 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
The economic impact of adverse events (AEs) for poly (ADP-ribose) polymerase inhibitors (PARPis) in ovarian or breast cancer has not been widely evaluated.
Objective
Compare PARPi-related AE management costs from a US payer perspective.
Methods
The frequency of treatment-related grade 3–4 AEs was obtained from published clinical trials of PARPis for the treatment of advanced ovarian cancer (AOC), platinum-sensitive recurrent ovarian cancer (PSROC), and metastatic breast cancer (MBC). AE management costs per patient (2020 USD) per treatment course were calculated by multiplying the AE unit costs by the frequency of AEs for each arm of each trial. Sensitivity analyses were conducted according to the lower and upper limits of the 95% confidence interval for AE rates and unit costs, respectively. Scenarios were also performed to explore the uncertainty of outcomes.
Results
Total AE management costs in AOC were: $3,904, olaparib; $5,595, olaparib plus bevacizumab; and $12,215, niraparib. In PSROC, total costs were: $3,894, olaparib; $6,001, rucaparib; and $11,492, niraparib, and in MBC: $3,574, olaparib; and $9,489, talazoparib. Hematological toxicities were the key drivers of AE management costs for PARPis.
Conclusions
The main AEs among PARPis were hematological. Olaparib was associated with lower AE costs compared to other PARPis.
Introduction
In the US, ovarian cancer has the fifth highest mortality rate of cancer in women. The survival rate at five years in patients with metastatic ovarian cancer is only 30.3% [Citation1]. Breast cancer is the most commonly diagnosed cancer and the second leading cause of cancer death among women[Citation2]. Six percent of patients are diagnosed at the stage of metastasis with a five-year relative survival rate of 29% [Citation1]. Historically, the treatment options for advanced/recurrent ovarian cancer and metastatic breast cancer (MBC) were limited to surgery and chemotherapy, which are often associated with early disease relapse and poor survival outcomes [Citation3,Citation4]. In recent years, poly (ADP-ribose) polymerase inhibitors (PARPis) have become an effective component of management strategies in advanced/recurrent ovarian cancer (as maintenance therapy; administered alone or in combination with bevacizumab) and MBC (in active therapy) [Citation5–7]. Multiple trials have showed that PARPis provide prolonged progression-free survival benefits for patients with advanced/recurrent ovarian cancer compared to placebo or bevacizumab as well as for patients with BRCA-mutated MBC compared to chemotherapy [Citation8]. A recent network meta-analysis has also raised the possibility that PARPi as a maintenance therapy is superior to bevacizumab in the survival gain for the treatment of platinum-sensitive recurrent ovarian cancer (PSROC) [Citation9].
Although existing economic studies have investigated the overall cost effectiveness of PARPis, modelling approaches such as the comparison of heterogenous trial populations, inconsistent efficacy and AE data for different treatment arms as well as mismatched cost data in the incremental cost-effectiveness ratio calculation have cast doubt on the validity of the cost-effectiveness result [Citation10–12]. Furthermore, limited research explores the specific cost impact of adverse event (AE) management [Citation10,Citation13]. Despite sharing a mechanism of action [Citation14,Citation15], individual PARPis demonstrate distinct safety profiles which may lead to meaningful variations in the costs of managing the AEs associated with these therapies [Citation16,Citation17]. The aim of this study was to explore AE management costs associated with the PARPis olaparib, niraparib, rucaparib and talazoparib relative to the comparators used in phase 3 trials, as well as investigate the consistency and differences of AE profiles across PARPis in the treatment of advanced/recurrent ovarian cancer and MBC.
Methods
Included studies
By the completion of this study, four PARPis olaparib, niraparib, rucaparib and talazoparib had been approved for cancer treatment. The frequency of AEs was sourced from publications of registered (phase 3) clinical trials of licensed PARPis for the treatment of advanced ovarian cancer (AOC), PSROC and MBC. These phase 3 clinical trials identified from the database clinicaltrials.gov are pivotal trials which led to FDA approval ().
Table 1. Phase 3 clinical trials of each PARPi therapy
Inclusion criteria for AEs
For each category, only grade 3–4 AEs were used for the analysis. Grade 1 and 2 AEs were excluded as they were assumed to have limited incremental management costs [Citation18,Citation19].
