ABSTRACT
Background
The economic consequences of the recent COVID-19 pandemic were substantial. However, direct medical costs in France have not been determined.
Objective
To describe patient characteristics, intensity of care, mortality, and direct medical costs in patients hospitalised for COVID-19 infections in France.
Study design
A retrospective study of the French national hospital claims database for 2020.
Setting
Hospital care.
Patients or other participants
All patients hospitalised for COVID-19 in 2020 were included and classified by hospitalisation duration into acute phase and prolonged COVID-19.
Intervention
Stratification by intensity of care (Level 1: no or low-flow oxygen support; Level 2: non-invasive ventilation; Level 3: mechanical ventilation).
Main outcome measure
Cost of hospital care in 2020 Euros from a payer perspective.
Results
199,455 patients were hospitalised for COVID-19 in France in 2020. 17,824 patients (8.9%) received mechanical ventilation and 32,602 patients (16.3%) died. Mean per patient cost was €5,510 ± 7,142. This cost was highest in patients receiving Level 3 care, patients aged >80 years and in those with prolonged COVID.
Conclusion
The economic burden of hospitalisations for COVID-19 infections in France during 2020 was substantial. The study provides robust baseline data to benchmark advances in the standard of care and to nurture epidemiological models.
Introduction
Coronavirus disease (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus. The disease can cause symptoms ranging from mild to critical. Patients with severe disease may require extended hospitalisation, including prolonged intensive care stays, and this has led to hospital services being overwhelmed in many countries, with demands for beds and care outstripping supply. In France, the first documented cases of COVID-19 were reported in January 2020. Over the year, the epidemic in France developed in two distinct waves, the first from February to June inclusive and the second from September inclusive, with an estimated 15% of the population having been infected with SARS-CoV2 over the course of the year [Citation1]. Early estimates indicated that 2.9% of infected individuals were hospitalised and 0.5% died [Citation2]. Older individuals, men, and patients with metabolic, respiratory, or cardiovascular comorbidities appear to be at higher risk of requiring hospitalisation or intensive care if they have COVID-19, and to be at higher risk of dying.
The availability of a single comprehensive healthcare insurance database in France, Programme de Médicalisation des Systèmes d’Information (PMSI) offers the possibility of identifying all patients in the country who were hospitalised with COVID-19 and evaluating the healthcare provisionthey received. In the present report, we have used the PMSI database to evaluate retrospectivelythe burden of COVID-19 in the hospital setting for the year 2020. The specific objectives of the study were to describe the characteristics of patients hospitalised, to describe the level of intensity of care that was required for their management, and to estimate the direct medical costs of these hospitalisations to the French national health insurance.
Methods
This was a retrospective study of healthcare resource consumption in the hospital setting performed in the French national health insurance claims database. The study population consisted of all patients hospitalised for COVID-19 in 2020. The costing analysis was performed from the payer perspective (national health insurance).
Data source
Data were obtained from the PMSI, the unique repository of data on all in-hospital resource consumption in acute stay hospitals in the public and private sectors. Stays in independent rehabilitation units or in long-term psychiatric hospitals were not covered.
For each hospital stay, a standardised discharge summary (SDS) is generated and used for tariffication of the stay. The SDS covers the entire stay, regardless of whether the patient has been hospitalised in different departments. For example, a patient initially hospitalised in a chest medicine department, then moved to intensive care before returning to the chest medicine ward will have a single SDS generated on discharge. Stays are identified on the SDS by the reason for hospitalisation as a diagnosis-related group (DRG), coded using the International Classification of Diseases 10th Edition. The DRG is associated with a categorical severity modifier determined by the intensity of care provided. Medical procedures and tests are classified by procedure codes and entered into the SDS. No information is available on the results of clinical examinations or tests. The only personal data available for the patient is the age, gender, and place of residence. If a patient dies, the date of death is documented.
Study population and identification of stays
The study included all patients hospitalised with an ICD-10 diagnostic code for COVID-19 documented on the SDS between 31st January 2020, the date when a DRG for COVID-19 was first introduced, and 31st December 2020. Some changes to the DRG codes, to allow entry of more precise information, were made between 31st January and the end of April. The diagnostic codes used are provided in the Supplemental Material. Due to overload of certain hospitals at the height of the epidemic, certain patients were transferred immediately after admission between hospitals. If this occurred, the stay with the longest duration was retained for the analysis.
