ABSTRACT
Introduction
Patients with coronavirus disease (COVID-19) and pneumonitis often have hypoxemic respiratory failure and a need of supplementary oxygen. Guidelines recommend controlled oxygen, for most patients with a recommended interval of SpO2 between 92 and 96%. We aimed to determine if closed-loop control of oxygen was feasible in patients with COVID-19 and could maintain SpO2 in the specified interval.
Methods
Patients were prospectively enrolled in an observational study on a medical ward dedicated to patients with COVID-19. Closed-loop controlled oxygen was delivered by O2matic® which can deliver 0–15 liters/min and adjusts flow every second based on 15 seconds averaging of SpO2 measured by pulse oximetry. Lung function parameters were measured at admission.
Results
Fifteen patients (six women, nine men) participated in the study. Average age was 72 years. Lung function was severely impaired with FEV1, FVC and PEF reduced to approximately 50%. The average stay on the ward was 3.2 days and O2matic was used on average for 66 hours, providing 987 hours of observation. O2matic maintained SpO2 in the desired interval for 82.9% of the time. Time with SpO2 > 2% below interval was 5.1% and time with SpO2 > 2% above interval was 0.6%.
Conclusion
Closed-loop control of oxygen to patients with COVID-19 is feasible and can maintain SpO2 in the specified interval in the majority of time. Closed-loop automated control could be of particular benefit for patients in isolation with decreased visibility, surveillance and monitoring. Further studies must examine the clinical benefits.
Introduction
A common feature of most patients admitted with coronavirus disease 2019 (COVID-19), pneumonitis and lower respiratory symptoms is hypoxemic respiratory failure and need of supplementary oxygen. Oxygen supplementation is a lifesaving treatment but is also associated with side effects. There is increasing awareness that oxygen, besides minor side effects such as dryness of the mucosa in the airways, also poses risks due to formation of reactive oxygen species (ROS), pulmonary toxicity and coronary and cerebral vasoconstriction [Citation1]. The pulmonary toxicity of oxygen has been known since it was demonstrated in a mouse model by Lorrain Smith in 1899 [Citation2]. Recently, a meta-analysis of 25 randomized controlled trials with 16,037 acutely ill patients admitted to hospital showed an increased mortality associated with liberal oxygen treatment compared to a conservative oxygen treatment [Citation3]. Most patients in these studies had myocardial or cerebral ischemia, but excess mortality with liberal oxygen has also been demonstrated in other patients. One randomized controlled trial with 480 patients admitted to a medical-surgical ICU found that a conservative strategy with SpO2 between 94 and 98% reduced mortality when comparing with a liberal strategy with SpO2 from 97 to 100%, and patients in the conservative group had fewer episodes of shock, liver failure and bacteremia [Citation4].
It is not known if it is critical to control SpO2 in a narrow interval for patients with COVID-19. In general, guidelines for oxygen treatment of patients with acute illness recommend that SpO2 is maintained within an interval from 94 to 98%, unless special conditions, such as risk of hypercapnic failure, dictates more conservative oxygen treatment [Citation5]. An ICU trial with acute respiratory distress syndrome (ARDS) randomized patients to a very conservative oxygen treatment (SpO2 at 88–92%) versus a liberal oxygen treatment with SpO2 > 96% [Citation6]. This trial was stopped early due to safety concerns and low likelihood of meeting the primary endpoint, which was mortality at 28 days. Five cases with mesenteric ischemia occurred in the group with a conservative oxygen strategy. Due to the above studies, the Surviving Sepsis Campaign’s guidelines for COVID-19 recommend starting oxygen therapy if SpO2 < 92% and recommend that SpO2 is maintained no higher than 96% [Citation7].
