ABSTRACT
Background:
Gene therapies can treat, prevent, or cure a disease by changing the expression of a person’s genes. They are an innovative strategy for treating genetic disorders; however, they are still emerging on the market access and in the healthcare system. Health technology assessment (HTA) agencies have not yet elaborated any standardised approach for assessing gene therapies; therefore, significant differences can be seen during HTAs carried out in various countries. In this review, we focused on submitted economic models of gene therapies approved for use by the US FDA and EMA with the aim to provide a comprehensive summary of how selected HTA bodies assessed the cost-effectiveness of gene therapies. An additional objective was to examine and discuss differences in the methods used in economic models across countries and drugs.
Methods:
We identified economic models of gene therapies from six countries (NICE, IQWiG, SMC, HAS, CADTH, ICER) and focused on nine agents (Glybera, Imlygic, Strimvelis, Yescarta, Kymriah, Luxturna, Zynteglo, Zolgensma, Tecartus). Details of cost-utility evaluations and budget impact models were reviewed and extracted.
Results:
Overall, 983 publications were identified, and 17 studies were included for the analysis. Reviewed evaluations of gene therapies differed in terms of the study perspective, discounting, extrapolation of outcomes based on limited and immature data, time horizon, and adequate estimation of benefits in terms of quality-adjusted life-years. Methods of economic evaluations were in line with the current recommendations; however, long-term follow-up studies are still missing.
Conclusions:
Discrepancies in an economic evaluation of gene therapies between different HTA bodies are rooted in a lack of general assessment frameworks specific to gene therapies. Although challenges were resolved by adjustments to the currently used value assessment framework, new methodological approaches would be useful. In addition, to improve the methods and quality of an evaluation, further research would be valuable.
Introduction
The American Medical Association defines gene therapies as ‘a novel approach to treat, cure, or ultimately prevent disease by changing the expression of a person’s genes.’ These therapies are an innovative strategy designed to cure genetic disorders, including rare and complex diseases, but are still emerging on the market access and in the healthcare system [Citation1]. Gene therapies have the potential to provide benefits not only to affected patients but also the society. As gene therapies are a novel treatment approach, they may be assessed by health technology assessment (HTA) agencies with different criteria, considering different perspectives. A standardised approach to gene therapies has not been elaborated yet. Moreover, gaining marketing authorisation does not imply a positive recommendation across each country. When observing the history of individual gene therapies, significant differences become apparent. In this review, we focused on nine gene therapies approved for use in the USA by the US Food and Drug Administration (FDA) and in the EU by the European Medicines Agency (EMA) with the aim to provide a comprehensive summary of how selected HTA bodies assessed the cost-effectiveness of gene therapies. An additional objective was to examine and discuss differences in the methods used in economic models across countries and drugs.
Methods
A review was conducted to identify submitted economic models of gene therapies across HTA bodies from six countries, namely the National Institute for Health and Care Excellence (NICE, UK), Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG, Germany), Scottish Medicines Consortium (SMC, Scotland), Haute Autorité de Santé (HAS, France), Canadian Agency for Drugs and Technologies in Health (CADTH, Canada), and Institute for Clinical and Economic Review (ICER, United States; referred further as ICER-US). We focused on the following gene therapies Glybera (alipogene tiparvovec, UniQure, EU/US authorisation), Imlygic (talimogene laherparepvec, Amgen Inc., EU/US authorisation), Strimvelis (autologous CD34+ cells transduced to express adenosine deaminase, GlaxoSmithKline, EU authorisation), Yescarta (axicabtagene ciloleucel, Gilead, EU/US), Kymriah (tisagenlecleucel, Novartis, EU/US authorisation), Luxturna (voretigene neparvovec, Spark Therapeutics Inc, EU/US authorisation), Zynteglo (betibeglogene autotemcel, bluebird bio, EU authorisation), Zolgensma (onasemnogene abeparvovec, Novartis, EU/US authorisation), and Tecartus (brexucabtagene autoleucel, Gilead, US authorisation). The included drugs are presented in .
Table 1. Type of gene therapies
Searches were performed independently by two analysts. The final inclusion of a certain document into a further review was based on a consensus between analysts or according to the recommendation of a senior co-author. The list of approved products was identified from the regulatory websites of the FDA for the USA and EMA for the European Union, and all approved gene therapies between October 2012 and July 2020 were recorded. All identified products were used as keywords for the purposes of the performed search. The search was complemented with additional keywords connected with gene therapies, like the names of gens or genetic disorders.
For each identified product, we searched for reports from the relevant HTA bodies listed above. Only full cost-utility evaluations and budget impact models were considered. An extraction grid was developed to collect information such as the name of the product, country and HTA agency, name of the report, year of publication, indication, key model assumptions, key scenario analysis, cost of the evaluated drug, cost of the comparator, incremental quality-adjusted life-year (QALY), incremental costs, incremental cost-effectiveness ratio (ICER), limitations, perspective, type of the model, data extrapolation, discount rate, and sensitivity analysis (Appendix 1).
Extraction was made by one analyst and the quality was checked by a second analyst. In case of any discrepancy, a senior co-author was consulted. Two types of analysis were performed for the purposes of this paper – a comparison of HTA assessments of the same product across countries, and a comparison of HTA assessments for different products within the same country.
The consolidation of findings per country across products and per product across countries was performed. Key features, limitations and reasons of uncertainty related to each economic evaluation were identified and analysed.
