ABSTRACT
Introduction and objective: Vernal keratoconjunctivitis (VKC) is a rare allergic eye condition that occurs in children and is characterised by a combination of debilitating symptoms. Repeated use of topical corticosteroid rescue therapy is often necessary in severe forms. This study aims to assess the validity of a new composite endpoint: the penalties-adjusted corneal staining score (PACS-S) proposed as primary endpoint in VEKTIS trial evaluating the efficacy of a new corticosteroid-sparing treatment, VERKAZIA® (ciclosporin 1 mg/ml eye drops), in severe VKC patients.
Methodology: This research comprised a systematic literature review to identify efficacy endpoints being proposed in clinical trials for pediatric patients with severe VKC, followed by a remote expert advisory board assessing the validity of the PACS-S.
Results: While no agreed or validated endpoint for assessing efficacy in VKC was identified when VEKTIS trial started, the experts’ board acknowledged a high face validity of PACS-S as a subjective integrated measure matching the current clinical practice. A fair external validity was considered with regards to VEKTIS trial secondary endpoints.
Conclusion: PACS-S appears to be a reliable, valid and clinically meaningful primary endpoint that allows significant improvement over existing endpoints in severe VKC trials. Additional research is needed to validate this endpoint.
Introduction
Vernal keratoconjunctivitis (VKC) is a severe form of ocular allergy with chronic ocular surface inflammation and seasonal exacerbations [Citation1,Citation2]. The disease is characterised by inflammatory responses on the ocular surface (conjunctiva and cornea) that lead to marked symptoms such as itching, tearing, eye irritation, mucous discharge and photophobia [Citation2]. Clinical signs of VKC include papillae on the tarsal or limbal conjunctiva, superficial keratopathy, subepithelial scarring, corneal opacity, plaques, erosions and ulcerations [Citation2,Citation3]. VKC is more often experienced in childhood and adolescence with symptoms occurring before the age of 10 in 80% of cases [Citation4]. Only 4% of newly diagnosed patients are older than 20 at the time of initial diagnosis [Citation5].
The condition is rare with very low incidence and prevalence rates and important geographical variation. In Europe, VKC prevalence was estimated to be 0.7 to 3.3 per 10,000 inhabitants and severe to very severe VKC prevalence was 0.3 to 1.4 per 10,000 in the general population [Citation6].
In the absence of standardized grading and diagnosis system for VKC, ophthalmologists rely on examination and observation of symptoms [Citation2] with tearing, itching and photophobia, blurry vision and mucous discharge considered as the hallmark symptoms of the disease [Citation7]. Since VKC severity encompasses a spectrum of manifestations, the definition of the severe form might vary according to clinical practice. However, the majority of severe VKC cases present a corneal involvement with punctate keratitis identified by corneal staining [Citation8].
A clinical grading system was proposed by Bonini et al., which aimed to provide a standardized classification system of signs and symptoms severity, offering a credible method for segmenting VKC patients [Citation9] ().
Table 1. VKC clinical presentation by severity based on the Bonini scale for grading VKC
Severe VKC defined as grade 3/4 on Bonini scale is associated with a combination of debilitating symptoms that have a significant impact upon children’s and their caregivers’ daily lives, social interactions and health-related quality of life (HRQoL) [Citation10]. Young patients with severe VKC require frequent visits to their specialist in order to try to manage their symptoms and prevent exacerbations. This places a large burden on healthcare resources and even impacts the parents or guardians of these children [Citation11].
VKC management
Treatment of VKC requires a multi-faceted approach that includes patient’s education and preventive measures together with the use of drugs and close collaboration between ophthalmologists, allergists and pediatricians [Citation12].
Drugs most commonly used to treat VKC include topically administered anti-histamines, mast-cell stabilizers, dual acting agents (with antihistaminic and mast cell stabilizing properties), and non-steroidal anti-inflammatory agents. However, these drugs are merely palliative; the disease is known for its recurrence when therapy is stopped, hence effective medications have to be used on a long – term basis [Citation12]. For severe VKC, current therapeutic strategies include:
Topical corticosteroids, however, signs and symptoms of severe VKC are not adequately controlled unless prolonged steroid-based therapies are used which is not without safety concern, especially in children [Citation9,Citation13].
Immunomodulatory agents such as topical ciclosporin have been proven to be effective in the long-term treatment of VKC, significantly improving signs and symptoms without significant side effects [Citation12]. VERKAZIA® (ciclosporin 1 mg/ml eye drops emulsion) is the first ciclosporin treatment to be specifically licensed in Europe and worldwide for severe VKC in children from 4 years of age and adolescents [Citation14]. Topical ciclosporin may be used as a corticosteroid-sparing treatment option in steroid-dependent patients after anti-allergic eye drops failure [Citation15].
Topical immunomodulatory agent tacrolimus also reported to be used in cases refractory to ciclosporin in severe VKC [Citation11,Citation16].