It is well known that it is difficult to maintain a stable level of SpO2 with manual oxygen titration. When compared to closed-loop automated oxygen titration, it has been demonstrated in randomized controlled trials that manual titration by nursing staff maintains SpO2 in the right interval in 38–56% of the time compared to closed-loop titration which maintains SpO2 in the right interval for 77–85% of the time [Citation8–10]. Closed-loop systems are based on continuous measurement of SpO2, which in a feed-back circuit controls the amount of oxygen delivered to the patient, and they have predominantly been used for neonates, patients with COPD, and patients with hypoxemic respiratory failure in the emergency ward [Citation8–Citation11].
In the management of patients with COVID-19, the task of manually controlling the oxygen supply becomes more cumbersome, due to the isolation regime, which requires a gown, face mask, gloves and goggles for even simple tasks at the patient bed. Thus, the benefits of closed-loop titration could be greater than with non-isolated patients in terms of use of nursing resources. However, it is not known if closed-loop automated oxygen control is feasible for patients with COVID-19. The pathophysiology behind COVID-19 is different from other etiologies to hypoxemic respiratory failure and ARDS. It is probably due to a combination of damage to the airway epithelium, especially type 2 alveolar cells, vascular endothelial damage and thromboinflammation [Citation12]. The result is impaired hypoxemic vasoconstriction in the pulmonary vessels, interstitial edema, and leakage of fluid to the alveoli. These key mechanisms contribute to a worsening of ventilation/perfusion (V/Q) mismatch and even shunting [Citation13]. In case of shunting, it is difficult to maintain normal SpO2, as well-oxygenated blood from areas with a high V/Q ratio cannot compensate for admixture with poorly oxygenated blood from areas with low V/Q or absent ventilation with high perfusion.
We aimed to determine if it was possible to maintain SpO2 in the desired interval for patients with mild to moderate hypoxemic failure admitted with COVID-19 pneumonitis, with a closed-loop automated oxygen control device, O2matic®, which has previously been tested on patients with a COPD exacerbation [Citation10]. Furthermore, we wanted to characterize the patients in terms of severity by doing spirometry at admission as supplement to radiology and biochemistry.
Methods
Study design
The study was performed as a prospective observational study at a university hospital in Copenhagen. Patients were recruited from a newly created medical ward, dedicated to patients admitted with COVID-19. Nursing staff and physicians who attended the patients were from all medical and surgical specialities and were not previously familiar with patients with COVID-19 or other severe infectious or pulmonary conditions with hypoxemic respiratory failure. It was decided to use O2matic as standard for oxygen treatment for patients who needed an oxygen supply of 0 to 15 liters of oxygen, which was delivered either by standard nasal cannula or high flow cannula. All nursing staff and physicians received training in use of O2matic. As O2matic was implemented as standard of care, the regional ethics committee did not require informed consent from the patients (20023238).
Patients
Patients were included from 15 April 2020 until the ward was closed down at the end of May due to lack of patients. Patients were included if they were older than 18 years, had a positive COVID-19 PCR analysis in pharyngeal swab or in tracheal secretion, needed oxygen supply to maintain SpO2 ≥ 92% and were able to comply with continuous measurement of SpO2 by pulse oximetry. Patients were excluded if they required oxygen supply >10 liters/min at admission. Furthermore, it was a prerequisite for inclusion that an investigator (LMK or CSB) was present to include the patient within the first 48 hours of admission.
The following parameters were registered at inclusion:
Hemoglobin, white blood cell count with differential, platelets, electrolytes, albumin, liver parameters, LDH, CRP.
Chest X-ray.
Bed-side spirometry with forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC) and peak expiratory flow (PEF).
Co-morbidities and symptoms at presentation.
Decisions on the extent of care and ceiling of treatment.
The patients participated in the study as long as they were admitted to the dedicated COVID-19 medical ward. The participation ended if they were weaned from oxygen supplementation and discharged, transferred to ICU for mechanical ventilation, to other ward in order to receive treatment with continuous positive airway pressure (CPAP), or died.