Only AE categories occurring above a selected incidence cut-off point were included in the analysis. However, AEs were reported at different cut-off points in each publication (). Therefore, for consistency in the analysis reported here, a cut-off point of 15% was applied to AOC, 10% to all PSROC trials, and 15% to all MBC trials. Across the trials in a given indication, any AE category that met the cut-off criteria at least once in any trial was included.
Frequency of AEs
Patients reported to have had multiple occurrences of the same AEs were only counted once. The frequency of AEs was expressed as a percentage of the number of patients in a given trial arm.
Calculation of costs
The inpatient unit costs for each grade 3–4 AE in the base case (where all patients were hospitalized) were obtained from 2017 Agency for Healthcare Research and Quality (AHRQ) Healthcare Cost and Utilization Project (HCUP) database [Citation20]. These unit costs were inflated to 2020 US dollar value according to the CPI-All Urban Consumers: Medical Care in US city average for all urban consumers, not seasonally adjusted [Citation21,Citation22]. Costs of AE management were calculated by multiplying the AE inpatient costs by the frequency of AEs for the intervention and comparator arms of each trial. Since AEs were assumed to occur independently of one another, their costs were added to derive the total cost.
Sensitivity and scenario analyses
Sensitivity analyses were conducted on inpatient unit costs and AE rates. In the sensitivity analyses, the 95% confidence intervals (CIs) of inpatient unit costs were calculated via bootstrapping with 1,000 iterations [Citation23]. The method for determining the 95% CIs of AE rates was chosen according to the criterion for approximate normality (CAN), defined as:
A normal distribution was applied to AEs with a CAN score greater than 5, a relationship between binomial and F distribution was applied to AEs with a CAN score smaller than 5 but greater than 0, the ‘rule of three’ (i.e., 95% CI is [0, 3/sample size]) was applied to AEs with a CAN score of 0 [Citation24]. Two sets of sensitivity analysis results were estimated for total AE management costs:1. AE cost varied by the upper and lower bounds of 95% CI of AE rates (i.e., 95% CI-AR [AE rates]); 2. AE cost varied by upper and lower bounds of 95% CI of inpatient unit costs (i.e., 95% CI-UC [inpatient unit costs]). These two variations of costs were reported along with the total costs from the base case.
Three scenarios (in addition to the base case) were implemented to explore uncertainty around outcomes. The base case assumed that all patients received inpatient treatment. In scenario 1, for each AE, the proportions of patients managed in the inpatient and outpatient setting were estimated to reflect clinical practice in the US (based on clinical values derived from clinical papers as well as assumptions made according to clinical guidelines [Supplementary Table 1]). In scenario 2, it was assumed that an equal number of patients were treated in inpatient and outpatient settings (50% vs 50%). For scenarios 1 and 2, the outpatient unit costs were identified via a targeted literature review. Only two sources of outpatient unit cost for AE management were identified (Supplementary Table 2) [Citation25,Citation26]. Weighted costs were computed according to the percentage of AE cases that were treated in an inpatient versus outpatient setting. Costs of AE management were computed by multiplying the AE weighted costs by the frequency of AEs for each arm. For scenario 3, the inpatient setting was assumed for 100% of patients (as per the base case). Since only two sources of inpatient unit costs were identified (one of which, the HCUP database, was used in the base case), costs from the second source, Biller et al., which through expert interviews estimated mean costs of treatment-related AEs for oncology in the US, were applied [Citation25]. Thus, eleven AE inpatient unit costs (ALT, AST, anemia, diarrhea, dyspnea, hypertension, nausea, decreased platelet count, rash, thrombocytopenia and vomiting [Supplementary Table 3]) were replaced.
Results
Safety profile
A number of randomized controlled trials (RCTs) were identified across the AOC, PSROC and MBC indications (). For AOC, this comprised of two placebo-controlled studies for olaparib (SOLO1) and niraparib (PRIMA), alongside a study which compared olaparib plus bevacizumab vs bevacizumab alone (PAOLA1). For PSROC, this comprised of placebo-controlled studies for olaparib (SOLO2), niraparib (NOVA) and rucaparib (ARIEL3). In MBC, the studies compared olaparib (OlympiAD) or talazoparib (EMBRACA) to chemotherapy treatment of physician’s choice (TPC).
