ABSTRACT
The lack of adequate treatment for many patients with opioid use disorder (OUD) has led to high medical costs ($90B in 2020). An analysis of the cost-effectiveness (cost-utility) of reSET-O, the first and only FDA-approved prescription digital therapeutic (PDT) for the treatment of OUD, is needed to inform value assessments and healthcare decision making. To evaluate the cost-utility of reSET-O in conjunction with treatment-as usual (TAU) compared to TAU alone. A third-party payer-perspective decision analytic model evaluated the cost-effectiveness of reSET-O + TAU relative to TAU (i.e., oral buprenorphine, face-to-face counseling, and contingency management [immediate rewards for negative drug tests logged]) alone over 12 weeks. Clinical effectiveness data (retention in therapy and health state utilities) were obtained from the peer-reviewed literature, while resource utilization and cost data were obtained from a published claims data analyses. Over 12 weeks, the addition of reSET-O to TAU resulted in a gain of 0.003 quality-adjusted life years (QALYs), and $1,014 lower costs, resulting in economic dominance vs. TAU. reSET-O + TAU’s was economically dominant (less costly, more effective) vs. TAU alone over 12 weeks, a result that was driven by a reduction in medical costs after initiation of reSET-O observed in a recent real-world claims analysis.
Introduction
Opioid use disorder (OUD) is a chronic disease characterized by a cluster of cognitive, behavioral, and physiological symptoms indicating that an individual continues using opioid substances despite significant substance-related problems[Citation1]. In the USA (US), since the 1990s, the incidence of OUD and overdose deaths involving opioids has reached epidemic proportions[Citation2]. In 2019, an estimated 9.7 million individuals in the US misused opioids and 1.6 million individuals in the US had an OUD[Citation3]. The current COVID-19 epidemic is compounding the opioid epidemic with increased isolation, risk of depression and increased barriers to care due to social distancing measures. Many states have reported an increase in overdoses since the start of the pandemic[Citation4].
Despite the increasing use of opioids and the increase in opioid-related deaths in the US, most individuals do not receive OUD treatment[Citation5]. Pharmacotherapy (i.e., buprenorphine, methadone, or naltrexone) combined with counseling and behavioral therapy is the recommended first-line treatment for OUD, and is known as medication-assisted therapy (MAT) [Citation6,Citation7]. Medications work to reduce cravings for illicit opioids and/or reduce withdrawal symptoms, while neurobehavioral therapy is needed to support long-term substance avoidance skills and to help restore patients’ enjoyment of healthy interpersonal, social, and vocational activities that have been displaced by substance use.
Retention in therapy is an outcome of paramount importance in the treatment of opioid use disorders, with research indicating that most individuals need at least 3 months in treatment to significantly reduce or stop their drug use and that the best outcomes occur with longer durations of treatment [Citation6,Citation8–14]. Barriers preventing broader access to OUD treatment include stigma, inadequate professional education and training related to the evidence base for using medication, and challenges in connecting individuals with appropriate OUD treatment (time, distance and financial challenges), and also work against patients being retained in treatment over the long term [Citation15,Citation16]. The lack of adequate treatment for many OUD patients has led to high medical costs associated with OUD (projected at $90B in 2020, equivalent to more than $40,000 per patient per year [Citation17]). OUD is responsible for approximately 585,000 emergency department (ED) visits each year, nearly half of which result in inpatient admissions, and the 30-day readmission rate for patients hospitalized with OUD is 24%[Citation18].
Even when patients have access to treatment, dropout rates are high (30% over one month and 50% or higher at three months and beyond) [Citation19–23]. This is why healthcare strategies to improve access and adherence to OUD treatment are of paramount importance to patients and payers.
reSET-O® is the first and only prescription digital therapeutic (PDT) currently authorized by the FDA for the treatment of OUD. The FDA authorization of reSET-O was based on a randomized-controlled trial (RCT) of its academic precursor, the Therapeutic Education System (TES)[Citation24]. The reSET-O therapeutic delivers treatment based on the community reinforcement approach (CRA), an intensive form of cognitive behavioral therapy (CBT) indicated as part of the gold standard treatment for OUD, along with other neurobehavioral therapies such as fluency training (to reinforce learning), and contingency management (to reward positive behaviors)[Citation25]. reSET-O, in conjunction with treatment as usual (TAU; i.e., oral buprenorphine, face-to-face counseling, and contingency management [CM; immediate rewards for negative drug tests logged]), showed significantly increased retention in OUD treatment vs. TAU alone over 12 weeks (80.4% vs 64.1%, respectively)[Citation24], and was ultimately FDA-authorized for this indication in 2018. An analysis of the likelihood of abstinence from opioids and cocaine in this study population showed reSET-O-treated patients were also more likely to be abstinent during weeks 9–12, the final month of treatment (reSET-O+ TAU: 75.9% abstinent, vs. TAU: 60.6%; OR: 2.08; 95% CI 1.10–3.95; P = 0.0248) [Citation26–29].