Systemic therapies may be used when appropriate with oral corticosteroids in very rare forms that are resistant to any other treatment with visual threat by limiting treatment duration as much as possible [Citation4,Citation17]. Systemic treatment with T-lymphocyte signal transduction inhibitors such as ciclosporin or tacrolimus may be used in severe patients who are refractory to conventional treatment [Citation12], as well as anti-IgE monoclonal antibody omalizumab in the most severe cases [Citation17,Citation18] and oral antileukotrienes such as montelukast in case of associated asthma [Citation17,Citation19].
Clinical efficacy assessment in severe VKC
To date, there is no agreed or validated criterion to clinically assess interventions in severe VKC while integrating the confounding impact of rescue therapy in a single index. A systematic review of all randomised clinical trials on topical therapy for VKC published up to December 2005 underlined the lack of standardised outcome measures in VKC [Citation20].
Obvious efforts have been undertaken by several researchers to propose a solution for a composite index that integrates signs and symptoms in a fair and balance way [Citation20]. However, the main limitation of all these scoring systems is that although signs and symptoms represent a reasonable overview of the clinical evolution, the evolution is driven by the combined efficacy of the tested product and rescue therapies that could not be avoided in most patients.
Therefore, if a patient is exposed in a clinical trial to an ineffective therapy, he/she may receive repeated rescue therapy that will improve the symptoms and eventually the signs and thus suggesting the tested treatment is effective. Rescue therapy appears to be a major confounding factor affecting composite endpoints making the results un-interpretable.
This is not unique to VKC but true for several disorders when effective rescue therapies are involved. For example, for allergic rhinitis (AR) it has become standard for regulators and payers to combine a scale with symptoms and penalty associated to the use of rescue therapies (). A combined symptom and medication score (CSMS) was also used as the primary endpoint for efficacy in clinical trials of house dust mite (HDM) allergen immunotherapy; it equally takes into account both symptom severity and the need for antiallergic medication [Citation21].
Box 1. AR scoring methods recommended by the European Medicines Agency’s (EMA) guidelines [Citation42]
In the absence of standardized criteria to assess severe VKC, and confounding effect of rescue therapy, a new penalties-adjusted corneal staining score (PACS-S) was developed in collaboration with VKC clinical experts and agreed during scientific consultation with EMA during the clinical development of VERKAZIA® (ciclosporin 1 mg/ml eye drops emulsion). However, the validation of this scoring system was restricted to face validity by regulatory, clinical, and methodologist experts, but no validation of the scoring per se was performed.
In the pivotal clinical VErnal KeratoconjunctiviTIs Study (VEKTIS) (ClinicalTrials.gov identifier, NCT01751126) [Citation22], corneal fluorescein staining (CFS) score was assigned at baseline for each patient based on the modified Oxford scale. Subsequently, at each monthly assessment visit during the four months randomization and double-masked period, a new CFS score was assessed; the need for rescue medication and any occurrence of corneal ulceration were noted. Based on these observations, a composite efficacy score for that particular month’s visit was calculated using the following formula:
Composite efficacy score at month X = CFS score (baseline)-CFS score (month X) + Penalty(ies)
Penalties of −1 were assigned to patients using one course of corticosteroids (i.e., rescue medication) and for development of corneal ulceration. A positive value in the patient composite efficacy score indicated an improvement. A 1 grade penalty for one course of rescue therapy is arbitrary but was considered a reasonable improvement in CFS ([0-5]-points scale, with 0 being the normal state) when rescue medication is administered. If it is 1 grade on average, then the penalty suppressed the improvement brought by the rescue medication. The primary endpoint of VEKTIS trial was the average of the four calculated composite efficacy score at each month for 4 months.
This article provides an overview of efficacy endpoints in clinical trials assessing treatments for pediatric patients with severe VKC and assesses the clinical relevance of the composite efficacy score PACS-S used in the VEKTIS study.
Materials and methods
This research was structured following two main steps: first, a search was conducted for existing efficacy endpoints used in VKC clinical trials; then subsequently, these findings were reviewed by a remote expert advisory board aiming at assessing the face validity of the new efficacy scoring system proposed in VEKTIS trial.
Step 1. Systematic literature review
The research was based on a systematic literature review conducted on Medline (year of publication ≥10 years) and Clinical Trials.gov website (last updated posted ≥10 years) to retrieve endpoints used to assess drug activity in completed and/or ongoing interventional clinical trials in VKC. The objective of this review was to identify the various scoring systems being proposed. Additional sources included Google Scholar, Science Direct, and conferences papers.
A search strategy for each database was developed. There were no restrictions on region. The search strategy in Medline combined free text and controlled vocabulary terms with results restricted to English language. Search filters to identify interventional trials of interest were used in Clinical Trials.gov. Full search strategies are provided in the supplementary material.