The study population was initially stratified into four subpopulations based on the intensity of care. These were hospitalisation with no oxygen support, hospitalisation with oxygen support, hospitalisation with non-invasive ventilation and hospitalisation with mechanical ventilation. The subgroups were identified from the relevant procedure codes documented on the SDS. These are provided in the Supplemental Material. Due to underreporting of medical procedures related to oxygen support during the study period, and since patient characteristics in the ‘no oxygen’ and ‘oxygen support’ subgroups were similar, these two subgroups were combined.
Data extraction
Information was extracted from the database on age, gender, and place of residence. Risk factors for complications of COVID-19 infections were identified. The choice of risk factors was based on the list defined by the French National Health Authority (Haute Autorité de la Santé; HAS) for use of neutralising monoclonal antibodies in ambulatory patients with mild or moderate COVID-19, as indicated in the French prescribing information for these products. These were identified principally on the basis of ICD-10 diagnostic codes on the SDS and include COPD or respiratory failure, hypertension, heart failure, diabetes, immunodeficiency, chronic renal failure, idiopathic pulmonary fibrosis, amyotrophic lateral sclerosis, myopathy, trisomy 21 and obesity. It should be noted that comorbidities are only identified on the SDS if they influence the cost or course of hospitalisation and may thus be under-reported. Patients receiving chemotherapy or radiotherapy for cancer were identified from the relevant DRG code. The diagnostic or DRG codes used to identify patients at risk are provided in the Supplemental Material. Finally, age >80 years was also considered a risk factor. On the basis of these risk factors, patients were divided into four groups, namely patients without risk factors, patients aged >80 years, patients who were immunosuppressed (patients with immunodeficiency syndromes, and patients undergoing chemotherapy) and patients with other medical risk factors (all other risk factors listed above).
Each hospital stay was identified by date and by length of stay. Stays were divided into those lasting up to 30 days inclusive and those lasting >30 days. Each stay was assigned to one of the three levels of intensity of care as described above; in cases where more than one level of care was provided during the same stay, the stay was defined by the most intense level of care provided. Rehospitalisations were defined as a second hospitalisation separated from the first by at least 14 days. Patients with either a first hospitalisation lasting >30 days or who required rehospitalisation were considered to have prolonged hospitalisation for COVID-19. If the patient died in hospital, this was documented.
Cost analysis
Since 2007, health care in France is financed through an activity-based tariffication system (tarification à l’acte). In this system, each hospital stay is costed upon discharge using fixed tariffs, and the total cost of the stay is documented on a Standardised Discharge Summary (SDS) which is accounted in the PMSI. For accounting purposes, costs for all hospitalisations are aggregated annually from data in the PMSI, firstly at the hospital level, and secondly at the national level by the Agence Technique de l’Information sur l’Hospitalisation (ATIH), a government agency in charge of assessment and planning of healthcare resources. Aggregated national data are used by the health authorities for planning resource attribution for future years.
The SDS lists a fixed stay cost based on the reasons for hospitalisation (including costs for any procedures performed during the stay). This cost is based on a specific tariff for the disease, classified according to the International Classification of Diseases (10th Edition) of the World Health Organization, adjusted for each individual stay by a number of modifiers describing comorbidities, disease severity, and procedures, such as operations, undergone during the hospitalisations. Each stay is thus assigned to a diagnosis-related group (DRG), which is allocated a daily tariff, which covers the cost of physician time, nursing care, board, and overnight stay costs and all medication and non-pharmacological treatments that are standard care for the condition. Stays in intensive care incur a supplemental cost. Certain expensive medical devices or drugs incur a top-up cost and are charged in addition to the stay cost. The most recent published DRG tariffs cover 2019, and these were updated to 2020 costs in Euros using the hospital consumer price index. Hospitalisations in the private sector may incur additional costs related to higher honoraria for physicians, and this information is captured in the French National Costs Study [Citation3].
