ABSTRACT
Flexible bronchoscopy and endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA) are the pulmonologists´ basic procedures for the biopsy of suspicious lung lesions. If inconclusive, other guiding-modalities for tissue sampling are needed, computed tomography performed by a radiologist, or – if available – radial EBUS or electromagnetic navigation biopsy.
We wanted to investigate if same-day X-ray fluoroscopy-guided transthoracic fine-needle aspiration biopsy (F-TTNAB) performed by the pulmonologist immediately after bronchoscopy and EBUS is a feasible alternative.
We retrospectively identified consecutive patients in whom F-TTNAB followed a bronchoscopy and EBUS in the same séance. Patients in whom the suspicion of malignancy was invalidated after complete work up were followed for six months to identify false-negative cases.
In total 125 patients underwent triple approach (bronchoscopy, EBUS and F-TTNAB) during the same séance. Malignancy was diagnosed in 86 (69%), and 77 of these (90%) were primary lung cancers. The diagnostic yield of F-TTNAB for malignancy was 77%, and sensitivity was 90%. Pneumothorax occurred in 35 (28%) patients, and was administered with pleural drainage in 22 (18% of all patients). No cases of prolonged haemoptysis were observed. The risk of pneumothorax differed insignificantly with lesion size ≤2.0 cm (27%) versus >2.0 cm (29%).
We conclude that it is feasible for pulmonologist to perform F-TTNAB immediately after endoscopy as a combined triple approach in a fast-track workup of suspected lung cancer.
Introduction
Invasive procedures represent a cornerstone in the diagnostic approach to patients with lung lesions. Distinction between a malignant and non-malignant cause of the lesion is fundamental for treatment planning. Therefore, the provision of a biopsy is essential. Centrally located lung lesions can be biopsied with the use of bronchoscopy or endosonography [Citation1], which is traditionally performed by the pulmonologist [Citation2]. Peripheral lesions can be biopsied endoscopically guided by radial endobronchial ultrasound (R-EBUS) [Citation3] or electromagnetic navigation bronchoscopy (ENB) [Citation4], or transthoracically guided by ultrasound [Citation5] or computed tomography (CT) [Citation6]. There is no clear correlation between diagnostic yield, complication risk or costs: using ENB results in fewer complications but increased costs compared to CT-guided biopsy [Citation7].
CT-guided biopsy often lies in the hands of the radiologist [Citation8,Citation9]. The radiologist also performs percutaneous lung biopsy guided by conventional X-ray fluoroscopy, which is a valuable alternative to CT guided biopsy [Citation10]. Most bronchoscopists are familiar with fluroscopy-guided bronchoscopy [Citation11]. However, the feasibility of fluoroscopy-guided transthoracic needle aspiration biopsy (F-TTNAB) in the hands of the pulmonologist has not been described in the literature before except for a single paper from our group [Citation12].
Objectives
On this background we decided to
Determine the diagnostic yield and complication rate of F-TTNAB in the hands of the pulmonologist.
To examine if it is feasible to perform F-TTNAB in combination with bronchoscopy and endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA).
Materials and methods
We retrospectively searched in the hospital’s electronical database for patients that underwent F-TTNAB, bronchoscopy and EBUS-TBNA at the same day from 1 September 2012 to 31 December 2013 in our pulmonary department because of suspected lung cancer. All patients had an initial evaluation with a medical history and physical examination, chest R ray, blood tests and electrocardiogram. Computer tomography (CT) scan with contrast enhancement and/or positron emission tomography- computer tomography (PET-CT) was performed. Lesion size, defined as the maximum axial diameter, location and distance to pleura was measured on CT images obtained on lung windows. Oral and written information concerning all procedures and possible complications were given to the patient and informed consent obtained.