The eligibility criteria were defined according to the population, outcomes and study design (PICOS) statement. All studies were reviewed against the eligibility criteria outlined in .
Table 2. Eligibility criteria for the systematic literature review
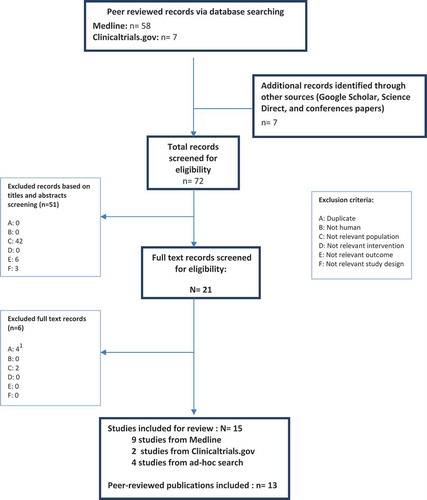
The search results were screened in a two-step selection process. In step one, abstracts of articles were assessed and categorized as ‘included’, ‘to be potentially included’ or ‘excluded’ by two independent reviewers based on the eligibility criteria. Discrepancies between reviewers were resolved by consensus; in the event of an unresolved dispute between reviewers, a third reviewer reviewed the questioned study and his/her judgment was considered final. In step two, two reviewers obtained and reviewed the full-text articles in the ‘included’ and ‘to be potentially included’ categories; the reviewers further reviewed the articles until all articles were ultimately categorized as ‘included’ or ‘excluded’. Reasons for rejections and exclusions of the studies were recorded. The study selection process was illustrated using a PRISMA flow diagram.
For full publications that met all inclusion criteria after the full-text review, data were extracted into a data extraction sheet designed a priori for each systematic review (in Microsoft Excel®) by a single reviewer. Errata and supplementary data were reviewed using the appropriate journal websites.
Step 2. Expert advisory board
A group of well recognized academic and clinical experts in VKC (n = 3) were gathered following several remote discussions conducted during November 2019 on how to address the face validity of the new efficacy scoring system proposed in VEKTIS trial. Experts’ feedback was discussed. Face validity was the first step of this validation process. The experts then reviewed all the endpoints used in VEKTIS trial to inform the external validity of the PACS-S.
During the discussions, experts:
assessed the relevance of the PACS-S with respect to their clinical practice in terms of the comprehensiveness of the item measured and the way they were scored. This served as face validity assessment.
were presented with the results of the VEKTIS study to appreciate how the score might contribute to discriminate two interventions.
were then presented with results of other endpoints to evaluate the consistency of therapeutic effect observed with the PACS-S scoring system.
Results
Systematic literature review findings
Searches for full records were run in Medline. The PRISMA diagram () details the numbers of abstracts and full publications identified and assessed at each stage of the review. From this database, nine publications of interventional clinical trials were identified and met the inclusion criteria for the review.
Searches were also conducted in Clinical Trials.gov to identify completed and/or ongoing trials that would meet the inclusion criteria. Two trials were identified in this process, but no published results were available and disease severity data were not reported.
Additionally, four relevant publications were identified by hand searching including one cohort study introducing a new VKC scoring system and were all included and extracted. In total, 15 studies are included in this report, with results published from 13 peer-reviewed articles.
Overview of endpoints used to assess drug activity in interventional clinical trials in severe VKC (full results in the supplementary material)
In about a third of studies, mean change in score of objective signs and/or subjective symptoms in severe VKC were used as primary endpoints [Citation23–Citation27].
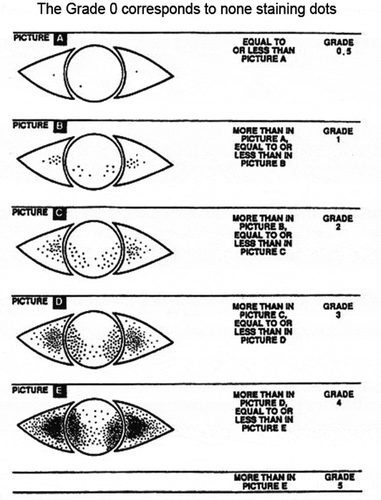
The clinical presentation of corneal damage in severe VKC was considered as an endpoint in one study, and assessed by CFS score graded on the modified Oxford scale [Citation22]. The Oxford grading system developed by Bron et al., is a well-established and standardised method to evaluate corneal health in which the count of corneal dots stained by fluorescein is scored against a set of categorised grades (). The modified Oxford scale is a seven-point ordinal scale (0, 0.5, 1, 2, 3, 4 and 5) with zero corresponding to complete clearing of the cornea [Citation28]. However, the modified Oxford scoring system used to assess CFS data was developed for use in patients with dry eye disease, rather than VKC. Although this system is validated for the grading of ocular surface disease, the pattern of corneal and conjunctival staining in dry eye disease is not the same as with VKC, in which there is a predominance of staining on the superior half of the cornea with mucus adhesion [Citation15].