The unit daily base tariff for hospitalisation for COVID-19 ranged from €2,385 to €8,516 in the public sector, depending on severity and other cost modifiers. The daily supplemental cost of a stay in an intensive care unit was €404.08 (without mechanical ventilation) or €807.21 (with mechanical ventilation).
Statistical analysis
Five analytical populations were considered in this study. The first consisted of all patients included in the study. The second consisted of all patients hospitalised for the first time for an acute phase COVID-19 infection. The third subgroup consists of patients presenting with a prolonged COVID-19 hospitalisation, as defined above. The fourth and fifth were subsets of the second and third subgroups and consisted of all patients with acute-phase COVID-19 or prolonged COVID-19 hospitalisation presenting a risk factor as defined above. Since there were two waves of the COVID-19 epidemic in France in 2020, patients hospitalised during each wave were described separately for certain analyses. The first wave was arbitrarily defined as lasting from 1st January 2020 until 30th June 2020 and the second wave from 1st July 2020 until 31st December 2020. The prolonged COVID-19 hospitalisation group was only analysed for the first wave due to the risk of incomplete data for the second wave, which was still in progress at the end of the data collection period (31st December 2020).
The study was purely descriptive, and no statistical hypotheses were tested. Continuous variables are described by mean values with their standard deviation (SD) or median values with their interquartile range (IQR). Categorical variables are presented as frequency counts and percentage.
Ethics
The study was conducted in accordance with all relevant regulatory requirements. Use of the PMSI database is regulated by the Health Data Hub (HDH). The study was authorised by ATIH (the French agency of hospital database) in July 2020 under the MR 006 process.
Results
Study participants
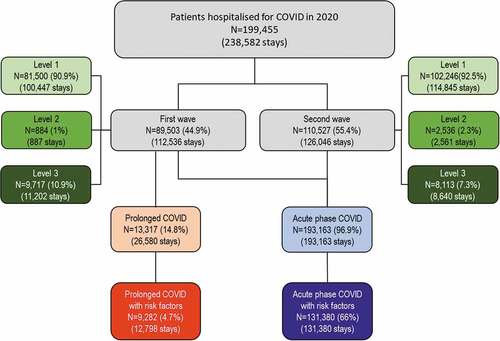
In 2020, 199,455 patients were hospitalised for COVID-19 in France, 89,503 during the first wave and 110,527 during the second (). In the majority of cases (183,142 patients; 91.8%), patients received the lowest intensity of care (Level 1: no or low-flow oxygen support), 3,420 (1.7%) patients received non-invasive ventilation (Level 2) whereas 17,824 patients (8.9%) received mechanical ventilation (Level 3). The distribution of patients over the three levels of care was similar between the two waves, with the exception of a small increase in the number of patients receiving non-invasive ventilation during the second wave, and a small decrease in those receiving mechanical ventilation. Overall, 193,163 patients were initially hospitalised for <30 days (acute phase COVID population) and 13,317 from the first wave were either initially hospitalised for >30 days or were hospitalised more than once (prolonged COVID population). The proportion of patients with risk factors was 68.0% in the acute phase COVID population and 34.9% in the prolonged COVID population.
Figure 1. Distribution of patients and stays by wave and intensity of hospitalisation.
Men were over-represented in the overall population hospitalised (), and the proportion of men increased with the intensity of care from 52.6% at Level 1 to 72.2% at Level 3. The mean age of the patients was 65.7 years (median: 69 years) and was relatively similar across all levels of intensity of care ().
Table 1. Patient characteristics by level of respiratory support.
With regard to risk factors, patients requiring more intensive care were more frequently at risk (). The proportion of patients with at least one identified risk factor increased from 63.4% at Level 1 to 90.6% at Level 3. In contrast, the proportion of patients aged >80 years was under-represented in patients receiving more intensive care (). The proportion of patients who were immunosuppressed was low (0.4% overall) at all care intensity levels.
The distribution by intensity of care level and by age in the different analysis subpopulations is provided in the Supplemental Material. Patients in the subgroups with risk factors were older (43.4% of acute-phase patients with risk factors aged >80 years compared to 29.5% of those without risk factors) and more frequently received more intense care (8.4% receiving Level 3 care versus 6.0%, respectively).