Equipment
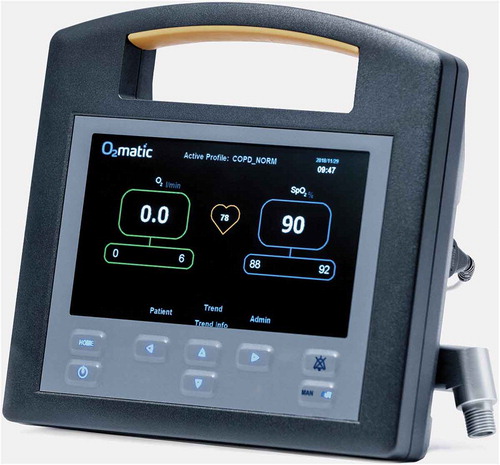
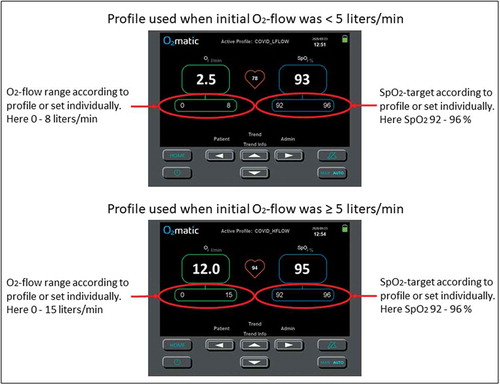
The O2matic® oxygen controller (O2matic Ltd., Herlev, Denmark) is a closed-loop system that, based on continuous monitoring of pulse rate and SpO2 by a standard wired pulse oximeter, adjusts oxygen flow to the patient (). The algorithm in O2matic samples the last 15 seconds of input from the pulse oximeter and calculates increments or decrements in oxygen flow every second based on the last 15 seconds’ average. Increments and decrements are proportionally increased relative to the difference between actual SpO2 and target SpO2. Target SpO2 is set as an interval, and in this study, it was set according to COVID-19 guidelines, which recommend SpO2 of 92 to 96% for most patients [Citation7]. Oxygen flow is also set as an interval. Patients with an initial need of oxygen <5 liters/min had oxygen delivered by a standard nasal cannula and a flow range set by O2matic from 0 to 8 liters/min (). Patients in need of 5–10 liters of oxygen had oxygen delivered by a high flow nasal cannula and a flow range set by O2matic from 0 to 15 liters/min (). Three kinds of sensors were used at nurses’ and physicians’ discretion: A Nonin® 8000 A multiple use finger sensor, a Nonin® 8000 Q2 multiple use ear sensor and a Nonin® 6000 C single patient finger sensor (Nonin Medical Inc., Plymouth, US). Spirometry was performed with a handheld spirometer, NDD EasyOne (NDD Medizintechnik AG, Zurich, Switzerland).
Statistical analysis
All data were managed and analyzed with IBM SPSS statistical package version 25. Demographic variables were analyzed using non-parametric statistics. Data from O2matic regarding SpO2, pulse rate and oxygen flow were aggregated with average values for each minute of observation, and fraction of time within SpO2 interval and fraction of time less than 2% and more than 2% outside SpO2 interval was calculated.
Results
Sixteen patients were included. One patient was excluded due to not having COVID-19. Fifteen patients provided data from use of O2matic. Demographics are presented in . Only abnormal biochemical values are presented. The most common comorbidities were hypertension (67%) and diabetes (47%). The most common symptoms were dyspnea (87%), cough (60%) and fever (60%). Twelve patients were able to perform spirometry within the first 5 days of admission. Lung function was in general severely impaired, around 50% of predicted. Lung function data are presented in .
Table 1. Demographics, comorbidities and symptoms.
Table 2. Lung function data.