Results
Identified economic evaluations
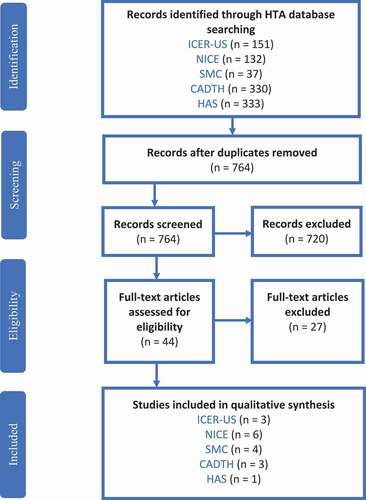
Overall, 983 publications were identified; 219 duplicate records were removed; and 722 papers were excluded after screening. After screening the table of content and introduction or abstract, further 27 records were excluded. Finally, 17 studies were included for the full-text review and data extraction. The Prisma diagram is shown in .
A limited number of documents were available from Germany and France. No evaluations were found for Tecartus probably because it is a relatively new therapy. Also, Glybera, because of the withdrawal, had no recent assessments. The HTAs are listed in . A part of the gathered information is presented in . Full extraction is available in Appendix 1.
Table 2. Health technology assessments
Table 3. Partial extraction
Model characteristics
Type of model
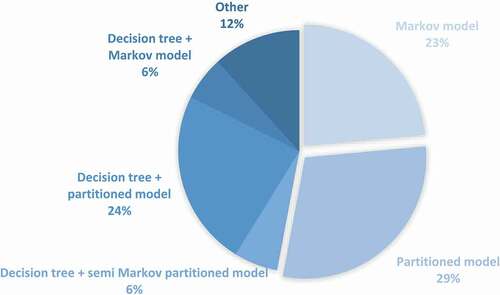
The most often used model types for gene therapies were partitioned survival and Markov models. Similar models were submitted for given drugs in different countries with some differences in case of models assessed by ICER-US. More details are presented in .
Data extrapolation
Common methods to extrapolate data were parametric survival models, hazard models and cure models. Data were extrapolated for approximately 83% of products based on visual inspection and statistical fit. The choice of appropriate curve range between Weibull, exponential, generalised gamma, Gompertz, log-logistic and log-normal distribution. Additional survival extrapolation scenarios were observed as well.
Perspective
All economic evaluations focused on a healthcare system and third-party payer perspective, except for HAS where a collective perspective was adopted. A societal perspective was included as a scenario analysis in all evaluations but HAS and NICE reports.
Time horizon and discount rate
The same discount rate for both cost and outcomes was used among all presented economic models of ICER (3%), NICE (3.5%), SMC (3.5%), CADTH (1.5%), and HAS (4%). In almost all economic evaluations, the lifetime time horizon was used. The one exception was Kymriah for patients with diffuse large B-cell lymphoma (DLBCL), for which CADTH used a 20-year time horizon and SMC, a 46-year time horizon.
Gene therapy across HTA bodies
Different recommendations () were made regarding the considered gene therapies in various countries.
Table 4. Gene therapy recommendation
For three gene therapies (Zolgensma, Imlygic, and Strimvelis), economic evaluations from only one HTA body was found. The ICER-US conducted an economic evaluation for Zolgensma, while NICE for Imlygic and Strimvelis. NICE is also working on an economic evaluation of Zolgensma, but the results have not yet been published.
ZOLGENSMA
ICER-US
The economic analysis was evaluated from a health care sector perspective with a 3% discount rate for cost and benefits. The de novo decision-analytic model including a short-term model and long-term model with a lifetime time horizon was made. The long-term risks of mortality, different parametric distributions (exponential, Weibull, gamma, Gompertz, long-normal, log-logistic, and generalised gamma) were fitted. Best fitting curves were based on statistical fit, clinical plausibility, and visual inspection. Despite a lack of long-term follow-up studies and a small number of patients in clinical trials, Zolgensma was found to be effective in treating patients with spinal muscular atrophy especially in improving motor function and reducing the need of using permanent ventilator support. Nevertheless, the price of $2 million is a reason why Zolgensma does not meet traditional cost-effectiveness benchmarks and needs to be reduced to meet a $150,000 per QALY threshold. The ICER ratio was $243,000 [Citation2].
IMLYGIC
NICE
A small sample of patients in the clinical trials and a lack of a direct comparator are the key limitations raised by NICE when assessing the model. No information about a discount rate, type of model, perspective, time horizon, or extrapolated data was published in the economic evaluation. Nevertheless, Imlygic was proven to have an improvement in the overall survival. The cost-effectiveness ratio versus the main comparator was not calculated because of the lack of suitable effectiveness inputs. However, the committee considered additional analyses on the cost-effectiveness of Imlygic compared with dacarbazine and best supportive care. The ICER was approximately £23,900 and £24,100 per QALY gained. Thus, NICE recommended Imlygic for adults for whom immunotherapies are not suitable [Citation3].
STRIMVELIS
NICE
The first-line treatment option for treating adenosine deaminase deficiency-severe combined immunodeficiency (ADA-SCID) is a haematopoietic stem cell transplant (HSCT) from the human leukocyte antigen (HLA)-matched related donor (MRD). If this is not available, the next option is an HSCT from an HLA-matched unrelated donor (MUD). Strimvelis is recommended as an option for ADA–SCID when no suitable human leukocyte antigen-matched related stem cell donor is available. In the economic evaluation, a decision tree and Markov model were used with a lifetime time horizon and discounted rate of 3.5% for costs and benefits. There is no information about survival extrapolation or perspective from which analysis was conducted. Despite a small sample size in the clinical trials, high cost, and uncertainties in the evidence, the plausible cost-effectiveness estimates for Strimvelis are within the range that NICE considers acceptable for highly specialised technologies. Moreover, Strimvelis is likely to provide benefits not captured in the economic analysis. The cost-effectiveness ratio in the company base case was £36,360 for MUD and £14,645 for MRD per QALY gained. However, after a few changes made by the evidence review group (ERG), ICER increased to £86,815 for MUD and £16,704 for MRD per QALY gained. With the committee final changes with a 3.5% discount rate, the ICER was £120,506 for MUD and £12,106 for MRD per QALY gained. At a 1.5% discount rate, the results of the model were £74,430 for MUD per QALY gained, and Strimvelis was dominant (that is more effective and less costly) for MRD [Citation4].