Hospitalisations
The 199,455 patients made a total of 238,582 hospital stays, with 10,321 patients (13.5%) being hospitalised more than once. The mean length of hospital stay was 8.32 ± 9.95 days (median: 6 days; IQR: 1–12; range: 0–360 days). Stay length increased both with the intensity of care and with the age of the patient ().
Figure 2. Length of stay.
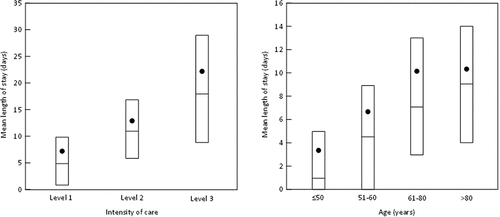
For the patients who survived their hospitalisation, the hospital stay lasted <30 days in 193,163 cases (69.3% of all stays). The proportion of stays lasting over 30 days in survivors increased as a function of age (26.5% for patients aged <50 years, 30.5% for the 51–60-year age group, 36.3% for the 61–80-year age group and 40.7% for the >80 year age group).
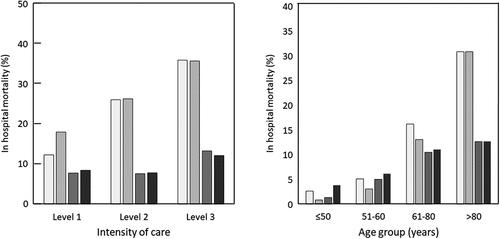
Overall, 32,602 patients (16.3%) died during the hospital stay, with the mortality rate increasing with the intensity of care (); for the patients receiving mechanical ventilation (Level 3), 36.3% of patients died during their stay. Around two-thirds of patients (67.2%) were discharged directly home, with this proportion decreasing with the level of intensity of care received (). The remaining patients were transferred to another care facility at the end of their hospitalisation.
Table 2. Discharge and in-hospital mortality.
Of the 76,282 patients hospitalised during the first wave who survived, 10,321 (13.5%) were subsequently rehospitalized for a COVID-19-related reason later in 2020. Rehospitalisation was most frequent in patients who had received mechanical ventilation during their first stay ().
Overall mortality was 16.3% in the overall population, 14.4% in the acute-phase analysis population and 20.3% in the acute phase with risk factors analysis population. Mortality rose as a function of both age and the level of intensity of care (). In the subgroup of acute-phase patients aged >80 years, the mortality rate in patients who required Level 3 intensity care rose to 69.0% (N = 671). For the patients who died, the mean duration of hospitalisation between admission and death was 9.96 ± 10.15 days (median [IQR]: 7 [3 to 13]). The mean stay length prior to death increased with the level of care from 7.97 ± 7.28 days at Level 1 to 17.9 ± 15.0 days at Level 3.
Costs
In the overall population, the mean per patient cost of hospitalisation for COVID-19 was €5,510 ± 7,142, corresponding to a total cost for all patients of €1.1 billion. Median (with IQR and range) and mean (± SD) costs according to the intensity of care provision are presented in . The spread of hospitalisation costs was very broad, reaching €219,661 in one patient. The mean cost increased six-fold between Level 1 and Level 3 care intensities, and was almost doubled in the >80-year age group compared to the group of patients aged ≤50 years (although the mean cost was in fact highest in the 60-80-year age group).
Table 3. Per patient costs of hospitalisation in the overall population.
In the different subpopulations, the cost also generally increased with intensity of care and with age (). The prolonged COVID hospitalisation subpopulation generated mean per patient costs that were nearly three times higher than those in the acute-phase COVID-19 subpopulation, even when restricting to patients with risk factors (€13,648 versus €4,753 for the whole subpopulations, €16,915 versus €5,954 for the subpopulations with risk factors).
Table 4. Mean per capita costs of hospitalisation in analysis subpopulations.
Discussion
This study in a national insurance claims database has demonstrated the high economic cost of hospitalisations for COVID-19 in France, with a mean per capita cost of €5,510. For the population of 199,455 patients hospitalised in 2020, this amounts to a total cost to national health insurance of over one billion euros. Although patients with prolonged COVID accounted for less than one-quarter of all patients hospitalised with COVID-19 during the first wave, on a per capita basis, the cost of their management was nearly three-fold higher than for patients with acute-phase COVID-19. The study documents all hospitalised patients with COVID-19 in France and yields a precise estimate of in-hospital mortality (16.3%) and the proportion of patients hospitalised for COVID-19 who require intensive care (10.9% received mechanical ventilation).