Bronchoscopy and EBUS-TBNA were performed under conscious sedation by the administration of midazolam and fentanyl intravenously in an ambulatory setting. Bronchoscopy was performed in all patients, since the surgeons demand an inspection of the bronchial tree in all patients who may be potential candidates for surgery. The patients received additional oxygen during the procedures. Oxygen saturation and pulse were monitored. The bronchoscopy was only supplemented with EBUS-TBNA if indicated according to the European Guidelines [Citation1] (for example abnormal hilar or mediastinal lymph nodes/centrally located lung tumor). Bronchoscopy was performed with an Olympus BF-1T180 (Olympus XBF-UC40P; Olympus Medical Systems Europe Ltd., Hamburg, Germany), and EBUS-TBNA was performed with a flexible ultrasound bronchoscope (Olympus XBF-UC40P; Olympus Medical Systems Europe Ltd., Hamburg, Germany) using a 22-G Olympus NA-200C needle with biopsy sampling according to guidelines [Citation13].
If no lung tumor or no abnormal lymph nodes were demonstrated by bronchoscopy or EBUS, it was considered unlikely that endoscopy would provide a conclusive diagnosis. Rapid onsite evaluation (ROSE) was not accessible. In these cases F-TTNAB was performed immediately (). The patient was placed in an appropriate supine position depending of the location of the lung lesion, which was localized in two planes with X ray fluoroscopy and the preferred route of the biopsy needle was chosen. X ray fluoroscopy was performed using a C-arm Ziehm exposcop 8000 (Ziehm GmBH, Nürnberg, Germany). The skin entry site was sterilized with standardized antiseptic solution and the cutaneous and subcutaneous tissue infiltrated with lidocaine up to a maximum dose of 20 ml of a 2% solution. Firstly, during lateral fluoroscopy and suspended patient respiration, a 7.5 mm guiding needle (Super 4 needle, (BD Angiomed GmBH, Karlsruhe, Germany) was placed adjacent to the lesion. Secondly, aspiration biopsies were obtained using a 220 mm Chiba 22G needle (BD Angiomed GmBH, Karlsruhe, Germany) via the guiding needle during shallow patient breathing and lateral fluoroscopy with at least two needle passes [Citation14]. Suction was applied to the Chiba-needle by a 10 mL syringe, and aspirated material was transferred to slides and a designated container for cell block preparation. The biopsy procedure was followed by observation for at least 60 minutes with measurements of respiration frequency, peripheral oxygen saturation, blood pressure, pulse. At 60 minutes, all patients underwent a standing, full inspiration chest X ray.
Figure 1. a) PET-CT showing a peripheral solid lesion in the anterior part of right upper lobe without pleural contact or bronchus sign (arrow). (b) With the patient in supine position, the operator locates the lesion in the anterior-posterior (A-P) projection with the C-arm with on-time fluoroscopic images on the screen.. (c) The borders of the lesion are marked on the skin with a filt pen and a metallic ruler. (d) Fine-needle aspiration with a Chiba needle via a guiding needle. The position of the needle tip can be checked with the C-arm in any angle different from the A-P-projection
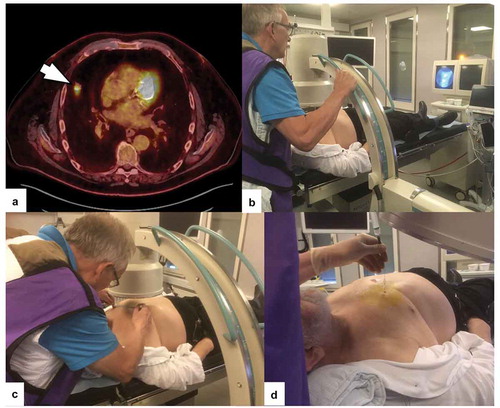
Performance status was described using the The Eastern Cooperative Oncology Group (ECOG) score, and comorbidity was summarized using the Charlson´s index of comorbidity [Citation8].
Standard of reference
Samples with malignant cells were considered true positive. For samples with non-malignant diagnoses at cytopathological evaluation, the results were considered true negative if the results were verified by at least six months follow up (clinical course possibly supplemented with computed tomography (CT).