The economic value of reSET-O has been increasingly studied. An analysis of the cost-utility of reSET-O from the perspective of increased retention in OUD treatment is needed to inform whether reSET-O will provide value beyond TAU, and at what cost. Therefore, the objective of this study was to evaluate the clinical and economic impact of reSET-O in conjunction with TAU compared to TAU alone based on treatment retention data from the pivotal RCT that supported reSET-O’s FDA authorization.
Methods
Study design and model structure
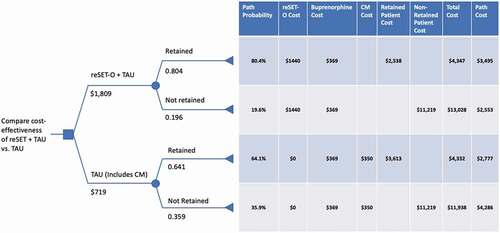
A decision analytic model evaluated the cost-effectiveness of reSET-O in conjunction with TAU (reSET-O + TAU) relative to TAU alone. The model’s perspective is that of the third-party payer, and the time horizon of the model was 12 weeks (the duration of one prescription for reSET-O). Patients treated with reSET-O + TAU or TAU alone were considered not retained in therapy based on their voluntary departure from the trial or after missing three consecutive visits as defined in the pivotal clinical trial by Christensen, et al. ().
Figure 1. Decision analytic model evaluating the cost-effectiveness of reSET-O with TAU (reSET-O + TAU) vs. TAU (i.e., oral buprenorphine, face-to-face counseling, and CM) alone CM, contingency management; OUD, opioid use disorder; TAU, treatment-as-usual
Decision analytic model inputs
Clinical inputs
Retention rates (reSET-O+ TAU: 80.4%; vs. TAU: 64.1%) were obtained from Christensen et al. (2014) ()[Citation24]. Health state utilities were obtained for retained and non-retained patients from Wittenberg et al., 201726 (0.761 for retained patients [similar to patients on stable buprenorphine therapy] vs. 0.694 for non-retained patients [similar to patients with active opioid use]) ().
Table 1. Clinical and economic model inputs for reSET-O+ TAU vs. TAU cost-effectiveness model
Economic inputs
The cost of a 12-week prescription of reSET-O was assumed to be $1,440 based on the Red Book wholesale acquisition cost (WAC) cost of $1,665 and assuming a discount of 13.5%. CM implementation and administration costs of $350 were only included for TAU alone as they are already included in the reSET-O cost. TAU costs over 12 weeks were obtained from an analysis of an early real-world (all-comer) cohort of reSET-O-treated patients (mean age 37 years, 60% female, 82.6% Medicaid) which evaluated total facility and medical services utilization in the 6 months prior to reSET-O initiation (cost input for TAU: $3,613) vs. the 6-months after reSET-O initiation (cost input for reSET-O+ TAU: $2,538)[Citation30]. Costs of non-retained patients ($11,219 over 12 weeks) was obtained from a recent analysis by Wang et al., 2017[Citation31Citation32]. Buprenorphine costs were $369 over 12 weeks (3 prescriptions at $123/prescription) ().
Analyses
Clinical and economic results (consequences) were presented in a simple, disaggregated form to provide decision makers with as broad a view as possible of the consequences of the two interventions. Clinical effectiveness was presented as the number of quality-adjusted life years (QALYs) for each treatment arm over 12 weeks. Disaggregated costs for the two treatment arms included the cost of reSET-O, CM costs, treatment intervention costs (i.e., facility and medical services costs), buprenorphine costs, and medical costs associated non-retention in treatment. One-way sensitivity analyses were performed by varying inputs by 5% to assess impact on cost outcomes.
Results
Base case
Over 12 weeks, the impact on costs and QALYs with reSET-O+ TAU vs. TAU was -$1,014 and 0.003, ().
Table 2. Base case clinical and economic consequences and cost-effectiveness of reSET-O + TAU vs. TAU
Sensitivity analysis
One-way sensitivity analysis showed that a 5% variation of cost and health utility inputs resulted in reSET-O being dominant in all cases; varying the cost of non-retained patients resulted in the largest variation in output although both high and low inputs resulted in reSET-O being economically dominant (low: -$78,615.80/QALY; high: -$94,199.13/QALY). Utility inputs for retained and not retained patients produced the largest overall changes in cost reductions per QALY gained ().