The total number of hospital stays in 2020 (15.9 million) was similar to the number in 2019, the year before the pandemic (15.7 million) [Citation8]. As a result, the care of patients hospitalised for COVID-19 could only be managed at the expense of a reduction in hospitalisations for other pathologies. It has been estimated that the number of these other hospitalisations fell by 13% between 2019 and 2020 [Citation8]. With respect to cost, the total cost of hospitalisations in 2020 was somewhat lower than the cost in 2019 (€71.3 billion versus €74.1 million), due to economies gained from cancelled hospitalisations, and in particular for elective procedures [Citation6,Citation9]. In 2020, the cost of hospitalisations for COVID-19 represented 0.1% of the total national healthcare expenditure and 2.2% of all expenditure on hospitalisation [Citation9]. It should, however, be noted that only hospitalization costs of COVID-19 are considered in this study, and community costs, notably those related to diagnostic tests (and, from 2021, vaccination) have been considerable [Citation7].
The cost incurred by national health insurance can be compared to the cost of hospitalisations for other diseases. For example, in 2016, the total costs of hospitalisation to French national health insurance were €0.6 billion for acute myocardial infarction, €1.3 billion for acute stroke and €1.0 billion for acute heart failure [Citation10]. Per capita hospitalisation costs are in the same range as those for other infectious diseases which can require critical care, but the total cost of COVID-19 infections is high due to the large number of patients concerned. In comparison, around 180,000 patients are hospitalised annually for the community acquired pneumonia [Citation11] and between 8,000 and 30,000 are hospitalised for influenza each year [Citation12]. However, the number of patients admitted to intensive care for COVID-19 (58,000 in 2020) was much higher than for influenza (19,000 patients between 2014 and 2019) [Citation5].
The mortality rate documented in this study underestimates the total COVID-related mortality in France since only in-hospital deaths are captured. Notably, deaths of residents in nursing homes are not included in the mortality rate. In 2020, the national strategy was not to hospitalise nursing home residents unless there was an imperative medical reason for providing respiratory support. Exactly how these patients were managed is not known, but it is probable that only low-intensity care, such as oxygen therapy, could be offered in the nursing home setting. This level of care would not be expected to have a major cost impact. It has been estimated that 145,914 residents had a symptomatic or virologically confirmed SARS-CoV2 infection in 2020, of whom 19,780 died [Citation13].
With respect to costs, only direct medical costs incurred in hospitals are documented. However, in 2020, community medical costs related to COVID-19 were likely to be principally limited to GP consultations, nurse visits, and diagnostic tests, since vaccines and treatment were not yet available. The present study was performed from a payer perspective and for this reason the high indirect costs of the COVID-19 epidemic in terms of sick leave, and of the general economic downturn associated with government measures to contain the epidemic are not considered. Indirect medical costs generated by pathologies that could not be managed optimally due to a switch of resources to COVID-19 management are also not considered, and estimating these costs would require a different methodological approach.
The data obtained come from the first year of the epidemic, and costs and outcomes in patients currently hospitalised for COVID-19 infections may have evolved. In particular, vaccination, which was introduced in January 2021, after the end of our study, has had a major impact on the hospital burden of COVID-19, notably with a major reduction on bed occupation in intensive care facilities [Citation4]. In addition, progress has been made over the past two years in improving standards of care. Current care involves prophylactic anticoagulation [Citation14,Citation15], use of glucocorticoids [Citation16,Citation17], and when appropriate, immunotherapeutic agents, such as tocilizumab [Citation18,Citation19] and neutralising monoclonal antibodies [Citation20,Citation21]. Systematic implementation of these therapeutic tools has improved patient outcomes, especially with regard to reducing the need for mechanical ventilation, and led to a decrease in mortality and more rapid hospital discharge. Even in 2020, we observed a reduction in the use of mechanical ventilation (Level 3 care) between the first and second waves. Repeating the analyses in the context of the more recent waves, such as those associated with the delta and omicron variants in 2022, will be of interest to assess formally the cost benefits of implementing these therapeutic strategies.