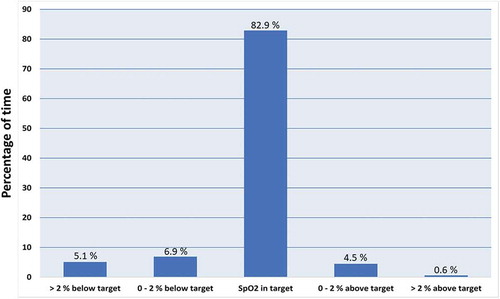
The 15 patients had automatic oxygen titration on average for 66 hours, providing a total of 987 hours of observation with O2matic. There was a missing signal in 8% of the time, either due to true missing signal despite sensor in place, or due to sensor intendedly removed during meals, personal hygiene, etc. Average flow of oxygen was 3.7 liters/min. Average pulse rate was 76 bpm (±14 bpm). Fourteen patients had the SpO2 target set to 92 to 96%, and one patient had the SpO2 target set to 88–92%, due to presence of COPD. Distribution of SpO2-values within target, not more than 2% outside target and more than 2% outside target is shown in . The average proportion of time spent within intended SpO2 target was 82.9%. The proportion of time spent within intended SpO2 target ± 2% was 94.3%, which means that patients with an SpO2 target of 92–96% were within an SpO2 interval of 90–98% in 94.3% of the time. The SpO2 was more than 2% below target in 5.1% of the time and more than 2% above target in 0.6% of the time. Time with SpO2 < 85% was on average 1.4%. The average duration of participation in the study was 3.2 days. Six patients were weaned from oxygen supplementation and discharged from the ward. Nine patients were transferred to more intensive care, of whom three later died.
Discussion
Our small observational study showed that supplementary oxygen titration in patients with COVID-19 pneumonitis and hypoxemic respiratory failure was feasible and resulted in satisfactory oxygenation for the majority of the time required.
Closed-loop oxygen control has been used in several settings but has until now not been used to patients with COVID-19. It is not evident that the principles can be extrapolated from one condition to another. In COPD, where the main body of evidence is for adult use of closed-loop control, the need for oxygen supplementation is often limited to a few liters of oxygen per minute, and the changes in oxygen need during an admission are often also minor and are due to worsening of V/Q mismatch resulting from regional impaired ventilation [Citation8,Citation10]. In COVID-19 another pathophysiology is causing a more severe V/Q mismatch and shunting due to a perfusion disorder with impaired hypoxic vasoconstriction and a vascular leakage into the interstitium and the alveoli impeding oxygen diffusion. Collapsed alveoli results in areas without ventilation, and if perfusion remains, the consequence is shunting, where oxygen supplementation has little effect [Citation14]. Thus, it is interesting that in patients handled on moderate oxygen supplementation, closed-loop control is able to maintain oxygenation within a 4% SpO2-interval, from 92 to 96%, in 82.9% of the time, which is at the same level that has been demonstrated in COPD [Citation8,Citation10]. Furthermore, an acceptable oxygen saturation within an 8% SpO2-interval, from 90 to 98%, was achieved in 94.3% of the time. We know from closed-loop studies that manual oxygen control only is able to achieve the target in approximately 50% of the time [Citation8–Citation10]. In COVID-19 the gain in terms of use of nursing staff resources is larger, as the isolation regime makes it difficult and time-consuming to do simple tasks such as manually adjusting the oxygen flow.
Some reservations regarding the study results must be made. The sample size was small and might not be representative for all patients admitted with COVID-19. However, compared to a larger cohort of all 175 patients admitted with COVID-19 to the same hospital during the same period, the patients in this study had comparable demographics in terms of age, smoking history and body mass index [Citation15]. Biochemistry at baseline was also similar. Compared to the larger cohort, our patients had more frequent dyspnea on admission, were more in need of oxygen supplementation, and more frequently had diabetes or hypertension. Our study was without a control group, and the benefit demonstrated is based on the assumption that manual oxygen control would not have done better than we have seen in other studies, resulting in around 50% of the time with optimal control of SpO2, compared to the 82.9% demonstrated in the actual study. Furthermore, the patients in this study were only moderately hypoxemic. When the need for oxygen supplementation exceeded 15 liters/min the patients were transferred to a unit where they could receive higher oxygen flow in combination with CPAP, where the latter demonstrated a very positive effect on oxygenation. It is important to emphasize that oxygen supplementation is only part of the ventilation strategy in COVID-19, and in more severe cases recruitment of alveoli and improvement in pulmonary compliance by instruments such as CPAP or mechanical ventilation is necessary [Citation16].