Across all studies, hematological events (primarily anemia, neutropenia, and thrombocytopenia) occurred more frequently in the PARPis treatment arm than the comparator arm except for olaparib in MBC. In the treatment of AOC, the combination of anemia, neutropenia and thrombocytopenia represented 31% of grade 3–4 AEs for olaparib, 73% for niraparib and 25% for olaparib plus bevacizumab, while this combination only accounted for 8%, 3% and 4% in the respective comparator arm. For PSROC, hematological events represented 26% of grade 3–4 AEs for olaparib, 31% for rucaparib and 79% for niraparib, while these were 7%, 2% and 2% in each placebo arm. In MBC, hematological AEs represented 25% and 75% of all grades 3–4 AEs for olaparib and talazoparib, compared to 31% and 41% for TPC respectively. Detailed summaries of AE rates in both the treatment and comparator arms of included studies are provided in the Supplementary Table 4–7.
Total cost of AE management
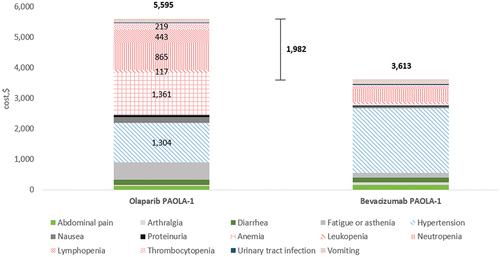
In AOC, the total AE costs per patient per course of treatment were $3,904 for olaparib monotherapy, $12,215 for niraparib () and $5,595 for olaparib in combination with bevacizumab (). The 95% CI-AR values for these total AE costs were $2,322 to $7,491 for olaparib, $9,388 to $15,750 for niraparib and $3,816 to $7,515 for olaparib in combination with bevacizumab. The 95% CI-UC values were $3,694 to $4,119, $11,290 to $13,287 and $5,159 to $6,047 respectively.
Figure 1. AE management (total and per category) costs per patient for PARPi monotherapy in AOC, by trial arm. Hematological AE categories are shown in patterned fill, with costs shown for treatment arms only.
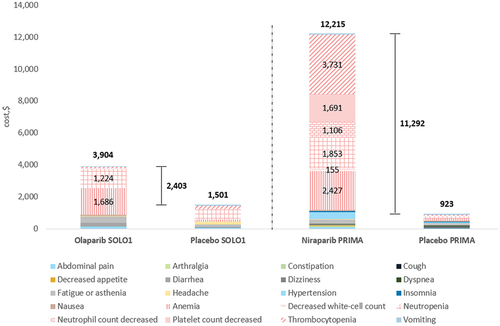
Figure 2. AE management (total and per category) costs per patient for olaparib plus bevacizumab in AOC, by trial arm. Cost drivers (hematological AE categories and hypertension) are shown in patterned fill, with costs shown for treatment arms only.
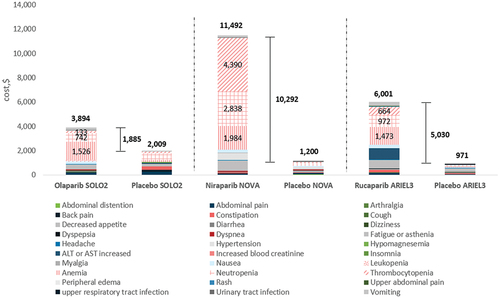
In PSROC, the total AE costs per patient per course of treatment were $3,894 for olaparib, $6,001 for rucaparib and $11,492 for niraparib (). The 95% CI-AR values for these total AE costs were $1,784 to $9,880 for olaparib, $3,659 to $9,953 for rucaparib and $8,808 to $15,958 for niraparib. Accordingly, the 95% CI-UC values for these were $3,622 to $4,180, $5,517 to $6,505, and $10,583 to $12,367.
Figure 3. AE management (total and per category) costs per patient for PARPi monotherapy in PSROC, by trial arm. Hematological AE categories are shown in patterned fill, with costs shown for treatment arms only.
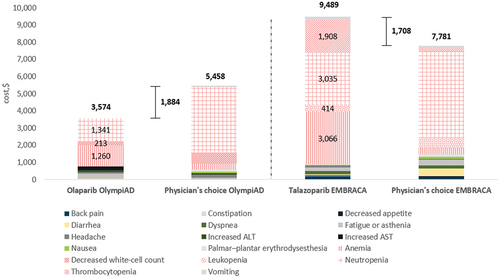
In MBC, the total AE costs per patient per course of treatment were $3,574 for olaparib and $9,489 for talazoparib (). The 95% CI-AR values for these total AE costs were $1,861 to $7,149 for olaparib and $6,854 to $13,234 for talazoparib. The 95% CI-UC values were $3,413 to $3,959 for olaparib and $8,783 to $10,193 for talazoparib.