The study has a number of limitations. Firstly, assessment of risk factors is incomplete, with a number of known risk factors inadequately documented in the PMSI. For example, smoking status is not mentioned on the SDS, and obesity is likely to be under-reported, as it is only mentioned if it generates additional cost or complexity to the hospital stay. In addition, since healthcare professionals were under great pressure due to work overload during this crisis, information on comorbidities may not have been recorded as rigorously as usual. Also, oxygen support with low-flow oxygen, particularly when of short duration (for a few hours or days) may not have been documented on the SDS because this would not have added extra cost to the hospital stay. Notably, certain patients might have been hospitalised, particularly at the beginning of the first wave for moderate covid-19, who did not require oxygen support or specific isolation measures. Since, in a preliminary analysis, patients in these two categories (no oxygen support at all and low-flow oxygen support) had similar characteristics, stay durations and outcomes, we decided to group them. In contrast, information on use of high flow oxygen, non-invasive ventilation, and mechanical ventilation is more reliable as these procedures are coded as complexity or severity factors on the SDS. Another limitation is that the distinction between acute-phase COVID-19 and prolonged COVID-19 that was used in the study was an arbitrary one (30-day stay duration). Finally, all cases of COVID-19 may not have been captured. The diagnostic code for COVID-19 was created on 31st January 2020, and the coding conventions were subsequently enriched over the following weeks. It is therefore possible that some early cases were missed through miscoding, or because a confirmed diagnosis was not made. However, it should be noted that less than ten cases of COVID-19 were notified prior to the middle of February. Similarly, patients who are treated with oxygen therapy in emergency departments without overnight hospitalisation are not captured in our study.
The possibility of other emerging diseases causing major epidemics in the coming years is not to be underestimated [Citation22,Citation23]. Data such as those collected in the present study of COVID-19 will be important in planning hospital resource provision and organisation for future epidemics. In particular, the economic data collected may be useful for modelling studies assessing the impact and cost-effectiveness of prevention and treatment strategies.
In conclusion, this study quantifies for the first time the medical and economic burden of hospitalisations for COVID-19 infections in France during the first year of the pandemic and provides robust baseline data to benchmark advances in the standard of care and to nurture epidemiological models of future COVID-19 outbreaks.
Disclosure statement
KB, VM, and KLL are employees of Roche SA France (Paris). AM, RS, CL, and CB are employees of Creativ-Ceutical (Paris) or were employed by Creativ-Ceutical (Paris) at the time of the study. Creativ-Ceutical performed analyses sponsored by Roche SA France. FR received research funding, honoraria or consultation fees for Astra-Zeneca, Gilead Sciences, Roche, MSD, Synairgen, Theratechnologies, ViiV Healthcare, Xenothera.
Data availability statement
The source database belongs to a third party, the ATIH (Agence Technique de l’Information sur l’Hospitalisation). Data are available exclusively from the ATIH to institutions who meet the criteria for access to confidential data, following procurement of consent from the French data protection authority (CNIL). Publication of individual patient data is not permitted. The ATIH, which is responsible for access to PMSI data in France, is a one-stop-shop window for access to the PMSI database. The contact address for the ATIH is 117 Bd Marius Vivier Merle, 69003 Lyon Telephone: +33 1 45 18 43 90; Email: [email protected]; Website: https://www.indsante.fr/fr.
Additional information
Funding
References
- Hozé N, Paireau J, Lapidus N, et al. Monitoring the proportion of the population infected by SARS-CoV-2 using age-stratified hospitalisation and serological data: a modelling study. Lancet Public Health. 2021;6(6):e408–9. DOI:10.1016/S2468-2667(21)00064-5
- Salje H, Tran Kiem C, Lefrancq N. Estimating the burden of SARS-CoV-2 in France. Science. 2020;369(6500):208–211. DOI:10.1126/science.abc3517
- Agence Technique de l’Information sur l’Hospitalisation. Guide de l’Etude Nationale de Coûts. Paris: ATIH; 2020.
- Joannes-Boyau O, Dahyot-Fizelier C, Albaladejo P. Lack of vaccination in ventilated patients for SARS-CoV-2 in France. Anaesth Crit Care Pain Med. 2022;41(2):101021.