A VKC-specific scoring system was recently introduced in an effort to address some of these limitations [Citation29]. This score was not available at the time of the VEKTIS study design. It consisted on a modification of the original CLEK scoring system introduced by the Collaborative Longitudinal Evaluation of Keratoconus study (CLEK). Authors modified the CLEK to the new VKC-CLEK to assign the equivalent clinical importance to the stained limbal area and to the central cornea. In this new scheme, the cornea and the limbal area, defined as 1 mm of peripheral cornea and 1 mm of the perilimbal conjunctiva, are divided into five zones (Zone A2-A3-A4-A5-A6): A2 represents the central corneal area, while the other zones correspond to the superior (A3), nasal (A4), inferior (A5) and temporal limbal areas (A6). In this scheme, the central corneal area (A2) has the approximately the same area of the sum of the four limbal areas (A3 + A4+ A5+ A6). The final score is given by the sum of the staining scores assigned for each area, considering a score 0–4 (0 = clear cornea; 1 = SPK less than half of the cornea; 2 = SPK more than half of the cornea; 3 = epithelial macro erosion or defect; 4 = corneal ulcer or plaque) for the central cornea (A2) and 0–1 for each limbal area. The score was given after the staining with both fluorescein and lissamine green. The total staining score (0–8) was also considered mild if less than 3, moderate if equal of more than 3 and less than 6 and severe if more than 6.
A composite score was used by Keklikci et al. and De Smedt et al. to jointly assess signs and symptoms in severe VKC [Citation30,Citation31]. Both authors used a scoring system described by Akpek et al. to assess the effect of topical resistant corticosteroid in atopic keratoconjunctivis and adapted for limbal VKC to produce a composite score for both symptoms and clinical signs [Citation32]. The composite score for the five symptoms (itching, tearing, discomfort, mucous discharge, and photophobia) and the 12 signs (bulbar conjunctival hyperemia, upper tarsal conjunctival papillae, punctate keratitis, corneal neovascularization, cicatrizing conjunctivitis, and blepharitis) could range from 5 to 62.
VKC signs and symptoms improvement, disease recurrence and the need for reinjection of investigational treatment as a rescue medication were considered as separate endpoints by Costa et al. [Citation24].
A single penalties-adjusted CFS score was proposed in VEKTIS trial by Leonardi et al., due to the lack of agreed or validated clinical endpoint for assessing efficacy in VKC [Citation22]. In the absence of any validated endpoint, it was determined that the efficacy of VERKAZIA® (ciclosporin A eye drops) should be assessed every month during the 4-month efficacy evaluation treatment period using a composite endpoint based on VKC signs and symptoms. It was proposed to use the CFS change from baseline in modified Oxford scale, with the possibility of penalties in case of rescue medication or corneal ulceration. Rescue medication and ulceration are post-randomisation variables and therefore could not be used as covariates [Citation22].
Integration of these items into a quantitative endpoint was challenging, which is why discussion and endorsement were sought with the Paediatric Committee (PDCO) in EMA during the regulatory process of VERKAZIA® (ciclosporin 1 mg/ml eye drops emulsion) [Citation33,Citation34].
PACS-S was selected as the primary endpoint in VEKTIS trial because it considers both the signs (keratitis, corneal ulceration) and symptoms (rescue therapy was used for worsening symptoms, with or without worsening signs) of VKC in the evaluation of the treatment efficacy. Severe VKC is associated with a combination of debilitating signs and symptoms that have a significant impact upon children’s daily lives and HRQoL [Citation10,Citation13] hence it is important to evaluate signs and symptoms as part of the primary endpoint.
This endpoint consisted of the mean of four penalties-adjusted CFS scores taken at each monthly visit from baseline for months 1–4. The composite score was based on improvement, stability or worsening of the CFS score, with penalty scores being applied for use of rescue medication and in cases of corneal ulceration.
CFS: is used to determine the degree of keratitis by assessing corneal damage on the modified Oxford grading system. In the VEKTIS trial, only patients with severe CFS (grade 4 or 5) were included. CFS was measured throughout the trial to objectively assess the effect of treatment at baseline, during the 4-month randomized phase (months 1, 2, 3 and 4) and during the 8-month, long-term, follow-up phase (months 6, 8, 10 and 12). A negative change from baseline indicated improvement.
Rescue therapy: The use of corticosteroid rescue medication was predefined in the study protocol since only severe patients were included and one arm of the study was placebo (vehicle)-treated. The use of corticosteroid rescue medication was monitored over the course of the study for each patient. After the baseline visit, patients were instructed to contact the investigator in cases of worsening VKC in order to assess the need for rescue medication. Patients could be treated with rescue therapy if they demonstrated either or both of the following criteria:
Worsening keratitis, defined as a change of at least one grade on the modified Oxford scale, or maintained baseline score for 2 months.