Figure 4. AE management (total and per category) costs per patient for PARPi monotherapy in MBC, by trial arm. Hematological AE categories are shown in patterned fill, with costs shown for treatment arms only.
Incremental AE costs
In the treatment of AOC, PARPi monotherapies had an incremental AE cost per patient per course of treatment of $2,403 for olaparib and $11,292 for niraparib versus placebo alone (). Compared with bevacizumab monotherapy, the incremental AE cost of olaparib plus bevacizumab was $1,982 ().
In the treatment of PSROC, incremental AE cost per patient per course of treatment was $1,885 for olaparib, $5,030 for rucaparib, and $10,292 for niraparib versus placebo alone ().
In the treatment of MBC, olaparib reduced the cost of AE management per patient per course of treatment by $1,884, whereas talazoparib incurred a $1,708 extra cost, compared with TPC (capecitabine, eribulin, vinorelbine or gemcitabine) ().
Hematological AEs
The cost burden across all PARPis and indications was predominantly driven by hematological toxicities. In the treatment of AOC with olaparib monotherapy, anemia ($1,686) and neutropenia ($1,224) were the most expensive hematological AEs to manage (). In the treatment of AOC with niraparib monotherapy, the costliest hematological AEs were thrombocytopenia ($3,731), anemia ($2,427), neutropenia ($1,853), decreased platelet count ($1,691) and decreased neutrophil count ($1,106) (). In the treatment of AOC with olaparib plus bevacizumab, anemia ($1,361) and hypertension ($1,304) incurred the highest costs amongst AEs (). However, it was stated that the high frequency of hypertension in the PARPis arm was considered to be primarily due to bevacizumab itself rather than olaparib [Citation27].
In the treatment of PSROC with olaparib, anemia was the most prevalent and costly AE to manage ($1,526). This was also true for rucaparib-treated patients ($1,473), whereas for niraparib, thrombocytopenia ($4,390), neutropenia ($2,838) and anemia ($1,984) were the most prevalent and costly AEs to manage ().
In the treatment of MBC with olaparib, anemia and neutropenia accounted for a large proportion of the cost burden of managing AEs ($1,260 and $1,341 respectively). When treated with talazoparib, anemia and neutropenia were again the primary cost drivers ($3,066 and $3,035), in addition to thrombocytopenia ($1,908) ().
Scenario analyses
In scenario 1 (estimated proportions from inpatient vs outpatient settings for each AE), the AE cost patterns across PARPi therapies and indications were slightly different from the base case. Particularly, anemia did not drive the cost any more as blood transfusion was assumed to be performed in an outpatient setting in scenario 1. Concerning the treatment of AOC, costs in the olaparib arm were mainly driven by the management of neutropenia ($480). Hypertension ($920) which was primarily caused by bevacizumab alone was the only significant cost driver in the olaparib plus bevacizumab arm. The management of hematological toxicities, excluding anemia, was the predominant driver in the niraparib arm ($3,103). In PSROC, neutropenia ($291) and vomiting ($207) were the two costliest AEs in the olaparib arm. The management of thrombocytopenia ($1,573) and neutropenia ($1,112) represented the largest proportion (72%) of AE management costs in the niraparib arm. In contrast to the base case, increased ALT/AST ($897) turned out to be the primary cost driver in the rucaparib arm. For MBC, neutropenia was the costliest AE to treat in both PARPi arms ($525 for olaparib and $1,189 for talazoparib) (Supplementary Table 8).
Across all indications and treatment therapies, the AE patterns and predominant cost drivers in scenarios 2 (50% inpatient and outpatient settings for all AE categories) and 3 (variation of AE inpatient unit costs) were mostly consistent with the base case. In both scenarios 2 and 3, hematological toxicities drove the substantial costs of managing AEs, which was in line with the results from the base case. Nevertheless, in scenario 3 for the treatment of MBC, it is noteworthy that the AE management cost in the olaparib arm was $160 higher than TPC ($6,612 vs $6,453), which was contrary to the base case where the corresponding incremental cost was negative. Additionally, the results from scenario 3 showed that the cost to treat thrombocytopenia surpassed the cost of managing neutropenia in the talazoparib arm, which contrasted with the outcomes of base case as well as scenarios 1 and 2.
