transplanted due to other cardiomyopathies [Citation2].
3. Conclusions
Cardiac sarcoidosis rarely presents as refractory cardiogenic shock. This case highlights the need to include CS in the differential diagnosis of patients with cardiomyopathy, even if there is no evidence of extracardiac disease. Findings on advanced cardiac imaging can help determine the potential etiology of a non-ischemic cardiomyopathy, including sarcoidosis. Though he presented acutely, in this case CS was likely progressive over a longer period of time than his months of symptoms, and perhaps could have been treated with immunosuppression if identified earlier.
4. Learning objectives
Cardiac sarcoidosis may be difficult to diagnose and can present as rapidly progressive heart failure with cardiogenic shock.
Young patients with an otherwise unknown etiology of cardiomyopathy would likely benefit from advanced cardiac imaging with FDG-PET or MRI, which may assist with diagnosis if a high diagnostic suspicion of cardiac sarcoidosis or other infiltrative disease is maintained.
The three most common presentations of cardiac sarcoidosis are heart failure, ventricular arrhythmias, and conduction abnormalities.
The mainstays of therapy for cardiac sarcoidosis include immunosuppression with corticosteroids and frequently steroid-sparing agents.
Amiodarone and sotalol are the most common antiarrhythmics used to suppress ventricular arrhythmias; ventricular ablation has demonstrated mixed results.
Severe refractory cardiac sarcoidosis may require heart transplantation though sarcoidosis can recur in the donor heart.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ribeiro Neto ML, Jellis CL, Joyce E, et al. Update in cardiac sarcoidosis. Ann Am Thorac Soc. 2019 Nov;16(11):1341–1350. .
- Rosenthal DG, Anderson ME, Petek BJ, et al. Invasive hemodynamics and rejection rates in patients with cardiac sarcoidosis after heart transplantation. Can J Cardiol. 2018 Aug;34(8):978–982. Epub 2018 Apr 6. .
- Jefic D, Joel B, Good E, et al. Role of radiofrequency catheter ablation of ventricular tachycardia in cardiac sarcoidosis: report from a multicenter registry. Heart Rhythm. 2009 Feb;6(2):189–195. Epub 2008 Oct 30.
- Koplan BA, Soejima K, Baughman K, et al. Refractory ventricular tachycardia secondary to cardiac sarcoid: electrophysiologic characteristics, mapping, and ablation. Heart Rhythm. 2006 Aug;3(8):924–929. Epub 2006 Mar 30.
- Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014 July;11(7):1305–1323. Epub 2014 May 9.