Symptoms worsening, defined as a change of ≥1 cm on a VAS on ≥1 of the four symptoms of VKC and the worsening of the mean of the four symptoms, or maintained baseline score.
In such situations, dexamethasone 0.1% eye drops were provided as rescue therapy. Patients were instructed to administer dexamethasone QDS for 5 days, with a maximum of two courses between scheduled visits during the 4-month randomized phase (months 1, 2, 3 and 4) and a maximum of four courses of rescue therapy between scheduled visits during the 8-month, long-term, follow-up phase (months 6, 8, 10 and 12). Use of rescue medication was documented in a diary by the patient. The use of corticosteroid rescue therapy was discussed as an alternative primary endpoint but was subsequently ruled out as the EMA’s human medicines committee (CHMP) acknowledged that it would not directly measure corneal health [Citation33].
Corneal ulceration: Severe VKC with corneal involvement is a concern for ophthalmologists because it can lead to corneal ulceration, scarring and, subsequently, visual impairment [Citation11,Citation35]. Furthermore, it is well established that decreased visual acuity is correlated with decreased HRQoL [Citation36]. The occurrence of corneal ulceration can signal worsening of disease [Citation37]; however, the modified Oxford scale does not capture this aggravation. Patients categorized at maximum CFS severity on the modified Oxford scale (grade 5) could show clinical worsening of disease by the development of corneal ulcers; however, their corneal staining would remain at maximum level and therefore they could be graded as having stable disease when clinically they might be deteriorating. Consequently, adding a penalty if an ulcer occurs allows the modified Oxford scale to be a broader measure of corneal health in VKC than CFS score alone. Occurrence of corneal ulceration was recorded throughout the study (months 1, 2, 3, 4, 6, 8, 10 and 12),
The penalties-adjusted CFS score was calculated as follows:
Penalties-adjusted CFS score at month X = CFS (baseline) – CFS (Month X) + penalty(ies)
Penalty for rescue medication: – 1 (per course, with a maximum of two courses between two scheduled visits)
Penalty for corneal ulceration: – 1 (per occurrence)
In VKC, a possible sign associated with worsening of the disease is the occurrence of corneal ulceration. However, this aggravation is not captured by the modified Oxford scale. Consequently, by adding a penalty if an ulcer occurs, the CFS score of the patient is negatively impacted, which contributes to discriminate between patients with or without corneal ulceration.
A sensitivity analysis was performed where the weighting of the penalty was modified in order to support the robustness of the primary endpoint results. Knowing that corticosteroids are very effective, a penalty of 2 by course of rescue medication was chosen (corresponding to a penalty of 2 grades of CFS at the corresponding visit) and one for ulceration.
Outcomes of the expert advisory board
The board of experts consistently agreed that the current proposed endpoints for VKC were insufficient to capture in a comprehensive way all the relevant information to assess an intervention in severe VKC. There was a need for a new integrated endpoint that is clinically relevant.
All experts considered that the PACS-S had a high face validity as it matched the clinical practice since the aim of treatment in VKC is to prevent corneal ulcerations, subsequent visual loss and to alleviate symptoms. Experts deemed that severe VKC is associated with a combination of debilitating signs and symptoms, hence it is important to evaluate signs and symptoms as part of a subjectively weighted integrated primary endpoint in the evaluation of treatment efficacy. The PACS-S allowed to assess both VKC signs (keratitis, corneal ulceration) and symptoms rendered by the use of corticosteroid rescue therapy to control worsening symptoms, with or without worsening signs. Ulceration was considered as a severe sign for risk of poor prognosis and rescue therapy was a last resort prescription signing the failure of all alternative therapies.
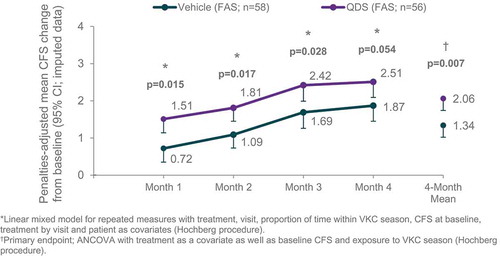
According to experts, PACS-S scoring system replicated the clinical behavior when weighting and assessing the patient clinical status thus providing a standardized tool that could be well used to define also clinical decision and patient prognosis under treatment. The analysis of contribution of the three components of the composite PACS-S endpoint also showed that the component of CFS score non-adjusted for penalties was the main driver of the magnitude of the treatment effect, together with the need for rescue medication to a lesser extent. PACS-S results were further supported by the analysis of the time course of the response. Using a linear mixed model for repeated measure instead of the average over 4 months, the difference was still significant versus vehicle. The time course of the response to the treatment was ascending and parallel to that of vehicle (). This means that the PACS-S improvement with respect to baseline increased on average over the 4 months of treatment, the onset of action was very fast and statistically significant (as early as month 1) and the observed benefit was constant over time.