KYMRIAH
The main differences in economic evaluations of Kymriah assessed in different countries were related to the way of calculating utilities and applied discount rates [Citation5–10]. The models were similar in structure: a decision tree followed by a partitioned survival model, description of stages, and included adverse events. Only CADTH did not consider a decision tree. To model survival, parametric survival curves or spline models were used, except for Scotland for patients with B-cell acute lymphoblastic leukaemia (ALL). Different distributions (exponential, Weibull, log-normal, log-logistic, Gompertz, generalised gamma) were selected to estimate cure fraction. The chosen one was influential on the cost-utility analysis.
ICER
ICER recommended treatment using Kymriah for patients with B-cell ALL and those with DLBCL. A decision tree and semi-Markov partitioned survival model were chosen for the economic evaluation with a lifetime time horizon. A 3% discount rate for both costs and benefits was taken. The analysis was made from a third-party payer perspective. Nevertheless, some limitations were underlined i.e., lack of head-to-head comparator, short-term follow-up on progression-free survival and overall survival, thus the long-term effectiveness is still unknown. The ICER was $45,871 per QALY gained for patients with B-cell ALL. The ICER did not model Kymriah for patients with DLBCL. However, a positive recommendation has been granted in this indication noticing that net health benefit compared with salvage chemotherapy may be substantial [Citation7].
NICE
Kymriah meets the NICE criteria to be considered as a life-extending treatment at the end of life for patients with DLBCL. In the economic evaluation, a decision tree and partitioned survival model were used. There is no information about a time horizon, discount rate or perspective. Key limitations in the submitted economic model refer to available clinical evidence, i.e., a small number of patients in the clinical trials, short-term follow-up, and single-arm study. However, more data on the overall survival, progression-free survival, and immunoglobulin usage can reduce this uncertainty. The cost-effectiveness ratio in the base case was £46,325 per QALY gained. The ERG made some changes around the administration of treatment, costs, adverse events of treatment, and utility for patients in different stages. After those changes, the cost-effectiveness ratio ranged between £42,991 and £55,403 per QALY gained. The committee agreed with all ERG’s assumptions and the value of ICER. NICE could not recommend Kymriah for routine use in the National Health Service (NHS) but recommend it for use within the Cancer Drugs Fund as an option for treating patients with DLBCL after two or more systemic therapies [Citation9].
For patients with B-cell ALL, the NICE end-of-life criteria were not met because of the uncertainty connected with life expectancy evidence. Also, evidence concerning the treatment efficacy is uncertain, and the cost of treating side effects is difficult to estimate. In the presented partitioned survival model with a discount rate of 3.5%, there is no information about perspective and time horizon. Additionally, a decision tree was chosen. To extrapolate survival, a standard parametric model with a generalised gamma was used. However, the committee considered using a mixture cure model, like it was done in SMC and selected a different distribution. The cost-effectiveness ratio was over £30,000 per QALY gained compared with blinatumomab. Kymriah cannot be recommended for routine use in the NHS. However, with the assumption that more data on the overall survival, immunoglobin usage and subsequent stem cell transplant rates will reduce the uncertainty, NICE recommended it as an option for treating patients with B-cell ALL in relapse post-transplant patients or in the second or later relapse in the Cancer Drugs Fund [Citation8].
CADTH
A lack of head-to-head comparative efficacy and safety data, underestimated costs of Kymriah, not taking into account heterogeneity in patients’ characteristics and small considered variation in input parameters are the key limitations in economic models for both indications of Kymriah. Nevertheless, CADTH recommended Kymriah for patients with DLBCL after two or more lines of systemic therapy and for paediatric and young adult patients from 3 to 25 years of age with B-cell ALL. In the economic model for patients with B-cell ALL, a lifetime time horizon (70 years) was used, and the higher costs of treatment were taken into consideration compared with the US model. For patients with DLBCL, the utilities were dependent on the health state and taken from a randomised multicentre study while the time horizon was set at over 20 years. Both partitioned survival models were conducted from the Canadian healthcare system perspective and assumed a discount rate of 1.5%. The incremental cost-utility ratio in the base case was $42,093 for patients with B-cell ALL and $131,716 for patients with DLBCL. CADTH made a reanalysis considering differently an indirect comparator, impact of stem cell transplantation and cost of bridging therapy for patients with DLBCL and impact of subsequent hematopoietic stem cell transplantation, data source for utility and also cost of bridging therapy for patients with B-cell ALL resulting in an ICER of $211,870 for patients with DLBCL and $53,269 for patients with B-cell ALL per QALY [Citation5].
SMC
SMC treated Kymriah as an ultra-orphan medicine and accepted greater uncertainty in an economic evaluation. Kymriah is recommended for adult patients with DLBCL after two or more lines of systemic therapy. In the economic model, a decision tree and partitioned survival model were chosen with a 46-year time horizon. The main comparators were two kinds of salvage chemotherapy. There is no information about a model perspective or discount rate. More important limitations underlined by SMS included a single-arm trial and ignorance of the impact of waiting times and bridging therapy on an efficacy analysis set. For both models, utility values were gained through the use of the EQ-5D questionnaire in patients with DLBCL. The time horizon considered was 46 years. The ICER was £44,330 and £44,151, depending on the comparator [Citation10].
