ABSTRACT
Background: Sub-aortic (station 5) and para-aortic (station 6) lymph nodes are not easily accessible due to the interposition of the aorta and the left pulmonary artery. Taking a biopsy from those stations could be of value when there are no other mediastinal lymph node stations or when biopsy in other sites failed to reach a diagnosis. Surgery is the gold standard technique in the evaluation of those stations; endoscopic ultrasound-fine needle aspiration (EUS-FNA) has been proposed as a minimally invasive technique through the trans-aortic approach, with an acceptable diagnostic yield and safety profile.
Objective: To evaluate diagnostic accuracy and safety of the trans-aortic EUS-FNA in lymph node stations 5 and 6.
Methods: We reviewed all patients who underwent trans-aortic EUS-FNA from 2010 to 2017, for mediastinal lymph node enlargement or positron emission tomography/computed tomography (PET/CT) positivity (integrated 2-deoxy-2-fluoro-d-glucose). Demographic characteristics, lesion site and size, needle, final diagnosis, and complications were collected.
Results: A total of 11 patients were included, 5 males, mean age 59 years. Samples were inadequate in two cases, a diagnosis of lung cancer was reached in four patients (two adenocarcinoma and two squamous cell carcinoma) and five cases were negative for malignancy (one confirmed by surgery, two were found to be cancer at percutaneous lung biopsy and transbronchial biopsy, one patient received a diagnosis of Langerhans cell histiocytosis by transbronchial lung cryobiopsy, and one patient was lost at follow-up). The sensitivity for malignancy was 57%, and the overall diagnostic accuracy was 45%. No complications occurred.
Conclusions: Trans-aortic EUS-FNA could be proposed as a valuable and safe approach for taking biopsy from mediastinal lymph node stations 5 and 6.
KEYWORDS:
Background
Endoscopic ultrasound-fine needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) are two relatively new, minimally invasive diagnostic techniques playing a key role in the diagnosis and in the management of several thoracic diseases, such as lung cancer [Citation1], granulomatous diseases [Citation2], lymphoproliferative disorders [Citation3], and vascular diseases [Citation4].
EBUS and EUS are complementary to each other: EBUS gives access to sites close to trachea and main bronchi, such as stations 2R, 2L, 4R, 4L, 7, 10, 11, and 12, while EUS gives access to sites close to the esophagus, such as stations 4R, 4L, 7, 8, and 9 and also structures below the diaphragm, for example, retroperitoneal lymph nodes, left liver lobe, and left adrenal gland. Moreover, EUS-FNA and EBUS-TBNA allow the visualization of stations 5 and 6, and a biopsy could be taken traversing the aorta [Citation1].
Traditionally, those stations are assessed with surgical procedures: Chamberlain procedure (also called anterior mediastinoscopy), video-assisted thoracic surgery (VATS), and transcervical extended mediastinal lymphadenectomy (TEMLA), while the role of endosonography is still under debate. To date, only few case series reported EUS-FNA in stations 5 and 6, with a favorable diagnostic accuracy but lower than in other lymph node stations and an acceptable safety profile [Citation5].
A recent study performed by Naur et al. [Citation6] found that 2-deoxy-2-fluoro-d-glucose positron emission tomography/computed tomography (PET/CT) and EBUS-TBNA missed an N2 disease in 12 out of 115 surgically treated patients, 5 of them having an N2 disease for metastatic sub-aortic or para-aortic lymph nodes.
Aim of the study
We aim to investigate the diagnostic yield and the safety of EUS-FNA in sub-aortic and para-aortic lymph node station biopsy with the trans-aortic approach.
Methods
We retrospectively analyzed all patients who underwent trans-aortic EUS-FNA on station 5 and/or 6 lymph nodes for enlargement or 2-deoxy-2-fluoro-d-glucose PET/CT scan positivity from January 2010 through January 2017, at the Pulmonology Unit of G.B. Morgagni – L. Pierantoni Hospital in Forlì (Italy). The collection of data was accepted by our local ethical committee.
Prior to each invasive investigation, clinical information (physical examination, medical history, laboratory tests, and electrocardiogram) was collected, and a written consent was obtained in each patient.
Patients underwent trans-aortic EUS-FNA only after a negative bronchoscopy and/or a negative EBUS-TBNA and/or a negative EUS-FNA in other standard locations (i.e. station 7. See and ).
Figure 1. The diagnostic protocol of our study. It has to be noted that one patient was lost in follow-up.