The clinical importance of maintaining SpO2 within an interval such as 92 to 96% as recommended by the Surviving Sepsis Campaign is uncertain [Citation7]. Pulmonary toxicity of oxygen is probably not present at a fraction of inspired oxygen (FiO2) less than 60%, which means that in terms of pulmonary toxicity 15 liters/min by nasal cannula can be administered safely [Citation17]. However, a high PaO2 can still be associated with myocardial vasoconstriction, which could be a problem in patients with COVID-19, where cardiovascular function also seems to be affected [Citation18]. At the lower end of the SpO2 interval, we have some documentation in similar conditions that hypoxemia is dangerous. The LOCO2 trial comparing very conservative oxygen treatment with liberal oxygen treatment in patients with ARDS, was stopped early, due to safety concerns, as more cases of mesenteric ischemia were seen with conservative oxygen strategy, indicating that longer periods with SpO2 below 90–92% could be associated with increased morbidity [Citation6].
We did not in this study examine patient compliance and discomfort related to the need for continuous measurement of SpO2. The average time with use of O2matic was 66 hours, which is close to the average stay at the ward, which was 3.2 days. The use of different pulse oximetry sensors made it possible to individualize this part of the treatment. The finger sensor for single patient use was convenient for patients as well as the nursing staff. The fraction of time with missing signal, which was 8%, is on the same level as in another closed-loop study of several days duration [Citation8], but longer than in a study of only a few hours duration [Citation10]. The reasons for loss of signal were in part that patients were allowed to remove the sensor while eating, visiting the bathroom, etc. In these periods, the oxygen flow was automatically fixed at the same level as when the signal was lost. Loss of signal during sleep, due to displacement of the sensor was probably also part of the reason for more frequent loss of signal compared to a daytime study (10). By visual inspection of graphs depicting SpO2 and oxygen flow versus time for individual patients, we found no indication that loss of signal was preceded by a clinically worsening of the condition with a decrease in SpO2 or increase in oxygen flow. Thus, we are confident that loss of signal was not a critical issue, and was handled properly by keeping oxygen flow fixed until signal was restored.
Lung function data showed a marked reduction in FEV1, FVC and PEF, all reduced to approximately 50% of predicted, reflecting a substantial loss of lung volume. It was not possible to measure diffusing capacity due to patients having dyspnea and tachypnea, which prevented the breath-holding maneuver. Neither do we have data on lung function before admission with COVID-19, but only three patients had known obstructive lung disease, which makes it unlikely that a severe reduction in dynamic volumes was present before admission. Some patients experienced difficulties in performing spirometry due to acute breathlessness and coughing, and acceptability criteria could not be met for all patients. However, we find it unlikely that low quality of the spirometry accounts for a substantial part of the reduction in FEV1, FVC and PEF. Another study has shown minor reduction in dynamic values at discharge from admission with COVID-19 [Citation19]. Our study was done during the first days after admission, and 93% of the patients had acute radiological abnormalities with infiltrates and interstitial changes which could account for the severely impacted lung function.
In conclusion, we find that it is possible to administer oxygen safely and effectively to patients admitted with COVID-19 with a closed-loop system, O2matic, based on continuous measurement of SpO2. The automatic adjustment of oxygen flow is advantageous, especially in patients isolated due to contagious disease, such as COVID-19. Further and larger studies must examine the clinical impact of optimized control of SpO2, in terms of possible reduction in adverse outcomes and faster weaning from oxygen supplementation and discharge from hospital.