Kymriah is also recommended for patients with ALL and it meets the SMC end-of-life criteria for this indication. A decision tree and partitioned survival model were chosen with a lifetime time horizon (88 years). No information about the model perspective and a discount rate were published. To model survival, a mixture cure model was chosen with the Weibull and generalised gamma distribution. The key weakness in the economic model raised by SMC concerned the use of indirect comparison and a relatively small number of patients in clinical trials. The cost-effectiveness ratio in the base case analysis was £25,238 per QALY gained [Citation11].
YESCARTA
A partitioned survival model was used in economic evaluations of Yescarta in every country; however, the ICER-US used, in addition, a decision tree before partitioned survival modelling was started [Citation7,Citation12–14]. The main differences in the models concerned applied discount rates and assumptions about costs, initial age, adverse events, data extrapolation, and clinical trials. According to the company’s preferences, a mixture cure model for overall survival extrapolation and parametric curve for progression-free survival were used. Except for ICER, base-case survival was derived using parametric survival modelling from the direct extrapolation of progression-free survival and overall survival curves for 5 years after therapy completion. Alternative scenarios and exploratory analyses were considered.
ICER-US
A short-term follow-up limited comparative evidence. The small size of the sample in the clinical trials and an unknown mechanism for payment of chimeric antigen receptor (CAR) T-cell therapies were the main limitations raised by ICER-US. Despite this, Yescarta met the commonly cited cost-effectiveness threshold in the USA and ICER-US rated it as beneficial in terms of overall survival, disease-free survival, and gains in QALY compared with chemotherapy. In comparison with other models, ICER-US also considered a decision tree before partitioned survival modelling was applied, and assumed additional costs for administration, hospitalisation, and adverse events. The model was conducted from a third-party payer perspective with a lifetime time horizon and discount rate of 3% for both costs and benefits. The ICER was $136,078 per QALY [Citation7].
NICE
Yescarta met the NICE criteria to be considered a life-extending treatment at the end of life. In the economic model with a lifetime time horizon, a 3.5% discount rate was used. There is no published information about a perspective used. The ERG proposed using different single parametric models for overall survival for Yescarta. However, based on the available data, it concluded that both approaches (company base case mentioned at the beginning) were appropriate. The main limitations in the economic model concerned the lack of direct comparison data, limited follow-up, immature survival data and unknown cost of side effects. A different assumption than this in other countries about the initial age was considered. However, this treatment seems to be cost-effective and further data from an ongoing study should reduce the uncertainty in the evidence. Therefore, Yescarta is recommended for use as an option within the Cancer Drugs Fund. The cost-effectiveness ratio in the company’s base case was above £50,000 per QALY gained. After ERG’s changes in extrapolating data, costs, time of treatment of an adverse event, and assumptions related to clinical inputs and outcomes, ICER increased to over £100,000 per QALY gained. The committee decided that the most plausible ICER would be the one between these two cases [Citation13].
CADTH
The economic evaluation was conducted from the Canadian healthcare system with a lifetime time horizon (44 years) and a discount rate of 1.5% for costs and benefits. Yescarta is recommended with the incremental cost–utility ratio of $84,030 per QALY gained for the manufacturer base case. However, CADTH highlighted the uncertainty in the cost-effectiveness model connected with a lack of head-to-head comparator, generalisability of the patient population, small size of the sample in the clinical trials, and long-term duration of remission. CADTH made a reanalysis taking into consideration alternative assumptions regarding costs, duration of an adverse event, initial age, and data extrapolation. The ICER was estimated to be $226,131 per QALY gained [Citation3].
SMC
SMC treated Yescarta as an ultra-orphan medicine. In the economic evaluation, there was no information about a perspective and discount rate. A lifetime time horizon (44 years) was used. Sensitivity analyses related to survival modelling were presented. Different single parametric distributions were used for progression-free survival and the assumption of a cure fraction parameter value for overall survival. Because of that, greater uncertainty in economic evaluation connected with high up-front costs, a single-arm study, and limited clinical data, was accepted. Compared to chemotherapy, this treatment significantly improved and extended the patient’s life. The ICER was £49,136 per QALY [Citation14].
LUXTURNA
All models for Luxturna, except for the one assessed by ICER-US, have the same structure and main assumptions. The primary outcome from the clinical trial was the multi-luminance mobility test, which was not used in the economic evaluation because of limited data linking this outcome with costs, utilities, and mortality. This was raised as a limitation by some authorities. In turn, visual acuity and visual field outcomes have been used in the economic analysis.
ICER
Luxturna is recommended for patients with confirmed biallelic retinal pigment epithelium-specific 65 kDa (RPE65) protein mutation. However, for the young population, Luxturna provided more health benefits and thus is likely to be more cost-effective for them. The key limitations related to the assessment of this drug concern a small sample size in clinical trials, and the fact that the natural history of the disease has not been thoroughly studied making the comparison difficult. Another restriction refers to measures of effectiveness considered outcomes in the clinical trials. It was difficult to determine which measures could quantify the quality of life. The Markov model was made from the US healthcare system perspective with a discount rate of 3% for both costs and benefit and a lifetime time horizon. To extrapolate visual acuity based on visual fit, an exponential function was created. Also, a linear form was chosen to calculate an average visual field. The cost-effectiveness ratio was $643,813 per QALY for patients with a mean age of 15 years. ICER-US is the only HTA body that made its own model with different health states, assumptions of initial age, and a function to calculate utilities. In the model, also an additional ICER per blindness-free year was calculated [Citation15].