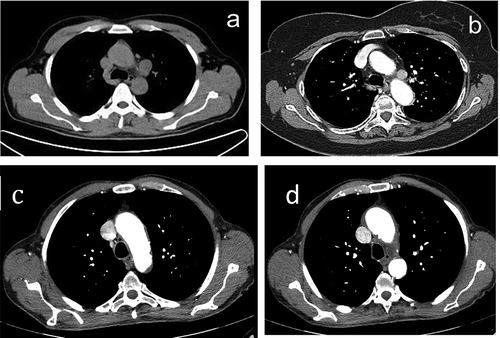
Figure 2. Lymph nodes in the aorto-pulmonary window: (a) enlarged and (b) positive to contrast CT scan, and (c and d) normal appearance.
Procedures were performed under deep sedation (propofol and remiphentanyl), with anesthesiologic assistance, while monitoring electrocardiogram and SpO2 traces, through the mouth using a linear esophageal endo-echoscope (Olympus GF UCT 160, EUS Exera, EU-C60, Hamburg, Germany) [Citation7]. When the lymph node was located under continuous real-time ultrasound (US) imaging, biopsies were taken with either a 21- or 22-gauge (G) needle (Olympus, Tokyo, Japan) traversing the aortic arch under real-time ultrasonic guidance, in the absence of intraluminal aortic plaques. Before sampling, a power Doppler examination was performed. An average of two specimens was obtained, and they were expelled onto glass slides, smeared, and air-dried. A rapid on-site evaluation was performed to assess the suitability of the sample. When possible, a sample was processed as a histology core (cell block); all remaining material was sent to the Department of Pathology for final diagnosis.
Patients were observed at least 6 h after the procedure. In case of a nondiagnostic biopsy, further investigations were performed or a clinical follow-up of at least 6 months was done.
Sensitivity for malignancy was calculated as the number of malignancies detected with trans-aortic EUS-FNA over the total number of patients with malignancy, while the diagnostic accuracy was calculated taking into account the number of diagnostic exams over the entire population.
Results
Eleven patients underwent trans-aortic EUS-FNA. Five of them (62%) were males, with a mean age of 59 years (range 40–73). EUS-FNA was performed with a 22-G needle in nine patients and with a 21-G needle in two. Four patients underwent EUS-FNA as outpatients.
Lymph nodes in para-aortic station were sampled in nine cases and in sub-aortic station in three (in one case both stations 5 and 6 were sampled). The mean lymph node size was 27 × 21 mm. EUS samples were adequate for cytological analyses in nine cases (82%). A diagnosis of lung cancer was reached in four patients: two adenocarcinomas and two squamous cells carcinomas. Five cases were negative for malignancy: one was confirmed to be nonmalignant by surgery, two were found to be cancer at percutaneous lung biopsy and transbronchial biopsy (one adenocarcinoma and one small cell carcinoma), one patient received a diagnosis of Langerhans cell histiocytosis by transbronchial lung cryobiopsy, and one patient was lost at follow-up. EUS samples were not adequate in two cases: one was negative at mediastinoscopy and one received the diagnosis of adenocarcinoma with transbronchial biopsy.
The sensitivity for malignancy was 57% (4/7), and the overall diagnostic accuracy was 45% (5/11). A summary of our results is shown in .
Table 1. Baseline characteristics of the 11 patients.
No complications occurred, neither during procedures nor in the post-procedural period.
Discussion
The systematic sampling of stations 5 (sub-aortic) and 6 (para-aortic) is not suggested routinely because their metastatic involvement does not preclude a surgical resection, rather it is important to identify cases in which the biopsy is really necessary [Citation1,Citation8]. Indeed, several studies confirmed that patients with left-sided tumors and sub- and para-aortic node metastases (N2 disease) who underwent surgical resection had an improved survival rate compared to patients with N2 diseases in other mediastinal lymph node stations that underwent resection [Citation9]. In the retrospective study by Defranchi et al. [Citation9], out of 59 patients with N2 disease, the most frequently affected lymph node station was station 7 in 22 patients (37%) and stations 5 and 6 in 18 (31%): all patients underwent lung resection followed by adjuvant therapy, but a better survival was found in patients with N2 disease in stations 5 and 6. Patterson et al. [Citation10] described 35 patients with primary bronchogenic carcinoma on the left upper lobe or in the left main bronchus with metastatic disease only in the sub-aortic lymph nodes that underwent surgical resection. The reported 3-year and 5-year survival was of 44 and 28%, respectively, suggesting that resection should be undertaken in these patients, especially when it is supposed to be complete. Thus, taking a biopsy from stations 5 and 6 may not change the management strategy in a selected population of patients; however, sometimes, this strategy could be the only possibility to reach the diagnosis.