Diagnostic yield was defined as the number of samples in which F-TTNAB provided a specific diagnosis (malignant or non-malignant) relative to the total number of F-TTNAB samples performed. Sensitivity was defined as the number of samples in which F-TTNAB provided a diagnosis of any malignancy relative to the total number of targeted lung lesions that turned out to be malignant. Patients in whom the suspicion of lung cancer was invalidated after complete work up were followed for six months to identify false-negative cases.
Statistics
Statistical analysis was performed using SPSS Statistics software for Windows, v.24 (IBM Corp., Armonk, N.Y., USA). Categorical variables were compared using Pearson’s Chi2-test or Fisher’s exact test (when expected n < 5 for any variable). Mann-Whitney U-test was applied for continuous variables. Results were collected in tables where continuous data were expressed as median (range) and binary data as percentages. Results were considered significant when p < 0.05.
Ethics
The study was a retrospective observational study without randomization or intervention in the treatment of the patients. These types of studies are exempt from approval by the local Research Ethics Committee according to Danish legislation. Patient data were anonymously collected, and The Danish Data Protection Agency approved the study.
Results
We identified 125 patients who underwent F-TTNAB after bronchoscopy and/or EBUS-TBNA. Baseline characteristics are presented in . shows that totally 86 patients (69%) received a final diagnosis of malignancy. F-TTNAB was conclusive in 96 patients, (overall diagnostic yield 77%). Of the remaining patients, two patients had a conclusive EBUS-TBNA, 23 underwent re-sampling, and four patients entered a CT control follow-up program. Sensitivity for a diagnosis of malignancy was 90% (71 cases diagnosed by F-TTNAB).
Table 1. Baseline characteristics and radiological features (n = 125)
Table 2. F-TTNAB biopsy results (n = 125)
Pneumothorax was by far the most common complication, and observed in 35 patients (28%). A pleural pig tail catheter was necessary in 22 (18% of total; 62% of patients with pneumothorax).
No cases of recurrent haemoptysis or pain were observed.
Lesions with an average diameter of ≤2 cm were not associated with a higher risk of pneumothorax (27%) compared with lesions >2 cm (29%; p > 0.8, Chi2-test).
Discussion
It is important to diagnose patients suspected of lung cancer as quickly as possible [Citation1,Citation15]. Firstly, a lung cancer can advance to a higher stage if curative intended treatment is delayed. Secondly, the waiting time for a diagnosis is a mental strain for both the patient and the patient´s relatives. Thirdly, individual and societal costs increase with number of visits [Citation16].
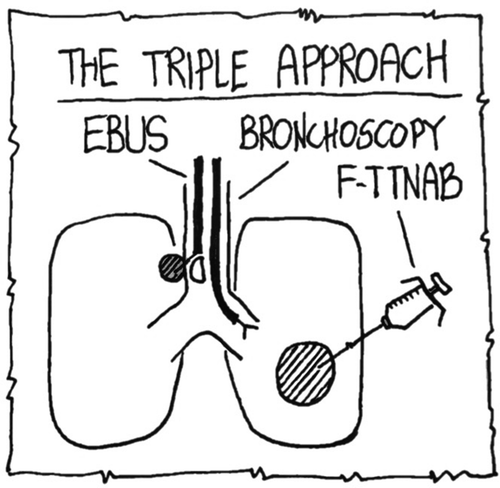
Bronchoscopy and EBUS-TBNA are basic procedures in the investigation of patients with lung lesions. If these techniques do not give access to the lung lesion, and a diagnosis is not obtained from extrapulmonary lesions, the next step may be referral to a radiologist for a CT guided lung biopsy later. We investigated if F-TTNAB performed by the pulmonologist in combination with bronchoscopy and EBUS-TBNA (a triple approach) could be an alternative. The principles of the triple approach are demonstrated in .
Figure 2. Schematic drawing of the principles of the triple approach. EBUS: endobronchial ultrasound, F-TTNAB: fluoroscopy-guided transthoracic fine-needle aspiration biopsy
Overall, F-TTNAB was able to establish a diagnosis in approximately three out of four patients with a malignant diagnosis, which is comparable and not significantly different from the results of previous studies assessing percutaneous biopsy of the lung [Citation9].