Experts found that the sensitivity analysis using different penalties was in favor of the active treatment (LS mean PACS-S with a penalty of 2 for rescue medication and 1 for ulceration = 0.99, 95%CI[0.37, 1.60], p = 0.004 versus vehicle; LS mean PACS-S with a penalty of 1 for rescue medication and 2 for ulceration = 0.76, 95%CI[0.24, 1.29], p = 0.009 versus vehicle) with the larger the penalty the larger the difference between active and control groups providing a good rationale of the penalty selection, and reassurance of the treatment benefit through the selected composite primary endpoint.
It was recognized that PACS-S happened to have a fair external validity as secondary efficacy endpoints including keratitis as measured by CFS score, CFS responders after 4 months of treatment, the use of rescue therapy, the change in four VKC symptoms of photophobia, tearing, itching, mucous discharge and quality of life were all statistically significant and clinically relevant in the VEKTIS trial ().
Table 3. VEKTIS trial outcomes
The panel of experts highlighted that the clinical relevance of the outcomes was further supported by a post-hoc responder analysis conducted to calculate the percentage of improvement in patient’ symptoms. Significantly greater proportions of VERKAZIA® versus vehicle-treated patients demonstrated at least 50% improvements in each VKC symptom and an average of all four symptom scores from baseline to month 4.
Discussion
When conducting a clinical trial to assess an intervention in a rare disease, it is critical to have appropriate efficacy endpoints to assess the clinical value of the intervention [Citation38]. The endpoint should be able to comprehensively represent relevant aspects of the disease to ensure its clinical relevance [Citation39]. This can be particularly challenging in rare diseases affecting pediatric populations [Citation40]. In the setting of severe VKC, a rare condition affecting children with several clinical signs and symptoms, corticosteroid rescue therapy might be required [Citation1], and would confound the appreciation of a new therapy tested in a clinical trial.
No agreed or validated clinical endpoint for assessing efficacy in VKC was available when the VEKTIS trial started. The development of the penalty-adjusted CFS represented therefore a clear step forward in this field.
The VEKTIS trial integrated in its primary endpoint VKC signs and symptoms which is a critical element as VKC signs and symptoms may not evolve within the same time horizon and they are equally important [Citation22]. The trial outcomes demonstrated the value of VERKAZIA® (ciclosporin 1 mg/ml eye drops emulsion) in reducing the need for rescue therapy [Citation22]. This corticosteroid-sparing effect is critical for pediatric patients with severe VKC as it reduces the severe undesirable effects of corticosteroid therapy [Citation12,Citation41]. It is therefore fair that such item is acknowledged in the primary endpoint considering its high relevance for patients and clinicians. The VEKTIS trial has also allowed, owing to an appropriate composite endpoint, to evidence the higher benefit of ciclosporin administered four times a day versus two times a day, while the clinical practice associated to hospital compounded ciclosporin was very often prescribed twice a day [Citation22].
These findings indicate that, the penalty-adjusted CSF score allowed integrating in the same endpoint the need for corticosteroid rescue therapy as well as the prognosis of ulceration complication. In addition, the proposed endpoint was consistent with the recommendations of the EMA guidance relating to the Treatment of Allergic Diseases, which suggests that a primary endpoint has to reflect both symptom severity and use of rescue medication [Citation42]. Although future research is needed to further validate the penalty-adjusted score, used in the VEKTIS trial, it has brought a significant improvement over existing efficacy endpoints used in VKC.
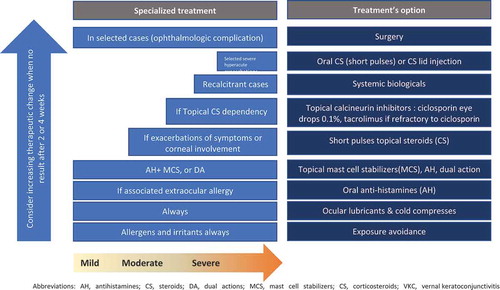
The positioning of VERKAZIA® (ciclosporin 1 mg/ml eye drops emulsion) as a corticosteroid-sparing option in the treatment of severe VKC was considered in the light of the treatment ladder illustrating the treatment intensification adapted from recent consensus position paper of the European Academy of Allergy and Clinical Immunology (EAACI) that is consistent with most published recommendations [Citation15] ().
As a high quality ciclosporin eye drops have become available, topical corticosteroids should be limited for short term use to treat acute exacerbations while topical ciclosporin is suitable for long term use in severe VKC after failure of lubricant eye drops, followed by failure of dual-acting eye drops (ketotifen, olopatadine) or cromones, and finally failure of antihistamines. Topical calcineurin inhibitors, preferentially cyclosporine A (0.1% on‐label treatment in the EU), may be used as a steroid‐sparing agent in steroid-dependent cases of VKC. Tacrolimus off‐label eye drops are reserved for use in severe VKC cases refractory to ciclosporin.