All three scenarios also showed that total costs to manage AEs were lower among olaparib-treated patients than other PARPis-treated, which kept consistency with the base case. A comparison of cost drivers across three scenarios for all PARPis has been presented in the supplementary material (Supplementary Table 8).
Discussion
In cancer settings where multiple effective therapeutic options currently exist, treatment choice is based on a wide range of factors in addition to clinical effectiveness – including quality of life, ease of administration, AE profile, and costs. Previous studies have explored the safety profiles and the overall cost effectiveness of PARPis across their licensed indications [Citation10,Citation13]. However, to our knowledge, this is the first study which specifically explores the costs associated with AE management within the context of the US healthcare system.
A comparison of the treatment and respective comparator arms of each trial showed that there was a comparable incidence of grade 3–4 fatigue, asthenia, and other gastrointestinal events including abdominal pain, constipation, and diarrhea. The addition of a PARPi maintenance therapy contributes to an increase in the incidence of hematological based AEs, including anemia, neutropenia and thrombocytopenia. Nonetheless, evidence shows that these common hematological AEs are largely transient and manageable [Citation28–30]. When exploring differences in AE rates between PARPis and their respective comparators, olaparib was found to be associated with lower incidences of neutropenia and thrombocytopenia compared with niraparib and rucaparib for both AOC and PSROC. For MBC, olaparib was associated with lower rates of anemia, neutropenia and thrombocytopenia than talazoparib.
The primary finding from our analysis is that despite better clinical efficacy, AE management costs with PARPis are higher compared with no maintenance therapy or bevacizumab alone (AOC only) in the AOC and PSROC populations. Olaparib had lower AE costs while talazoparib had higher AE costs compared to TPC in the MBC population. Incremental AE costs were primarily attributed to hematological AEs. However, olaparib is associated with lower AE rates than other PARPis, which suggests the favorability of olaparib with respect to safety profile. Consequently, olaparib had both lower total and incremental AE costs than the other PARPis.
Previous studies of grade 3–4 AE management costs typically assume that these events will always be managed within an inpatient setting [Citation18,Citation19], thus potentially overstating the associated costs. In our scenario analyses, we sought to adjust for this by applying weights derived from the literature to the proportion of each type of AE which were managed within an inpatient or outpatient setting. The scenario analyses also confirmed that hematological AEs remained the costliest AEs to manage for PARPis across all indications. However, their relative cost impact versus other non-hematological AEs was reduced when a lower proportion of AEs were assumed to be managed in the inpatient setting.
Limitations
Our study has several potential sources of methodological limitations. Firstly, real-world AE patterns, and therefore their associated management costs, may differ from those in clinical trials because real-world treatment patterns may differ from those in clinical trials. For instance, the occurrence of grade 3–4 AEs among patients with PSROC who initiated niraparib 200 mg/day in the real-world setting was much lower than those patients who were given niraparib 300 mg/day, as per the clinical trial protocol [Citation31,Citation32]. This implies that in our analysis (which is based on clinical trials), the AE management cost associated with niraparib is likely to be overestimated. However, the clinical trials remain the best source to describe and compare AE profiles and costs between PARPis until sufficient real-world data are available.
Secondly, assumptions were made about AE management in real-world settings. The management of 1–2 AEs was assumed to be minimal as only mild or minimal intervention is required [Citation18,Citation19]. In scenario 1 of the sensitivity analysis, for each AE, the proportions of patients managed in the inpatient and outpatient setting were estimated to reflect clinical practice in the US based on values derived from clinical papers as well as assumptions made according to clinical guidelines. For example, grade 3–4 PARPi-related hematological AEs are generally managed through dose discontinuation or dose reduction only compared to other chemotherapies [Citation33]. Indeed, scenario 1 assumes that 100% of patients with anaemia, 73% of patients with neutropenia and 70% of patients with thrombocytopenia were managed in the outpatient setting (Supplementary Table 1). Whether these assumptions led to our analysis overestimating the real-world costs for haematological AEs would require external validation, since the management of AEs in real-world settings is currently only sparsely documented.
Additionally, a variety of cut-off points for grade 3–4 AEs were applied to achieve consistency within the same indication. This implied that some more serious AEs incurring large costs that did not meet the inclusion criteria might have been excluded in this analysis. For example, pneumonia and pulmonary embolism for PSROC were removed due to their rare occurrence despite their potentially large management costs.