The most common complication to CT guided lung biopsy is pneumothorax, which occurs in 0–61% of the cases [Citation9]. Between 3.3% and 15% of all patients will require a chest drain [Citation9]. Yeow et al found a risk for pneumothorax and hemoptysis of 23% and 4%, respectively, in a cohort of 660 consecutive procedures [Citation17]. The risk for pneumothorax was increased risk when the lesion diameter was ≤ 2 centimeters [Citation17]. In our study, pneumothorax risk was 28% and 18% of all patients required a chest drain, thus slightly increased. We did not find an increased risk with tumor size ≤ 2 centimeters, and we observed no cases of protracted haemoptysis. Unfortunately, there are no systematic reports in the literature concerning the risk of pneumothorax and chest tube requiring pneumothorax in connection with F-TTNAB.
It could be argued, that non-diagnostic bronchoscopy and EBUS should not be followed by F-TTNAB but by more modern sampling guided by electromagnetic navigation bronchoscopy (ENB) and radial ultrasound probe [Citation1,Citation13]. However, in many centres – especially in non-Western countries – these techniques are not available due to the substantial costs for single-purpose equipment. Fluoroscopy is used in several disciplines of medicine and is readily available even in low-income countries. The sampling of peripheral lung lesions guided by CT or fluoroscopy normally requires referral to a radiologist [Citation16], which will cause a delay in the diagnostic workup. A combined, same-day invasive triple approach aiming at both diagnosis and staging will speed up the process. The equipment for fluoroscopy is routinely available in the operating room of the pulmonologist, since it is used when performing transbronchial lung biopsies [Citation13].
A randomized study comparing a) the triple approach with b) FB and EBUS followed by referral of the patient to a radiologist for percutaneous lung biopsy seems redundant if the purpose were to investigate if the former approach decreases the waiting time for a lung biopsy. Of course, it could be argued that suspicious lung lesions should be removed by the surgeons without waiting for a malignant biopsy. However, this is not recommended as a suspicious lesion might be for example pneumonia or other localized inflammation [Citation18]. One could claim that F-TTNAB could appear to be superfluous in selected cases where EBUS-TBNA unexpected reveals diagnostic material from the mediastinum or the hilar regions. This was the case in two of our 125 patients (1.6%). We think that this risk of performing an F-TTNAB procedure, that retrospectively shows up to be superfluous, is acceptable and relatively small.
Though inclusion in this study was consecutive, a limitation is a selection bias due to the retrospective design. Subsequent studies should include larger cohorts in a prospective multi-centre design including exclusively consecutive patients. A strength to this study is that it shows the results of F-TTNAB as an integrated part of the triple approach in everyday clinical workup, supporting our findings in another [Citation17].
There are no guidelines or standardized training programs for learning F-TTNAB. Prior to the implementation of the procedure, the physicians were experienced in other transthoracic procedures (e.g. pleural drainage and medical thoracoscopy) The education in the F-TTNAB was based on traditional apprenticeship training.
We conclude that it is feasible to perform F-TTNAB immediately after a non-diagnostic FB and EBUS-TBNA in the investigation of patients with suspicious lung lesions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Jatinder Singh Sidhu
Jatinder Singh Sidhu is a specialist registrar in respiratory medicine with special interest in interventional pulmonology.
Geir Salte
Geir Salte is a young doctor with interest in respiratory medicine.
Ida Skovgaard Christiansen
Ida Skovgaard Christiansen is a resident doctor and PhD-fellow. She has an interest in interventional pulmonology and pathology.
Therese Marie Henriette Naur
Therese Maria Henriette Naur is a resident doctor. She has an interest in interventional pulmonology, oncology and simulation based training, and she has published several papers in these areas.
Asbjørn Høegholm
Asbjørn Høegholm is a consultant in internal and respiratory medicine and particularly interested in invasive procedures for the diagnosis and staging of suspected cancer in lungs or pleura, and member of the Danish Lung Cancer Group steering committee working for improved survival in lung cancer.