In recalcitrant cases, supra‐tarsal injections of dexamethasone sodium phosphate, triamcinolone acetonide, or hydrocortisone sodium succinate have been proposed, but should only be used by specialists with caution in severe patients unresponsive to other treatments [Citation15].
Restricting corticosteroids use for flares is consistent with their mode of action, their short term use, and their very limited safety especially in children and the risk of corticosteroid-dependence [Citation9,Citation13].
Conclusion
In conclusion, the penalties-adjusted CFS score, in combination with the responder analysis on signs and symptoms provide a validated simplified interpretation of treatment effect and is scientifically and clinically meaningful in severe VKC. It has a well-established face validity and appears to be consistent with several other endpoints of keratitis, VKC symptoms, patient’s quality of life and reduced need for rescue therapy, additionally supporting its external validity.
Financial disclosures
A. L.: Consultant or research grant – Bausch & Lomb, Dompe, Medivis, Novartis, Santen, Thea; Investigator – VEKTIS study. D. B-G.: Consultant – Alcon, Allergan, Horus, Santen, Thea; Investigator – VEKTIS study. This research is sponsored by Santen GmbH
Supplemental Material
Download MS Word (42.7 KB)Acknowledgments
The authors thank Prof. Bruno Scherrer for statistical and methodology advice, Imen Soussi (Creativ-Ceutical- France) for medical writing services.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Leonardi A, Bonini S. Is visual function affected in severe ocular allergies? Curr Opin Allergy Clin Immunol. 2013;13(5):558–12.
- Vichyanond P, Pacharn P, Pleyer U, et al. Vernal keratoconjunctivitis: A severe allergic eye disease with remodeling changes. Pediatr Allergy Immunol. 2014;25(4):314–322.
- Kraus, Courtney.Vernal Keratoconjunctivitis. American academy of Ophthalmology; 2016 [cited 2019 Nov 12]. Available from: https://www.aao.org/disease-review/vernal-keratoconjunctivitis-5
- De Smedt S, Wildner G, Kestelyn P. Vernal keratoconjunctivitis: an update. Br J Ophthalmol. 2013;97(1):9–14.
- Leonardi A, Busca F, Motterle L, et al. Case series of 406 vernal keratoconjunctivitis patients: a demographic and epidemiological study. Acta Ophthalmol Scand. 2006;84(3):406–410.
- Bremond-Gignac D, Donadieu J, Leonardi A, et al. Prevalence of vernal keratoconjunctivitis: a rare disease? Br J Ophthalmol. 2008;92(8):1097–1102.
- Ono SJ, Abelson MB. Allergic conjunctivitis: update on pathophysiology and prospects for future treatment. J Allergy Clin Immunol. 2005;115(1):118–122.
- Zicari AM, Capata G, Nebbioso M, et al. Vernal Keratoconjunctivitis: an update focused on clinical grading system. Ital J Pediatr. 2019;45(1):64.
- Bonini S, Sacchetti M, Mantelli F, et al. Clinical grading of vernal keratoconjunctivitis. Curr Opin Allergy Clin Immunol. 2007;7(5):436–441.
- Sacchetti M, Baiardini I, Lambiase A, et al. Development and testing of the quality of life in children with vernal keratoconjunctivitis questionnaire. Am J Ophthalmol. 2007;144(4):557–563.
- Santen. The QANT study. Vernal Keratoconjunctivitis Physician Survey; 2017 June.
- Leonardi A. Management of vernal keratoconjunctivitis. Ophthalmol Ther. 2013;2(2):73–88.
- Bonini S, Bonini S, Lambiase A, et al. Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term followup. Ophthalmology. 2000;107(6):1157–1163.
- VERKAZIA.Summary of product characteristics(SmPC). [ cited 2019 Nov 13]. Available from: https://www.ema.europa.eu/en/documents/product-information/verkazia-epar-product-information_en.pdf.
- Leonardi A, Silva D, Perez Formigo D, et al. Management of ocular allergy. Allergy. 2019;74(9):1611–1630.
- Vichyanond P, Kosrirukvongs P. Use of cyclosporine A and tacrolimus in treatment of vernal keratoconjunctivitis. Curr Allergy Asthma Rep. 2013;13(3):308–314.
- Pisella P-J, Baudouin C, Hoang-Xuan T. Société Française d’Ophtalmologie-Rapport 2015 SURFACE OCULAIRE. art. L. 122-4, L. 122-5 et L. 335-2. [ cited 2019 Nov 13] Available from: https://www.em-consulte.com/em/SFO/2015/html/index.html
- Heffler E, Picardi G, Liuzzo MT, et al. Omalizumab treatment of Vernal Keratoconjunctivitis. JAMA Ophthalmol. 2016;134(4):461–463.