The Chamberlain procedure, also called anterior mediastinoscopy, is considered the gold standard procedure for the invasive assessment of stations 5 and 6 [Citation11]: it consists of an incision in the second or third intercostal space, just to the left of the sternum and has a sensitivity of around 71% and a good negative predicted value of 91%. Other surgical procedures to assess the aorto-pulmonary window are transcervical extended mediastinal lymphadenectomy and VATS: the first is not used routinely due to high invasiveness and high complications rate; Cerfolio et al. [Citation5] performed a retrospective study on 39 patients with VATS for the analysis of N2 disease in the aorto-pulmonary window and demonstrated a sensitivity of 100%.
Tumors and mediastinal lymph nodes located in the para-aortic region can easily be identified by esophageal EUS, because the aorta provides an excellent medium to transfer US waves, and a biopsy could be taken traversing the aorta.
Guidelines do not propose EUS-FNA as an alternative to these surgical procedures, but few studies documented that it can be done with considerable diagnostic yield and few or no complications.
In the study of Von Bartheld and colleagues [Citation12], EUS-FNA was diagnostic in 9 cases out of 14: eight were nonsmall-cell lung cancer and one small-cell lung cancer; in one case, sample showed reactive nodal tissue, and in four cases, the material was not representative (accuracy 10/14 = 71%; sensitivity 9/12 = 75%). Further investigations revealed malignancy in three of the false-negative EUS procedures. In two patients, a small para-aortic hematoma was suspected, but the patients recovered uneventfully; no other complications occurred.
Wallace et al. described a patient with an enlarged mass in the superior segment of the left lower lobe of the lung, in close contact with the descending thoracic aorta [Citation11]. EBUS and EUS with a 25-G needle were proposed as first diagnostic approach because patient’s comorbidities rendered the indication for surgery borderline. The cytological on-site evaluation demonstrated malignant cell, and a final diagnosis of nonsmall-cell lung cancer was done. The patient underwent surgery for the resection of the involved segment, and during the operation, the EUS needle puncture was noted, and its dimension was 5 mm with no significant surrounding inflammation or bleeding.
Liberman et al. reported three cases in which lymph nodes in station 6 were biopsied without traversing the aorta, deflecting the needle with the wheels of the endoscope to avoid the trans-aortic approach [Citation13]. The diagnosis was reached in all three patients, and no major complications were reported. There are some potential dangers of this procedure due to the long trans-mediastinal trajectory (risk of bleeding, pneumothorax, lung laceration, esophageal laceration with fistula formation, or mediastinal abscess) and the proximity of the needle to the left subclavian artery (risk of arterial puncture, laceration, and pseudoaneurysm formation). Furthermore, this procedure cannot be possible in case of enlarged diameter of the transverse or descending aortic arch, early takeoff of the left subclavian artery, aneurysmal dilation of the left subclavian artery, and lymph nodes caudal on the descending aortic arch.
Even EBUS has been used to take a biopsy through the mediastinal vessels, with a favorable diagnostic yield and an acceptable safety. In the retrospective study by Panchabhai et al., EBUS was used to sample lung lesions, hila, and mediastinal lymph nodes through the trans-vascular approach, traversing the pulmonary artery or its branches [Citation14]. Out of 10 patients, a final diagnosis was reached in nine patients: five nonsmall-cell lung cancer, one small-cell lung cancer, one metastatic colon cancer, and two normal lymphoid tissue. In one patient, EBUS did not reach the diagnosis, and a VATS procedure was necessary to establish the diagnosis of histoplasmosis. No clinically relevant bleeding or other complications occurred. Boujaoude et al. described two cases of right hilar tumor biopsied with EBUS-TBNA through the pulmonary artery: the diagnosis was reached in both cases, and no complications were reported [Citation15]. Kazakow et al. described 15 consecutive patients who underwent EBUS- and EUS-guided trans-vascular biopsy in an outpatient setting [Citation16]. Eight biopsies were done through the pulmonary artery and in seven through the aorta. The overall diagnostic yield was 71%, and no complications were seen in the immediate post-procedural period, and all 15 patients were discharged home the same day.