Table 3. Sensitivity analysis of 5% variation in model inputs
Discussion
This analysis found reSET-O + TAU was shown to be economically dominant (i.e., 0.0027 QALYs more effective and $1,014 less costly) compared to TAU alone over 12 weeks. Reductions in medical costs after initiation of reSET-O exceeded the amount needed to offset the cost of the PDT, while greater retention in treatment drove QALY gains. One-way deterministic sensitivity analyses showed the model results to be robust, and reSET-O was economically dominant in all cases. Variation of health utility scores for retained and non-retained patients had the biggest impact on the results of the model although reSET-O remained economically dominant given its cost-reducing effect.
The challenge to healthcare payers and providers is to maximize the net benefits obtained from healthcare expenditures. Comparative effectiveness research intends to help identify cost-effective medical treatments and, in turn, help curb spending for expensive illnesses such as OUD. This change in spending trajectory is to be achieved, in part, through the selection of therapies that have been proven effective while providing value for investments.
Pharmacotherapy alone is rarely sufficient treatment for substance use disorders[Citation33]. TAU in this modeling evaluation included face-to-face counseling (6 visits over 12 weeks) and CM, which may be challenging for many practices to implement due to limitations in available time, resources, and personnel. There is a significant gap of limited time and resources to provide adequate evidence-based neurobehavioral and pharmacological care to all patients in need of recovery treatment [Citation34,Citation35].
The addition of reSET-O to TAU increases the proportion of patients who are retained in therapy and ultimately results in improved quality of life and a gain of QALYs. These findings are consistent with prior studies which have shown an association between better patient OUD treatment retention and improved clinical and humanistic patient outcomes [Citation36–38]. The burden associated with diminished quality of life from OUD extends beyond the individual, affecting the physical and mental health of the individual’s family; however these QALY benefits were not captured in this analysis.
Relapse from OUD may lead to the transmission of infectious disease, criminal activity, or death[Citation39]. As such, the ultimate treatment goal of patients with OUD is sustained abstinence and recovery of their lives[Citation40]. Treatment is critical for achieving this goal as research has shown that individuals who begin and remain in treatment stop using opioids, decrease their criminal activity, and improve their occupational, social, and psychological functioning[Citation40]. However, less than 35% of adults with OUD in 2019 received treatment for opioid use in the past year, highlighting the need for expanded access to comprehensive OUD treatment[Citation41].
The limitations of this study are mostly those inherent to all decision analytic modeling studies. Economic models combine data from many different sources to inform decisions about resource allocation. They provide more explicit details regarding the potential implications of alternate decisions and therefore can be a valuable input for the decision-making process. However, the model represents a simplification of the complex factors involved in the clinical and economic outcomes of patients with OUD. Although every effort has been made to identify the most relevant inputs for inclusion in this model, the results may not be generalizable to all populations of patients with OUD, or to all regions, and should therefore be interpreted with care.
The utilization of a 12-week time horizon in this modeling evaluation may also be regarded as a limitation as OUD often requires long-term management. However, this use of a shorter time horizon is likely a conservative approach for estimating cost-effectiveness as the benefits of reSET-O, reflected in new learned behaviors, drug refusal skills, and coping mechanisms, can be expected to continue to accrue long after treatment, as has been observed with other PDTs [Citation42,Citation43], with no additional cost incurred due to the device. Hence, the incremental cost-effectiveness would become more favorable. The 12-week time horizon has also been studied in two other published health economic evaluations of reSET-O; the first (Wang et al., 2020) [Citation31] evaluated the impact of adherence with reSET-O but did not include impact on health utilities and therefore could not calculate a cost/QALY outcome, while the second (Velez, et al., 2021) [Citation44] measured health utilities and calculated a cost/QALY based on abstinence rates from the pivotal clinical trial for reSET-O. Future studies should include economic analyses and real-world evidence of the impact of reSET-O beyond 12 weeks, and beyond third-party payers, as it is likely that other public costs related to criminal activity and lost productivity also would be impacted by increasing treatment adherence through PDTs[Citation45].
Lastly, it should be noted that the TAU comparator in this analysis represents a level of care which was implemented in a clinical trial and which is seldom available to patients in usual-care settings. As a result, there is the potential for even greater QALY gains with reSET-O vs TAU, which should also be evaluated in future studies.