NICE
In the published economic evaluation, there was no information about a time horizon and perspective. The Markov model was conducted with a 3.5% discount rate for both costs and benefits. During the long-term phase of the model, a parametric function determined the probability of transition to the next health state (parametric multistate model). Across different distributions, the Weibull model was selected based on visual inspection and statistical fit. According to NICE, even if there is no long-term clinical evidence, Luxturna seems to improve the vision of patients and maintain this effect for decades. However, the duration of treatment effect and its impact on a patient’s quality of life are the key limitations in the cost-effectiveness model. Nevertheless, Luxturna is likely to provide clinical benefits. The cost-effectiveness ratio in the company base case was £86,635 per QALY gained. After ERG’s changes in calculated transition probabilities, duration of treatment effect, mortality, utilities, and costs, the ICER increased to £155,750 per QALY gained. Finally, changes approved by the committee gave an ICER range between £114,956 and £155,750 per QALY gained. NICE recommended Luxturna for patients with RPE65-mediated inherited retinal dystrophies [Citation16].
SMC
Luxturna is recommended for patients with confirmed biallelic RPE65 protein mutation. However, there are some limitations of the assessment raised by SMC: small sample size in the clinical trial, lack of inclusion of the primary outcome from the clinical study and high cost in relation to health benefit. Luxturna, however, meets the SMC ultra-orphan criteria. In the cohort-based state transition, a Markov model with a healthcare system perspective and a lifetime time horizon was used. There is no published information about a discount rate. To model long-term effectiveness, a parametric multistate survival model was fitted to the data and the Weibull distribution was used in the base-case due to its performance in terms of statistical fit and visual inspection. The ICER was £89,871 per QALY [Citation17].
HAS
Construction of health state, limited data for utilities and small sample of patients in the clinical trial were the key uncertainties impacting the assessment of the economic analysis. A Markov model was conducted from a collective perspective with a lifetime time horizon (85 years). In the analysis, 4% for costs and benefits was used. The approach to modelling survival was the same as in the NICE evaluation. In the HAS assessment, adverse events that were not connected to visual ability were included. Indirect costs were also taken into consideration, but only in the scenario analysis. Also, additional ICER per blindness-free years was calculated. Luxturna was considered to have a high clinical benefit. With an ICER of €191,811 per QALY, HAS recommended Luxturna for use in inherited retinal dystrophies caused by RPE65 gene mutations [Citation18].
HTA bodies across therapy
During assessments of gene therapies, HTA bodies were often trying to fit the specific needs of gene therapies in existing assessment frameworks in order to accept greater ICER thresholds. As those treatments are innovative with potential curative benefits, it was difficult to adjust currently available criteria to specific features of these novel drugs. To manage with higher ICERs and greater uncertainty, different approaches were introduced across countries.
NICE
Six economic evaluations of gene therapies were assessed by NICE. Most of the identified models were based on data for which no long-term follow-up was available, hence, extrapolation was necessary [Citation8,Citation9,Citation13]. Thus, this extrapolation and, in particular long-term survival, is one of the main reasons for uncertainty. Also, the necessity of different assumptions regarding the health states utilities considered in various models was often noticed by NICE. For example, in the Strimvelis model, health states utilities were derived from people with congenital hearing loss and may not reflect patients with ADA-SCID whose hearing loss is acquired during infancy [Citation4].
NICE does not have any specific framework for the assessment of gene therapies. Two drugs, Luxturna and Strimvelis, were appraised using a NICE interim process and methods of the highly specialised technology programme. For some other drugs, supplementary advice to the appraisal committee was considered when treatment could be life-extending for patients and when indications affected a small number of patients with incurable illnesses [Citation19]. So-called end-of-life criteria allow the use of lower discount rates and accept higher ICER when a threshold range that is normally approved is exceeded. In the majority of the models, because of high uncertainty that gene therapies would meet these criteria for using a discount rate of 1.5%, both rates (3.5% and 1.5%) were considered in different scenarios. Only Kymriah in relapsed or refractory B-cell ALL was recognised as not curative, thus a 3.5% discount rate was used [Citation8].
Two drugs met both criteria to be considered a life-extending treatment at the end of life (Kymriah for DLBCL and Yescarta) [Citation9,Citation13]. Thus, in the economic evaluation higher uncertainty could be considered.
The ICER ranged between £14,645 and £86,635 across manufacturer models and £12,106 and £155,750 in NICE revised base cases, which shows a significant interference of authorities in the economic evaluation.
Highly specialised technologies with an ICER below £100,000 are normally accepted as cost-effective treatments. In the cases when the ICER was above £100,000 judgment about acceptability was satisfied. There was sufficient evidence that both Luxturna and Strimvelis offer significant QALY gains [Citation4,Citation16]. In the submitted model, the ICER was difficult to determine for Strimvelis compared with ipilimumab because of uncertainties in the relative clinical effectiveness of these agents. However, using a different comparator than proposed by the company, NICE concluded that this drug is a cost-effective option [Citation4]. All oncology drugs met the criteria to be included in the Cancer Drugs Fund [Citation8,Citation9,Citation13].
SMC
Four assessments of gene therapy products were found in SMC. All these economic models were evaluated according to the ultra-orphan drug framework. Only oncology drugs (Kymriah and Yescarta) were also analysed considering the end-of-life criteria [Citation14–16]. The Task and Finish GroupFootnote1 noted that there is considerable overlap between the end-of-life criteria and orphan medicines, while ultra-orphan medicines were a separate, distinct category [Citation20].
For end-of-life medicines, a Patient and Clinician Engagement (PACE) meeting was held to consider the added value of these drugs in the context of treatments currently available in the NHS in Scotland. A key output from the PACE meeting is more clarity on the clinicians and patients views on the need for the medicine, e.g., based on a ‘value’ which is not fully captured by the QALY, such as disease severity and the level of unmet need in that population, and the impact on carers. Implicitly, this support acceptance of medicines means a higher cost per QALY. For ultra-orphan drugs, the preferred approach is to include the nature of the condition, the impact of the medicine, the impact of the technology beyond direct health benefits, and value for money. This approach is consistent with the interim methods being explored by NICE in England [Citation21]. These criteria allow accepting greater uncertainty for decision-making.