Furthermore, a variety of patient populations were included in PARPi trials. For instance, the trial EMBRACA (talazoparib vs TPC) included patients who had advanced or metastatic breast cancer even though the locally advanced breast cancer merely accounted for 5% while OlympiAD (olaparib vs TPC) only comprised patients with metastatic disease[Citation34], which necessitated within-trial comparisons of AE frequency and management costs only.
To our knowledge, no studies have investigated the costs of management of AEs for PARPis in the real world. Further evidence is therefore required to validate and support our findings, such as a network meta-analysis and real-world comparative effectiveness studies.
Further research including external validation around the unit costs and weighting assumptions is required to validate and support our findings. Real world evidence relating to AE patterns, hospitalization rates and actual unit costs is needed to improve the reliability of our results.
Conclusions
Hematological events were the most prevalent AEs across PARPis, yet they were normally transient and manageable. Predominantly driven by hematological toxicities, PARPis increased AE management costs relative to routine surveillance and conventional therapies in the treatment of advanced/recurrent ovarian cancer and MBC in general. Compared with placebo, olaparib had lower incremental AE costs than other PARPis. Due to a lower frequency of hematological AEs occurring in olaparib-treated patients compared to niraparib, rucaparib and talazoparib, the total costs of AE management were lower for olaparib.
This analysis can inform clinical decision making on optimizing treatment in ovarian and breast cancer, providing information on AEs and associated management costs which can supplement considerations of clinical effectiveness.
Supplemental Material
Download MS Word (118.5 KB)Disclosure statement
LF, DM, MM and JRE are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. YZ and PM are employed by Wickenstones Ltd, a company that received consultancy fees from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. AM and PG are employees of AstraZeneca PLC.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20016689.2022.2078474
Additional information
Funding
References
- SEER. Cancer stat facts. 2021; https://seer.cancer.gov/statfacts/more.html
- USCS. U.S. cancer statistics data visualizations tool, based on 2020 submission data (1999-2018). 2021; https://gis.cdc.gov/Cancer/USCS/#/AtAGlance/
- Bahena-González A, Toledo-Leyva A, Gallardo-Rincón D. PARP inhibitors in ovarian cancer: evidence for maintenance and treatment strategies. Chin Clin Oncol. 2020;9(4):51.
- Cortesi L, Rugo HS, Jackisch C. An overview of PARP inhibitors for the treatment of breast cancer. Target Oncol. 2021;16(3):255–9.
- Scott CL, Swisher EM, Kaufmann SH. Poly (ADP-ribose) polymerase inhibitors: recent advances and future development. J Clin Oncol. 2015;33(12):1397–1406.
- Du Y, Yamaguchi H, Hsu JL, et al. PARP inhibitors as precision medicine for cancer treatment. Natl Sci Rev. 2017;4(4):576–592.
- Palleschi M, Tedaldi G, Sirico M, et al. Moving beyond PARP inhibition: current state and future perspectives in breast cancer. Int J Mol Sci. 2021;22(15):7884.
- Rose M, Burgess JT, O’Byrne K, et al. PARP inhibitors: clinical relevance, mechanisms of action and tumor resistance. Front Cell Dev Biol. 2020;8(879). DOI:10.3389/fcell.2020.564601
- Bartoletti M, Pelizzari G, Gerratana L, et al. Bevacizumab or PARP-inhibitors maintenance therapy for platinum-sensitive recurrent ovarian cancer: a network meta-analysis. Int J Mol Sci. 2020;21(11):3805.
- Guy H, Walder L, Fisher M. Cost-effectiveness of niraparib versus routine surveillance, Olaparib and Rucaparib for the maintenance treatment of patients with ovarian cancer in the USA. PharmacoEconomics. 2019;37(3):391–405.
- Guy H, Walder L, Fisher M. Response to ‘comment on “cost-effectiveness of Niraparib versus routine surveillance, Olaparib and rucaparib for the maintenance treatment of patients with ovarian cancer in the USA”’. PharmacoEconomics. 2019;37(7):965–967.
- Wallace K, Goble S, Isaacson J, et al. Cost-effectiveness of niraparib versus routine surveillance, Olaparib and Rucaparib for the maintenance treatment of patients with ovarian cancer in the USA. PharmacoEconomics. 2019;37(8):1065–1067. Comment on.