Conclusion
reSET-O + TAU’s economic dominance (reduced costs, greater effectiveness) vs. TAU alone over 12 weeks was driven by a reduction in medical costs observed in a real-world claims analysis of reSET-O-treated patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- American Psychiatric Association (APA). Diagnostic and statistical manual of mental disorders (DSM-5). Arlington, VA, American Psychiatric Association;2013
- Rudd RA, Seth P, David F, et al. Increases in Drug and Opioid-Involved Overdose Deaths - USA, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051):1445–6.
- Substance Abuse and Mental Health Services Administration (SAMHSA). Key substance use and mental health indicators in the USA: results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54). 2019; https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf Accessed 2020 Sept 30
- American Medical Association (AMA). Issue brief: reports of increases in opioid-related overdose and other concerns during COVID pandemic. 2020. https://www.ama-assn.org/system/files/2020-09/issue-brief-increases-in-opioid-related-overdose.pdf Accessed 2020 Sept 30.
- Wu L-T, Zhu H, Swartz MS. Treatment utilization among persons with opioid use disorder in the USA. Drug Alcohol Depend. 2016;169:117–127.
- U.S. Department of Health & Human Services. Facing Addiction in America - The Surgeon General’s Spotlight on Opioids. Washington, DC: HHS, September 2018..
- Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Network Open. 2020;3(2):e1920622. e1920622-e1920622.
- Hubbard RL, Marsden ME, Rachal JV, et al. Drug abuse treatment: a national study of effectiveness. Chapel Hill, NC, US: University of North Carolina Press; 1989.
- Sells SB, Simpson DD. The case for drug abuse treatment effectiveness, based on the DARP research program. Br J Addict. 1980;75(2):117–131.
- Anglin MD, Hser Y-I. Treatment of drug abuse. Crime and Justice. 1990;13: 393–460.
- Ball JC, Ross A. The effectiveness of methadone maintenance treatment: patients, programs, services, and outcome. New York, NY, US: Springer-Verlag Publishing; 1991.
- De Leon G. Psychopathology and substance abuse: what is being learned from research in therapeutic communities. J Psychoactive Drugs. 1989;21(2):177–188.
- Gerstein DR, Harwood HJ, eds. Treating Drug Problems: volume 1: a Study of the Evolution, Effectiveness, and Financing of Public and Private Drug Treatment Systems. Washington DC: © 1990 by the National Academy of Sciences; 1990.
- McLellan AT, Alterman AI, Metzger DS, et al. Similarity of outcome predictors across opiate, cocaine, and alcohol treatments: role of treatment services. J Consult Clin Psychol. 1994;62(6):1141–1158.
- National Academies of Sciences Engineering and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on Medication-Assisted Treatment for Opioid Use Disorder. Barriers to Broader Use of Medications to Treat Opioid Use Disorder. Washington, DC: National Academies Press (US); 2019.
- National Academies of Sciences Engineering and Medicine. Medications for Opioid Use Disorder Save Lives. Washington, DC: The National Academies Press.2019.
- Murphy SM. The cost of opioid use disorder and the value of aversion. Drug Alcohol Depend. 2020 Dec 1;217:108382. Accessed June 25, 2021
- Premier. Opioid Overdoses Costing U.S. Hospitals an Estimated $11 Billion Annually. 2019. https://www.premierinc.com/newsroom/press-releases/opioid-overdoses-costing-u-s-hospitals-an-estimated-11-billion-annually Accessed 2020 Jul 28.
- Stark MJ. Dropping out of substance abuse treatment: a clinically oriented review. Clin Psychol Rev. 1992;12(1):93–116.
- Simpson DD. Treatment for drug abuse. Follow-up outcomes and length of time spent. Arch Gen Psychiatry. 1981;38(8):875–880.
- Kang SY, Kleinman PH, Woody GE, et al. Outcomes for cocaine abusers after once-a-week psychosocial therapy. Am J Psychiatry. 1991;148(5):630–635.
- Harris PM. Attrition revisited. American Journal of Evaluation. 1998;19(3):293–305.
- Simpson DD, Joe GW, Brown BS. Treatment retention and follow-up outcomes in the Drug Abuse Treatment Outcome Study (DATOS). Psychol Addict Behav. 1997;11(4):294–307.
- Christensen DR, Landes RD, Jackson L, et al. Adding an Internet-delivered treatment to an efficacious treatment package for opioid dependence. J Consult Clin Psychol. 2014;82(6):964–972.