Disclosure statement
EFH is one of the inventors of O2matic® and have participated in the development and testing of the device since 2011. The partnership, which was built during the development funded by Innovation Fund Denmark, formed a new company (O2matic Ltd., Herlev, Denmark) in which EFH is shareholder. CSB and LMK do research involving O2matic, which is partly funded by Innovation Fund Denmark. The authors report no other conflicts of interest in this work.
Additional information
Funding
Notes on contributors
Ejvind Frausing Hansen
Ejvind Frausing Hansen is a Respiratory Consultant at the Department of Respiratory Medicine, Hvidovre University Hospital, Hvidovre, Denmark.
Charlotte Sandau Bech
Charlotte Sandau Bech is a Specialist Nurse, Master of Science and Ph.D. student at the Department of Respiratory Medicine, Hvidovre University Hospital, Hvidovre, Denmark.
Jørgen Vestbo
Jørgen Vestbo is a Professor of Respiratory Medicine at the Division of Infection, Immunity & Respiratory Medicine, University of Manchester, Manchester, United Kingdom.
Ove Andersen
Ove Andersen is a Professor and Head of Department at Department of Clinical Research, Hvidovre University Hospital, Hvidovre, Denmark.
Linette Marie Kofod
Linette Marie Kofod is a Specialist Physiotherapist, Master in Rehabilitation and Ph.D. student at Department of Physio-and Occupational therapy, Hvidovre University Hospital, Hvidovre, Denmark.
References
- Cornet AD, Kooter AJ, Peters MJL, et al. The potential harm of oxygen therapy in medical emergencies. Crit Care. 2013;17:313.
- Smith JL. The pathological effects due to increase of oxygen tension in the air breathed. J Physiol. 1899;24:19–8.
- Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391:1693–1705.
- Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen-ICU randomized clinical trial. JAMA. 2016;316:1583–1589.
- O’Driscoll BR, Howard LS, Earis J, et al. British thoracic society emergency oxygen guideline group. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(Suppl1):ii1–ii90.
- Barrot L, Asfar P, Mauny F, et al. LOCO2 investigators and REVA research network. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382:999–1008.
- Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID‑19). Intensive Care Med. 2020. DOI:10.1007/s00134-020-06022-5.
- Lellouche F, Bouchard PA, Roberge M, et al. Automated oxygen titration and weaning with FreeO2 in patients with acute exacerbation of COPD: a pilot randomized trial. Int J Chron Obstruct Pulmon Dis. 2016;11:1983–1990.
- L’Her E, Dias P, Gouillou M, et al. Automatic versus manual oxygen administration in the emergency department. Eur Respir J. 2017;50:1602552.
- Hansen EF, Hove JD, Bech CS, et al. Automated oxygen control with O2matic during admission with exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3997–4003.
- Sturrock S, Williams E, Dassios T, et al. Closed loop automated oxygen control in neonates-a review. Acta Paediatr. 2020;109:914–922.
- Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nature Med. 2020. DOI:10.1038/s41591-020-0968-3.
- Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102.
- Gattinoni L, Coppola S, Cressoni M, et al. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300.
- Israelsen SB, Kristiansen KT, Hindsberger B, et al. Characteristics of patients with COVID-19 pneumonia at Hvidovre Hospital, March-April 2020. Dan Med J. 2020;67:A05200313.
- Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020. DOI:10.1001/jama.2020.6825
- Hedley-Whyte J. Pulmonary oxygen toxicity: investigation and mentoring. Ulster Med J. 2008;77:39–42.
- Moccia F, Gerbino A, Lionetti V, et al. COVID-19-associated cardiovascular morbidity in older adults: a position paper from the Italian society of cardiovascular researches. Geroscience. 2020;42:1021–1049
- Mo X, Jian W, Su Z, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55:2001217.