- Muston D, Hettle R, Monberg M, et al. Cost-effectiveness of olaparib as a maintenance treatment for women with newly diagnosed advanced ovarian cancer and BRCA1/2 mutations in the USA. Gynecol Oncol. 2020;159(2):491–497.
- Brown JS, O’Carrigan B, Jackson SP, et al. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov. 2017;7(1):20–37.
- Zhu H, Wei M, Xu J, et al. PARP inhibitors in pancreatic cancer: molecular mechanisms and clinical applications. Mol Cancer. 2020;19(1):49.
- LaFargue CJ, Dal Molin GZ, Sood AK, et al. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019;20(1):e15–e28.
- Madariaga A, Bowering V, Ahrari S, et al. Manage wisely: poly (ADP-ribose) polymerase inhibitor (PARPi) treatment and adverse events. International Journal of Gynecologic Cancer. 2020;30(7):903–915.
- Wu L, Zhong L. Budget impact analysis of niraparib and olaparib for maintenance treatment of platinum-sensitive, recurrent ovarian cancer in the US. J Med Econ. 2019;22(2):187–195.
- Zhong L, Tran AT, Tomasino T, et al. Cost-effectiveness of Niraparib and Olaparib as maintenance therapy for patients with platinum-sensitive recurrent ovarian cancer. J Manag Care Spec Pharm. 2018;24(12):1219–1228.
- AHRQ. HCUPnet. 2017; https://hcupnet.ahrq.gov/#setup, 2021.
- Bureau of Labor Statistics. The economics daily, consumer price index: 2017 in review. 2018; https://www.bls.gov/opub/ted/2018/consumer-price-index-2017-in-review.htm?view_full, 2021.
- Bureau of Labor Statistics. The economics daily, consumer price index: 2020 in review. 2021; https://www.bls.gov/opub/ted/2021/consumer-price-index-2020-in-review.htm?view_full
- Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1(1):54–75.
- Jovanovic BD, Zalenski RJ. Safety evaluation and confidence intervals when the number of observed events is small or zero. Ann Emerg Med. 1997;30(3):301–306.
- Bilir SP, Ma Q, Zhao Z, et al. Economic burden of toxicities associated with treating metastatic melanoma in the USA. Am Health Drug Benefits. 2016;9(4):203–213.
- Wolford JE, Bai J, Eskander RH, et al. A Markov model to evaluate cost-effectiveness of the PARP inhibitor, olaparib, for fourth-line treatment of recurrent ovarian cancer. J Clin Oncol. 2016;34(15_suppl):5563.
- Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus Bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381(25):2416–2428.
- Nooka AK. Management of hematologic adverse events in patients with relapsed and/or refractory multiple myeloma treated with single-agent carfilzomib. Oncology. 2013;27(3):11–18.
- Harvey RD. Incidence and management of adverse events in patients with relapsed and/or refractory multiple myeloma receiving single-agent carfilzomib. Clin Pharmacol. 2014;6:87–96.
- Colombo N, Moore K, Scambia G, et al. Tolerability of maintenance olaparib in newly diagnosed patients with advanced ovarian cancer and a BRCA mutation in the randomized phase III SOLO1 trial. Gynecol Oncol. 2021;163(1):41–49.
- Gallagher JR, Heap KJ, Carroll S, et al. Real-world adverse events with niraparib 200 mg/day maintenance therapy in ovarian cancer: a retrospective study. Future Oncol. 2019;15(36):4197–4206.
- Berek JS, Matulonis UA, Peen U, et al. Safety and dose modification for patients receiving niraparib. Ann Oncol. 2018;29(8):1784–1792.
- Madariaga A, Bowering V, Ahrari S, et al. Manage wisely: poly (ADP-ribose) polymerase inhibitor (PARPi) treatment and adverse events. Int J Gynecologic Cancer. 2020;30(7):903–915.
- McCrea C, Hettle R, Gulati P, et al. Indirect treatment comparison of olaparib and talazoparib in germline BRCA-mutated HER2-negative metastatic breast cancer. J Comp Eff Res. 2021;10(13):1021–1030.
- Moore K, Colombo N, Scambia G, et al. Maintenance Olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495–2505.
- González-Martín A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391–2402.
- Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–1284.
- Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–2164.
- Coleman R, Oza A, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10106):1949–1961.
- Robson M, S-A I, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533.
- Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763.