- Naouri D, Vuagnat A, Beduneau G. Covid-19 : prise en charge des patients en soins critiques au cours des trois premières vagues de l’épidémie. Etudes et résultats. 2022;1226:1–8.
- L’Assurance Maladie, Améliorer la qualité du système de santé et maîtriser les dépenses : propositions de l’assurance maladie pour 2022. 2021.
- Ministre de l’Économie des Finances et de la Souveraineté industrielle et numérique. Projet de loi de financement de la sécurité sociale pour 2023; 2020. https://assurance-maladie.ameli.fr/sites/default/files/2021-07_rapport-propositions-pour-2022_assurance-maladie_3.pdf
- Naouri D. En 2020, le nombre de séjours hospitaliers hors Covid-19 a diminué de 13 % par rapport à 2019. Etudes et résultats. 2021;1204:1–8.
- L’Assurance Maladie. Améliorer la qualité du système de santé et maîtriser les dépenses : propositions de l’assurance maladie pour 2023; 2022. https://assurance-maladie.ameli.fr/sites/default/files/2022-07_rapport-propositions-pour-2023_assurance-maladie_1.pdf
- Tuppin P, Rivière S, Rigault A, et al. Prevalence and economic burden of cardiovascular diseases in France in 2013 according to the national health insurance scheme database. Arch Cardiovasc Dis. 2016;109(6–7):399–411. DOI:10.1016/j.acvd.2016.01.011
- Dupuis C, Sabra A, Patrier J, et al. Burden of pneumococcal pneumonia requiring ICU admission in France: 1-year prognosis, resources use, and costs. crit care. 2021;25(1):24. DOI:10.1186/s13054-020-03442-z
- Pivette M, Nicolay N, Lauzun V, et al. Characteristics of hospitalizations with an influenza diagnosis, France, 2012-2013 to 2016-2017 influenza seasons. Influenza Other Respir Viruses. 2020;14(3):340–348. DOI:10.1111/irv.12719
- Santé Publique France. COVID-19 : Point épidémiologique hebdomadaire du 31 décembre 2020. Saint Maurice; 2020. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/bulletin-national/covid-19-point-epidemiologique-du-31-decembre-2020
- Rentsch CT, Beckman, J. A., Tomlinson, L., Gellad, W. F., Alcorn, C., Kidwai-Khan, F., & Freiberg, M. S. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2021;372:n311.
- Tacquard C, Mansour, A., Godon, A., Godet, J., Poissy, J., Garrigue, D., & Zufferey, P. Impact of high-dose prophylactic anticoagulation in critically ill patients with COVID-19 pneumonia. Chest. 2021;159(6):2417–2427. DOI:10.1016/j.chest.2021.01.017
- Pulakurthi YS, Pederson, J. M., Saravu, K., Gupta, N., Balasubramanian, P., Kamrowski, S., & Evanson, K. W. Corticosteroid therapy for COVID-19: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2021;100(20):e25719. DOI:10.1097/MD.0000000000025719
- van Paassen J, Vos JS, Hoekstra EM, et al. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. crit care. 2020;24(1):696. DOI:10.1186/s13054-020-03400-9
- Hermine O, Mariette X, Tharaux P-L, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):32–40. DOI:10.1001/jamainternmed.2020.6820
- Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344. DOI:10.1056/NEJMoa2028836
- Corti D, Purcell, L. A., Snell, G., & Veesler, D. Tackling COVID-19 with neutralizing monoclonal antibodies. Cell. 2021;184(12):3086–3108. DOI:10.1016/j.cell.2021.05.005
- Taylor PC, Adams AC, Hufford MM, et al. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol. 2021;21(6):382–393. DOI:10.1038/s41577-021-00542-x
- Tetro JA. From hidden outbreaks to epidemic emergencies: the threat associated with neglecting emerging pathogens. Microbes Infect. 2019;21(1):4–9.
- Lefrançois T, Malvy D, Atlani-Duault L, et al. After 2 years of the COVID-19 pandemic, translating one health into action is urgent. Lancet. 2022; DOI:10.1016/S0140-6736(22)01840-2.