The economic analyses for Yescarta and Kymriah for patients with B-cell ALL were based on a ‘mixture cure’ model; this structural assumption implies that those gene therapies potentially offer long term curative benefits (without the need to link better outcomes through providing a bridge to potentially curative transplants as it was for Kymriah (or patients with relapsed or refractory DLBCL) [Citation10,Citation11,Citation14]. On the other hand, the duration of treatment effect for Luxturna is highly uncertain, as no long-term data are available, with only tangential evidence on potential lifetime effects from non-human studies [Citation17].
In each assessment, SMC highlighted uncertainty regarding health-related quality of life data, costs sources, and methods of data extrapolation.
According to SMC, the extremely high upfront acquisition cost for one-time administration of gene therapies is associated with a financial risk if long-term predicted clinical benefits do not materialise. But high comparative benefits were highlighted against available treatment. The ICER ranged between £25,238 and £89,871 across all submitted models. A higher threshold was accepted in the case of the evaluation according to an ultra-orphan pathway.
ICER-US
The ICER-US acknowledged that the currently used assessment framework may not work with ongoing costs and a higher ICER [Citation21].
So far, ICER-US assessed five economic evaluations for gene therapies. Zolgensma and Luxturna were analysed according to the value assessment framework for ultra-rare diseases [Citation2,Citation15]. For these treatments, ICER-US adapted its analyses to provide the willingness-to-pay threshold results for a broader range, from $50,000 per QALY to $500,000 per QALY. Similarly, to help payers decide whether a price is reasonable, ICER-US calculated a health-benefit price benchmark for these treatments using the standard range from $100,000 to $150,000 per QALY [Citation22]. On the other hand, Kymriah and Yescarta met the commonly cited threshold. To manage uncertainty, a threshold scenario analysis was performed to get a clear idea of whether the new treatment price comes below the ICER threshold of $150,000 per QALY gained over alternatives [Citation7].
The critique of all models relates to the lack of robust data and comparative evidence. There were no long-term follow-up clinical trials, hence the duration of benefit and treatment effect remain uncertain. Moreover, utility data applied in economic evaluations were limited. For Luxturna, the majority of available quality of life literature has used a visual acuity; therefore, the primary outcomes from a clinical study measured with the multi-luminance mobility test were not used. However, for Zolgensma, utility data derived from several sources were considered by ICER-US to be coherent [Citation2,Citation15]. Similarly, cost data were uncertain for CAR T-cell therapies as no uniform source was available, which could lead to variation between sources in a cost calculation. A modified societal perspective performed in the scenario analysis of different models was restricted. In CAR T-cell therapies, models focused only on productivity losses and patient-level costs (transportation, lodging, etc.) during treatment administration [Citation7]. On the other hand, in the Zolgensma economic evaluation, the modified societal perspective did not include the quality of life burden associated with caregivers [Citation2]. The ICER ranged between $45,871 and $261,000 across all models. Zolgensma came close to meeting the ICER thresholds [Citation2].
CADTH
The Canadian HTA body analysed and expressed objective assessment only for CAR T-cell therapies [Citation5,Citation6,Citation12]. Given the unique aspects of these health technologies, CADTH reviewed CAR T-cell therapies through its health technology assessment process for medical devices and clinical interventions, and not through its pan-Canadian Oncology Drug Review (pCODR) or Common Drug Review (CDR) [Citation23].
There is little documentation available on the CADTH website regarding this HTA process. CADTH identified a potential limitation and key uncertainty relating to the economic models of the manufacturer. The main weakness was a lack of head-to-head comparative efficacy and safety. The generalisability of patient characteristics, differences in studies and risk factors under- or overestimated the cost-effectiveness results. Similarly, costs become the determinant for the ICER factor. What distinguishes CADTH from the other HTA bodies is that the analysis comparing Yescarta and Kymriah was conducted [Citation12]. The price of acquisition of both drugs was assumed to be the same in the absence of the published Canadian public price list. Results presented that Yescarta is more costly and less effective. The ICER ranged between $41,028 and $211,870 across manufacturers’ models and $53,269 and $226,131 in CADTH revised base cases.
CADTH undertook a price-reduction analysis based on the manufacturer and CADTH revised base cases. The price reduction scenarios indicated that 83%, 10%, 70% price reduction, respectively, for Yescarta, Kymriah (for patients with B-cell ALL), Kymriah (for patients with DLBCL) would result in the ICER to be below $50,000 per QALY [Citation5,Citation6,Citation12].
Discussion
This study aimed to review economic evaluations assessed to date by HTA bodies in several countries across the world. We focused on similarities and differences of the same assessments between countries as well as on different evaluations within the same country. Several uncertainties related to data extrapolation, perspective, type of model, time horizon and discount rate were highlighted in the analysed model.
Challenges related to the reliable evaluation of gene therapies in the context of their cost-effectiveness have been recently widely discussed in the literature. The same issues are being raised by different authors who are trying to discuss them and provide some recommendations. A lot has been already done in this area; however, we are only in the middle of the way as no general assessment framework specific to gene therapies has been developed. The only available official document was released by ICER-US [Citation22].
Despite the lack of specific assessment frameworks, HTA bodies in different countries had to perform evaluations of gene therapies that had already received marketing authorisation. Almost all of the already known challenges of economic evaluations of gene therapies have been visible in studied assessments. They concerned the study perspective, appropriate discounting, extrapolation of outcomes based on limited and immature data, proper time horizon, and adequate estimation of benefits in terms of the QALYs.