A pulmonary artery intramural hematoma during an EBUS-TBNA was described by Botana-Rial et al.: the hematoma occurred after the puncture of the artery and resolved spontaneously [Citation17].
Our case series confirmed the feasibility of trans-aortic aspiration of para-aortic lymph nodes under real-time controlled EUS guidance, but along with a low diagnostic accuracy. No complications occurred in the entire cohort of patients, nor during the procedure nor immediately after. More caution should be taken when echo images show aortic atherosclerotic plaques, which could eventually be dislocated during the trans-aortic FNA [Citation12,Citation18]. Furthermore, we found low sensitivity for cancer and a low diagnostic accuracy, of course lower than reported with EUS-FNA in other lymph node stations or in centrally located lung tumor [Citation19–Citation21], suggesting that when the suspicion for malignancy is high, further investigations are needed in case of a negative trans-aortic EUS-FNA. This low diagnostic yield may be explained by the fact that only a single attempt at trans-aortic FNA was made in all patients: the number of needle passes should be reduced to the minimum, because it is unknown whether hematogenous tumor seeding can occur if a lesion is biopsied through a vessel [Citation15].
Our report has several limitations: first of all, the number of patients is small, and significant conclusions cannot be drawn from our study; moreover, we included in the study only patients who underwent EUS-FNA, without including unsuccessful attempts. Finally, it is a retrospective single-center study.
In conclusion, even if the theoretical risk of bleeding exists, EUS-FNA, as well as EBUS-TBNA, could be proposed, beyond their conventional indications to evaluate lung tumor or mediastinal lymph node abnormalities close to the big vessels and to evaluate vascular abnormalities, both of thrombotic and nonthrombotic origins. EUS biopsy through big vessels could be performed safely, also in an outpatient setting, especially when there are no other locations eligible for taking biopsy or when prior investigations in other locations failed to get the diagnosis; however, in case of negative or inadequate material, further exams should be performed to exclude the possibility of false-negative results.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Claudia Ravaglia
Claudia Ravaglia, MD, is a Consultant at Department of Thoracic Diseases, G.B. Morgagni – L. Pierantoni Hospital, Forlì, Italy. She is a specialist in Pulmonology and her main research focus is in interventional pulmonology and interstitial lung diseases.
Sara Colella
Sara Colella, MD, is a Consultant at Pulmonology Unit, C. & G. Mazzoni Hospital, Ascoli Piceno, Italy. She is a specialist in Pulmonology. Main interests: interventional pulmonology, lung cancer and interstitial lung diseases. Currently, she is the National Representative for the Early Career Members for the “Associazione Italiana Pneumologi Ospedalieri” (AIPO).
Sara Tomassetti
Sara Tomassetti, MD, is a Consultant at Department of Thoracic Diseases, G.B. Morgagni – L. Pierantoni Hospital, Forlì, Italy. She is a specialist in Pulmonology and her main research focus is in interventional pulmonology and interstitial lung diseases.
Christian Gurioli
Christian Gurioli, MD, is a Consultant at Department of Thoracic Diseases, G.B. Morgagni – L. Pierantoni Hospital, Forlì, Italy. He is a specialist in Pulmonology and he is expert in interventional pulmonology and interstitial lung diseases.
Sara Piciucchi
Sara Piciucchi, MD, is a Consultant at Department of Radiologist, G.B. Morgagni – L. Pierantoni Hospital, Forlì, Italy. She is a specialist in Radiology, her main focus is on thoracic radiology.
Dubini Alessandra
Dubini Alessandra, MD, has a qualification as Surgical Pathologist and she is a Consultant at Pathology Department, G.B. Morgagni – L. Pierantoni Hospital in Forlì, Italy, since 1997, working on lung pathology during the last 13 yrs.
Carlo Gurioli
Carlo Gurioli, MD, is a Consultant at Department of Thoracic Diseases, G.B. Morgagni – L. Pierantoni Hospital, Forl, Italy. He is a specialist in Pulmonology and he is expert in interventional pulmonology and interstitial lung diseases.
Venerino Poletti
Venerino Poletti Full Professor of Pulmonary Medicine & Chair Department of Diseases of the Thorax, Ospedale GB Morgagni, Forlì (I) and Department of Respiratory Diseases & Allergy, Aarhus University Hospital, Aarhus (DK). Head of Assembly 12 (Diffuse Parenchymal Lung Disease), European Respiratory Society, President of “Associazione Italiana Pneumologi Ospedalieri” (AIPO). Board certified in Pulmonary Medicine and in Anatomic Pathology Reserch. Interests: diffuse parenchymal lung disease, interventional pulmonology, lung pathology.