Paul Frost Clementsen
Paul Frost Clementsen is a consultant in pulmonary medicine and particularly interested in simulator based training, supervised training programs and assessment of competence in endoscopic ultrasonography and fine-needle aspiration and other invasive procedures for the diagnosis and staging of lung cancer.
Uffe Bodtger
Uffe Bodtger is a consultant in respiratory medicine and particularly interested in invasive and non-invasive procedures for the diagnosis suspected cancer in lungs or pleura, as well as non-pharmacological treatment in COPD or asthma.
References
- Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer. A clinical guideline by the European Society of Gastrointestinal Endoscopy (ESGE), European Respiratory Society (ERS) and European Society of Thoracic Surgeons (ESTS). Eur Respir J. 2015;46(1):40–6.
- Herth F, Shah P, Gompelmann D. Interventional pulmonology. ERS monography. European Respiratory Society; 2017. ISBN 978-1-84984-091-0.
- Ali MS, Trick W, Mba BI, et al. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: A systematic review and meta-analysis. Respirology. 2017 Apr;22(3):443–453.
- Folch EE, Pritchett MA, Nead MA, et al. Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: one-year results of the prospective, multicenter NAVIGATE study. J Thorac Oncol. 2019 Mar;14(3):445–458.
- Laursen CB, Naur TM, Bodtger U, et al. Ultrasound-guided lung biopsy in the hands of respiratory physicians: diagnostic yield and complications in 215 consecutive patients in three centers. J Bronchology Interv Pulmonol. 2016;23(3):220–228.
- Ashraf H, Kragh-Andersen S, Naqibullah M, et al. Computer tomography lung biopsy using interactive breathhold control. A randomized study. Ann Transl Med. 2017;5(12):253.
- Dale CR, Madtes DK, Fan VS, et al. Navigational bronchoscopy with biopsy versus computed tomography-guided biopsy for the diagnosis of a solitary pulmonary nodule: a cost-consequences analysis. J Bronchology Interv Pulmonol. 2012 Oct;19(4):294–303.
- Tavare AN, Hare SS, Miller FNA, et al. A survey of UK percutaneous lung biopsy practice: current practices in the era of early detection, oncogenetic profiling, and targeted treatments. Clin Radiol. 2018 Jun 16;73:800–809.
- Manhire A, Charig M, Clelland C, et al. Guidelines for radiologically guided lung biopsy. Thorax. 2003;58(11):920–936.
- Kurban LA, Gomersall L, Weir J, et al. Fluoroscopy-guided percutaneous lung biopsy: a valuable alternative to computed tomography. Acta Radiol. 2009;49(8):876–882.
- Georgia Hardavella and Jeremy George. Breathe (Sheff). Interventional bronchoscopy in the management of thoracic malignancy. 2015 Sep;11(3):202–212.
- Madsen KR, Høegholm A, Bodtger U. Accuracy and consequences of same-day, invasive lung cancer workup - a retrospective study in patients treated with surgical resection. Eur Clin Respir J. 2016;Nov(3):32590.
- Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176(1):36–41.
- Khouri NF, Stitik PF, Erozan YS, et al. Transthoracic needle aspiration biopsy of benign and malignant lung lesions. Am J Radiol. 1985;144:281–288.
- Gaga M, Powell CA, Schraufnagel DE, et al. An official American thoracic society/European respiratory society statement: the role of the pulmonologist in the diagnosis and management of lung cancer. Am J Respir Crit Care Med. 2013 Aug 15;188(4):503–507.
- Jacobs P, Hall EM. Estimating the cost of outpatient hospital care. Healthcare Manage Forum. 1995 Winter;8(4):36–38.
- Yeow KM, Su IH, Pan KT, et al. Multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest. 2004;126(3):748–754.
- Callister MJ, Baldwin DR, Akram AR, et al. BTS guidelines for the investigation and management of pulmonary nodules. Thorax. 2015 August;70(Supplement 2):ii1–ii54.