The chosen methods of economic evaluations were generally appropriate according to the indication of gene therapies and available evidence through the clinical study [Citation8]. However, long-term follow-up studies for gene therapies are still not available. Also, heterogeneity and a small patient sample size were emphasised by HTA bodies considering clinical trials for gene therapies. Because of these limitations, data extrapolation is an important source of uncertainty in economic evaluations of gene therapies. There are no definitive extrapolation techniques that could reduce uncertainty and maximise health outcomes. However, it seems that using more sophisticated survival methods will be more effective because there are no long-term data showing the survival plateaus after a certain time [Citation15].
It is believed that economic evaluations should present the potential of future benefits and risks associated with the patient community more effectively. Generally, it is quite impossible to include a full consideration from a patient’s point of view and often requires wide and uncertain assumptions, not only for gene therapy [Citation24]. It has been acknowledged that some models for gene therapies are only able to provide a restricted societal perspective based on the evidence available at this time. Their restricted societal perspective focused on productivity losses and patient-level costs [Citation7]. But the perspective of gene therapies is a more critical topic. An inclusion of functional ability, productivity, and impact on caregivers and social services may have a significant effect on whether the treatment is cost-effective at a given price. For example, in the case of drugs providing more health benefits when given to a younger population, educational costs can be important in cost-effectiveness results [Citation25].
Discounting was carried out mainly in line with general guidelines for economic evaluations in particular countries. NICE proposed a more flexible approach. For example, it included a discount rate of 1.5% for costs and benefit, which may be considered when treatment effects are both substantial in restoring health and sustained over a very long period and if the introduction of the technology does not commit the NHS to substantial irrecoverable costs [Citation3,Citation4,Citation8,Citation9,Citation13,Citation16]. Overall, all HTA bodies consider a different discount rate in sensitivity analyses to examine the results. Gene therapy involves high treatment costs before all health outcomes have occurred, so the choice of the proper discount rate has a particularly significant impact on their cost-effectiveness [Citation26]. Gene therapies require monitoring of the patient’s health changes during their entire lives. Thus, the time horizon considered in economic evaluations was determined as the lifetime in all assessments. However, ICER-US stated that this approach overestimates and biases the long-term benefits of the treatment. A shortened long-term extrapolation would better estimate the value of gene therapies but would also exclude long-term benefits. To deal with this obstacle, ICER-US proposed additional scenario analyses. Assuming a different time horizon model, these scenario analyses reduce uncertainty [Citation27]. This should establish results due to the changing conditions.
HTA bodies in different countries were trying to meet the challenges encountered in various ways, according to the country-specific assessment frameworks. In general, the specific nature of gene therapies along with their significant benefit were well recognised by all the agencies. The other commonly present aspect was, however, related to great uncertainty. This uncertainty cannot be abolished by any robust clinical data in the near future, while the reimbursement decisions must be made already now. HTA bodies tend to take a risk and recommend using gene therapies with a relatively high threshold. They are trying to use already available frameworks for rare diseases or lifesaving treatments. This approach can be a temporary solution for now when only several gene therapies are available. The need for the development of a new assessment framework specific for gene therapies seems to be necessary looking at the long-term perspective.
Our review found that similar economic models were submitted for given drugs in different countries. Some differences were identified especially in the case of models assessed by ICER-US. ICER-US is a unique HTA body that is developing its own economic models. The type of economic model, its structure, inputs, and assumptions made have a great impact on the results. This can be seen in our analysis of different results of the same model between countries and within the country between the submitted and the finally accepted base case. The challenges of the economic evaluation of gene therapies and related to that high uncertainty translate into difficulties in developing and assessing the economic models. It is important to better understand the impact of using different models and assumptions on the conclusions on the cost-effectiveness of gene therapies. Further research is necessary in this regard based on available sources. Based on the results of this review, an interesting object for such analyses is the economic model for Luxturna, for which two different models were used in the USA and other countries. Any further research, however, as well as this review is limited by the availability of data. As economic models are strictly connected with pricing, not all information is always publicly available. This availability differs significantly between countries, which is an additional difficulty.
Conclusions
Some of discrepancies in economic evaluation of gene therapies between different HTA bodies were recognised. Although challenges were resolved by adjustments to the currently used value assessment framework, new methodological approaches would be useful. In addition, to improve the methods and quality of an evaluation, further research would be valuable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes
1 On behalf of the Scottish Medicines Consortium, a group consists of representative key stakeholders, including clinicians, Patient Interest Groups, the pharmaceutical industry and the SMC Patient and Public Involvement Group which undertake a review of the assessment of medicines for end-of-life care and very rare conditions (orphan and ultra-orphan medicines) in Scotland.