References
- Silvestri GA, Gonzalez AV, Jant MA, et al. Methods for staging non-small cell lung cancer diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013 May;143(5):Suppl e211S–e250S.
- von Bartheld MB, Dekkers OM, Szlubowski A, et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis: the GRANULOMA randomized clinical trial. JAMA. 2013 Jun 19;309(23):2457–6.
- Senturk A, Babaoglu E, Kilic H, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Asian Pac J Cancer Prev. 2014;15:4169–4173.
- Li P, Zheng W, Zhao L. Convex probe endobronchial ultrasound: applications beyond conventional indications. J Thorac Dis. 2015 Sep;7(9):E289–E297.
- Cerfolio RJ, Bryant AS, Eloubeidi MA. Accessing the aortopulmonary window (#5) and the paraaortic (#6) lymph nodes in patients with non-small cell lung cancer. Ann Thorac Surg. 2007;84:940–945.
- Naur TMH, Konge L, Clementsen PF. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of patients with non-small cell lung cancer without mediastinal involvement at positron emission tomography-computed tomography. Respiration. 2017;94(3):279–284.
- Ulivi P, Romagnoli M, Chiadini E, et al. Assessment of EGFR and K-ras mutations in fixed and fresh specimens from transesophageal ultrasound-guided fine needle aspiration in non-small cell lung cancer patients. Int J Oncol. 2012 Jul;41(1):147–152.
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014 May;45(5):787–798.
- Defranchi SA, Cassivi SD, Nichols FC, et al. N2 disease in T1 non-small cell lung cancer. Ann Thorac Surg. 2009 Sep;88(3):924–928.
- Patterson GA, Piazza D, Pearson FG, et al. Significance of metastatic disease in subaortic lymph nodes. Ann Thorac Surg. 1987 Feb;43(2):155–159.
- Wallace MB, Woodward TA, Raimondo M, et al. Transaortic fine-needle aspiration of centrally located lung cancer under endoscopic ultra- sound guidance: the final frontier. Ann Thorac Surg. 2007;84:1019–1021.
- von Bartheld MB, Rabe KF, Annema JT. Transaortic EUS-guided FNA in the diagnosis of lung tumors and lymph nodes. Gastrointest Endosc. 2009;69:345–349.
- Liberman M, Duranceau A, Grunenwald E, et al. New technique performed by using EUS access for biopsy of para- aortic (station 6) mediastinal lymph nodes without traversing the aorta. Gastrointest Endosc. 2011;73:1048–1051.
- Panchabhai TS, Machuzak MS, Sethi S, et al. Endobronchial ultrasound-guided transvascular needle aspiration: a single-center experience. J Bronchology Interv Pulmonol. 2015;22:306–311.
- Boujaoude Z, Pratter M, Abouzgheib W. Transpulmonary artery needle aspiration of hilar masses with endobronchial ultrasound: a necessary evil. J Bronchology Interv Pulmonol. 2013 Oct;20(4):349–351.
- Kazakov J, Hegde P, Tahiri M, et al. Endobronchial and endoscopic ultrasound-guided transvascular biopsy of mediastinal, hilar, and lung lesions. Ann Thorac Surg. 2017 Mar;103(3):951–955.
- Botana-Rial M, Núñez-Delgado M, Pallarés-Sanmartín A, et al. Intramural hematoma of the pulmonary artery and hemopneumomediastinum after endobronchial ultrasound-guided transbronchial needle aspiration. Respiration. 2012;83(4):353–356.
- Scolari F, Tardanico R, Zani R, et al. Cholesterol crystal embolism: a recognizable cause of renal disease. Am J Kidney Dis. 2000;36:1089–1109.
- Annema JT, Veselic M, Rabe KF. EUS-guided FNA of centrally located lung tumours following a non-diagnostic bronchoscopy. Lung Cancer. 2005;48:357–361.
- Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasound- guided fine-needle aspiration for non-small cell lung cancer staging: a systematic review and meta-analysis. Chest. 2007;131:539–548.
- Varadarajulu S, Hoffman BJ, Hawes RH, et al. EUS-guided FNA of lung masses adjacent to or abutting the esophagus after unrevealing CT- guided biopsy or bronchoscopy. Gastrointest Endosc. 2004;60:293–297.