- Pear Therapeutics Inc. reSET-O - Clinician Instructions for Use. 2019. https://peartherapeutics.com/wp-content/uploads/2019/08/PEAR-MKT-025-reSET-O-Clin-Brief-Sum_Dec2019.pdf Accessed 2020 Aug 17.
- Wittenberg E, Bray JW, Aden B, et al. Measuring benefits of opioid misuse treatment for economic evaluation: health-related quality of life of opioid-dependent individuals and their spouses as assessed by a sample of the US population. Addiction. 2016;111(4):675–684.
- Pyne JM, Tripathi S, French M, et al. Longitudinal association of preference-weighted health-related quality of life measures and substance use disorder outcomes. Addiction. 2011;106(3):507–515.
- Sindelar JL, Olmstead TA, Peirce JM. Cost-effectiveness of prize-based contingency management in methadone maintenance treatment programs. Addiction. 2007;102(9):1463–1471.
- Petry NM, DePhilippis D, Rash CJ, et al. Nationwide dissemination of contingency management: the veterans administration initiative. Am J Addict. 2014;23(3):205–210.
- Velez FF, Colman S, Kauffman L, et al. Real-world reduction in healthcare resource utilization following treatment of opioid use disorder with reSET-O, a novel prescription digital therapeutic. In: Expert Review of Pharmacoeconomics & Outcomes Research (Under Review). In review; 2020.
- Wang W, Gellings-Lowe N, Jalali A, et al. Economic Modeling of reSET-O, a Prescription Digital Therapeutic for Patients with Opioid Use Disorder. J Med Econ. 2021 Jan-Dec;24(1):61-68.
- Institute for Clinical and Economic Review (ICER). Extended-Release Opioid Agonists and Antagonist Medications for Addiction Treatment (MAT) in Patients with Opioid Use Disorder: effectiveness and Value. 2018. https://icer-review.org/wp-content/uploads/2018/04/ICER_Opioid_Use_Disorder_Draft_Evidence_Report_090718-1.pdf Accessed 2020 Sept 23.
- McLellan AT, Arndt IO, Metzger DS, et al. The effects of psychosocial services in substance abuse treatment. JAMA. 1993;269(15):1953–1959.
- Marsch LA, Dallery J. Advances in the psychosocial treatment of addiction: the role of technology in the delivery of evidence-based psychosocial treatment. Psychiatr Clin North Am. 2012;35(2):481–493.
- Pincus HA, England MJ. Improving the Quality of Psychosocial Interventions for Mental and Substance Use Disorders: a Report From the IOM. JAMA. 2015;314(12):1227–1228.
- Ruetsch C, Tkacz J, Nadipelli VR, et al. Heterogeneity of nonadherent buprenorphine patients: subgroup characteristics and outcomes. Am J Manag Care. 2017;23(6): e172-e179.
- Ronquest NA, Willson TM, Montejano LB, et al. Relationship between buprenorphine adherence and relapse, health care utilization and costs in privately and publicly insured patients with opioid use disorder. Subst Abuse Rehabil. 2018;9:59–78.
- Tkacz J, Volpicelli J, Un H, et al. Relationship between buprenorphine adherence and health service utilization and costs among opioid dependent patients. J Subst Abuse Treat. 2014;46(4):456–462.
- National Institute on Drug Abuse (NIDA). Effective Treatments for Opioid Addiction. 2016; www.drugabuse.gov/publications/effective-treatments-opioid-addiction/effective-treatments-opioid-addiction Accessed 2020 Sept 20.
- National Institute on Drug Abuse (NIDA). Principles of Drug Addiction Treatment: a Research-Based Guide (Third Edition). 2018. https://d14rmgtrwzf5a.cloudfront.net/sites/default/files/675-principles-of-drug-addiction-treatment-a-research-based-guide-third-edition.pdf. Accessed 2020 Sept 20.
- Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78–82.
- Christensen H, Batterham PJ, Gosling JA, et al. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): a randomised controlled trial. Lancet Psychiatry. 2016;3(4):333–341.
- Ritterband LM, Thorndike FP, Ingersoll KS, et al. Effect of a Web-Based Cognitive Behavior Therapy for Insomnia Intervention With 1-Year Follow-up: a Randomized Clinical Trial. JAMA Psychiatry. 2017;74(1):68–75.
- Velez FF, Luderer HF, Gerwien R, et al. Evaluation of the cost-utility of a prescription digital therapeutic for the treatment of opioid use disorder. Postgrad Med. 2021;133(4):421–427.
- The Council of Economic Advisers. The underestimated cost of the opioid crisis. 2017. https://www.whitehouse.gov/briefings-statements/cea-report-underestimated-cost-opioid-crisis/