References
- Marsden G, Towse A, Pearson SD, et al. Gene therapy: understanding the science, assessing the evidence, and paying for value. ICER; 2017. Available from: https://www.ohe.org/publications/gene-therapy-understanding-science-assessing-evidence-and-paying-value
- ICER. Spinraza® and Zolgensma® for Spinal Muscular Atrophy: effectiveness and Value. Final Evidence Report; 2019. Available from: https://icer-review.org/material/sma-final-evidence-report/
- NICE. Talimogene laherparepvec for treating unresectable metastatic melanoma. Technology appraisal guidance [TA410]; 2016. Available from: https://www.nice.org.uk/guidance/ta410
- NICE. Strimvelis for treating adenosine deaminase deficiency–severe combined immunodeficiency.Highly specialised technologies guidance [HST7]; 2018. [cited 2020 Oct 20]. Available from: https://www.nice.org.uk/guidance/hst7
- CADTH. Tisagenlecleucel (Kymriah) for Pediatric Acute Lymphoblastic Leukemia and Diffuse Large B-Cell Lymphoma; 2019. Available from: https://www.cadth.ca/tisagenlecleucel-kymriah-pediatric-acute-lymphoblastic-leukemia-and-diffuse-large-b-cell-lymphoma
- CADTH. Tisagenlecleucel for Acute Lymphoblastic Leukemia: economic Review Report; 2019 [cited 2020 Nov 27]. Available from: https://cadth.ca/sites/default/files/pdf/car-t/op0538-tisagenlecleucel-economic-report-pALL-jan2019.pdf
- ICER. Chimeric Antigen Receptor T-Cell Therapy for B-Cell Cancers: effectiveness and Value. Final Evidence Report; 2018 [cited 2020 Oct 20]. Available from: https://icer-review.org/material/car-t-therapies-evidence-report/
- NICE. Tisagenlecleucel for treating relapsed or refractory B-cell acute lymphoblastic leukaemia in people aged up to 25 years. Technology appraisal guidance [TA554]; 2018 [cited 2020 Oct 20]. Available from: https://www.nice.org.uk/guidance/ta554
- NICE. Tisagenlecleucel for treating relapsed or refractory diffuse large B-cell lymphoma after 2 or more systemic therapies. Technology appraisal guidance [TA567]; 2019 [cited 2020 Oct 20]. Available from: https://www.nice.org.uk/guidance/ta567
- SMC. tisagenlecleucel (Kymriah) - SMC2200; 2019 [cited 2020 Oct 20]. Available from: https://www.scottishmedicines.org.uk/medicines-advice/tisagenlecleucel-kymriah-resubmission-smc2200/
- SMC. voretigene neparvovec (Luxturna) - SMC2129; 2019 [cited 2020 Oct 20]. Available from: https://www.scottishmedicines.org.uk/medicines-advice/tisagenlecleucel-kymriah-fullsubmission-smc2129/
- CADTH. Axicabtagene Ciloleucel for adults with relapsed or refractory large B-cell lymphoma; 2019 [cited 2020 Oct 20]. Available from: https://www.cadth.ca/axicabtagene-ciloleucel-adults-relapsed-or-refractory-large-b-cell-lymphoma
- NICE. Axicabtagene ciloleucel for treating diffuse large B-cell lymphoma and primary mediastinal large B-cell lymphoma after 2 or more systemic therapies. Technology appraisal guidance [TA559]; 2019. Available from: https://www.nice.org.uk/guidance/ta559
- SMC. axicabtagene ciloleucel (Yescarta®) - SMC2189; 2019 [cited 2020 Oct 20]. Available from: https://www.scottishmedicines.org.uk/medicines-advice/axicabtagene-ciloleucel-yescarta-resubmission-smc2189/
- ICER. Voretigene Neparvovec for Biallelic RPE65-Mediated retinal disease: effectiveness and value. Final Evidence Report; 2018 [cited 2020 Oct 20]. Available from: https://icer-review.org/material/voretigene-evidence-report/
- NICE. Voretigene neparvovec for treating inherited retinal dystrophies caused by RPE65 gene mutations. Highly specialised technologies guidance [HST11]; 2019 [cited 2020 Oct 20]. Available from: https://www.nice.org.uk/guidance/hst11
- SMC. voretigene neparvovec (Luxturna) - SMC2228; 2019 [cited 2020 Oct 20]. Available from: https://www.scottishmedicines.org.uk/medicines-advice/voretigene-neparvovec-luxturna-uoia-smc2228/
- HAS. LUXTURNA (voretigène néparvovec); 2019 [cited 2020 Oct 20]. Available from: https://www.has-sante.fr/jcms/c_2964759/fr/luxturna
- NICE. Appraising life-extending, end of life treatments; 2009 [cited 2020 Oct 20]. Available from: https://www.nice.org.uk/guidance/GID-TAG387/documents/appraising-life-extending-end-of-life-treatments-paper2
- SMC. Assessment of medicines for end of life care and very rare conditions (orphan and ultra-orphan medicines) in Scotland; 2013 [cited 2020 Oct 20]. Available from: ssessment of medicines for end of life care and very rare conditions (orphan and ultra-orphan medicines) in Scotland
- Gardner J ICER draws new gene therapy pricing framework; 2019.
- ICER. Modifications to the ICER value assessment framework for treatments for ultra-rare diseases; 2020 [cited 2020 Oct 20]. Available from: https://34eyj51jerf417itp82ufdoe-wpengine.netdna-ssl.com/wp-content/uploads/2020/10/ICER_URD_Framework_Adapt_013120.pdf
- CADTH. CADTH to Evaluate CAR T-Cell Therapies; 2018 [cited 2020 Nov 27]. Available from: https://www.cadth.ca/news/cadth-evaluate-car-t-cell-therapies-0
- ICER. 2020-2023 value assessment framework; 2020 [cited 2020 Oct 20]. Available from: https://icer.org/wp-content/uploads/2020/10/ICER_2020_2023_VAF_102220.pdf
- Drummond MF, Neumann PJ, Sullivan SD, et al. Analytic considerations in applying a general economic evaluation reference case to gene therapy. Value Health. 2019;22(6):661–18. eng
- Jönsson B, Hampson G, Michaels J, et al. Advanced therapy medicinal products and health technology assessment principles and practices for value-based and sustainable healthcare. Eur J Health Econ. 2019;20(3):427–438. eng
- ICER. Value assessment methods and pricing recommendations for potential cures; 2019 [cited 2020 Oct 20]. Available from: https://icer.org/wp-content/uploads/2020/10/ICER_SST_Report_Response_to_Comments_111219.pdf