- Gane J, Buckley R. Leukotriene receptor antagonists in allergic eye disease: a systematic review and meta-analysis. J Allergy Clin Immunol. 2013;1(1):65–74.
- Mantelli F, Santos MS, Petitti T, et al. Systematic review and meta-analysis of randomised clinical trials on topical treatments for vernal keratoconjunctivitis. Br J Ophthalmol. 2007;91(12):1656–1661.
- Pfaar O, Gerth van Wijk R. Mite-allergic rhinitis: how to evaluate clinical efficacy in allergen-specific immunotherapy trials? Curr Treat options Allergy. 2015;2(1):1–9.
- Leonardi A, Doan S, Amrane M, et al. A randomized, controlled trial of cyclosporine a cationic emulsion in pediatric vernal keratoconjunctivitis: the VEKTIS study. Ophthalmology. 2019;126(5):671–681.
- Zanjani H, Aminifard MN, Ghafourian A, et al. Comparative evaluation of tacrolimus versus interferon alpha-2b eye drops in the treatment of vernal keratoconjunctivitis: a randomized, double-masked study. Cornea. 2017;36(6):675–678.
- Costa AXD, Gomes JAP, Marculino LGC, et al. Supratarsal injection of triamcinolone for severe vernal keratoconjunctivitis in children. Arq Bras Oftalmol. 2017;80(3):186–188.
- Pucci N, Caputo R, Di Grande L, et al. Tacrolimus vs. cyclosporine eyedrops in severe cyclosporine-resistant vernal keratoconjunctivitis: A randomized, comparative, double-blind, crossover study. Pediatr Allergy Immunol. 2015;26(3):256–261.
- Muller GG, Jose NK, de Castro RS. Topical tacrolimus 0.03% as sole therapy in vernal keratoconjunctivitis: a randomized double-masked study. Eye Contact Lens. 2014;40(2):79–83.
- Tesse R, Spadavecchia L, Fanelli P, et al. Treatment of severe vernal keratoconjunctivitis with 1% topical cyclosporine in an Italian cohort of 197 children. Pediatr Allergy Immunol. 2010;21(2 Pt 1):330–335.
- Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–650.
- Leonardi A, Lazzarini D, La Gloria Valerio A, et al. Corneal staining patterns in vernal keratoconjunctivitis: the new VKC-CLEK scoring scale. Br J Ophthalmol. 2018;102(10):1448–1453.
- Keklikci U, Dursun B, Cingu AK. Topical cyclosporine a 0.05% eyedrops in the treatment of vernal keratoconjunctivitis - randomized placebo-controlled trial. Adv Clin Exp Med. 2014;23(3):455–461.
- De Smedt S, Nkurikiye J, Fonteyne Y, et al. Topical ciclosporin in the treatment of vernal keratoconjunctivitis in Rwanda, Central Africa: a prospective, randomised, double-masked, controlled clinical trial. Br J Ophthalmol. 2012;96(3):323–328.
- Akpek EK, Dart JK, Watson S, et al. A randomized trial of topical cyclosporin 0.05% in topical steroid-resistant atopic keratoconjunctivitis. Ophthalmology. 2004;111(3):476–482.
- European Medicines Agency. Follow-up protocol assistance, Ciclosporin (VEKACIA). 2012 Apr 19. (EMA/CHMP/SAWP/225272/2012).
- European Medicines Agency. Positive opinion of the paediatric committee on compliance with a paediatric investigation plan. 2016 Apr 29. (EMEA-C-000575-PIP01-09-M03).
- Kumar S. Vernal keratoconjunctivitis: a major review. Acta Ophthalmol. 2009;87(2):133–147.
- Brown M, Gordon WA. Quality of life as a construct in health and disability research. Mt Sinai J Med. 1999;66(3):160–169.
- Sacchetti M, Lambiase A, Mantelli F, et al. Tailored approach to the treatment of vernal keratoconjunctivitis. Ophthalmology. 2010;117(7):1294–1299.
- Cox GF. The art and science of choosing efficacy endpoints for rare disease clinical trials. Am J Med Genet Part A. 2018;176(4):759–772.
- Roever L Endpoints in clinical trials: advantages and limitations. Evidence based medicine and practice 1: e111.2016. [ cited 2019 Nov 13]. Available from: https://www.omicsonline.org/open-access/endpoints-in-clinical-trials-advantages-and-limitations-ebmp-1000e111.pdf
- Mulberg AE, Bucci-Rechtweg C, Giuliano J, et al. Regulatory strategies for rare diseases under current global regulatory statutes: a discussion with stakeholders. Orphanet J Rare Dis. 2019;14(1):36.
- McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25(1):33–55.
- European Medicines Agency.Guideline on the clinical development of products for specific immunotherapy for the treatment of allergic diseases. 2008 Nov 20. (CHMP/EWP/18504/2006).