ABSTRACT
The evaluation and management of severe asthma patients require collection of comprehensive information, which is often a challenge in a busy outpatient clinic. The Danish Severe Asthma Register (DSAR) was designed as an electronic patient record form that captures operational clinical data and provides a clinical overview of the severe asthma patient. DSAR is a nationwide register; all patients in Denmark who are treated with biologics for severe asthma are included, and data are as a minimum entered at start of biological treatment, after four and 12 months of treatment, and hereafter annually. Currently, there are data from 621 treatment courses with biologics included in DSAR, with 71% of patients treated with anti-IL-5 drugs and 29% with an anti-IgE drug. Patients enter Patient Reported Outcome Measures electronically on tablets when they arrive in the outpatient clinic and their answers are immediately available to the clinician during the consultation. Nurses and doctors enter clinical data into DSAR during the consultation. DSAR offers immediate access to well-presented longitudinal overview and automatically creates a journal output that can be copy-pasted into the hospital’s existing health record form. DSAR is also currently expanding with an app, to be used for monitoring of home-treatment. In addition to serving as an electronic patient record form, DSAR will also provide opportunities to monitor the real-life efficacy of biological treatment for severe asthma in Denmark, and it will be a valuable research platform that will aid in answering important research questions on severe asthma in the future.
Introduction
In the European Respiratory Society (ERS) and American Thoracic Society (ATS) guidelines, severe asthma is defined as asthma, which requires treatment with high doses of inhaled corticosteroids in combination with a second controller (long-acting bronchodilators, leukotriene-antagonists, xanthines) or treatment with systemic corticosteroids to prevent it from becoming uncontrolled, or which remains uncontrolled despite this treatment [Citation1]. Patients with seemingly severe asthma may also have other modifiable causes of poor asthma control, including lack of adherence to maintenance therapy, inadequate inhalation technique, exposure to asthma triggers, and a battery of co-morbidities [Citation2–4]. Consequently, guidelines emphasize that a systematic assessment of patients with uncontrolled, and by that possibly severe, asthma by respiratory specialists is necessary to differentiate between true severe asthma and poor asthma control due to external factors. The systematic assessment is recommended to include objective confirmation of the asthma diagnosis, phenotypic evaluation, identification of treatment barriers, and assessment of comorbidities and environmental exposures [Citation1–3,Citation5,Citation6]. This is a comprehensive process that is time-consuming, not always feasible in a busy clinical setting and is therefore may not always be performed; a diagnosis of severe asthma should only be given to patients that either are poorly controlled, or lose control when treatment is down-titrated, despite having optimal adherence, correct inhalation technique, removal of exposures and management of co-morbidities [Citation1,Citation3]. In two Danish studies examining patients seen by asthma specialists in outpatient clinics, it was found that among patients treated with high dose asthma medications – thus fulfilling one of the characteristics of severe asthma – systematic assessment to confirm the severe asthma diagnoses was infrequently performed [Citation7], and most patients could be classified as having ‘difficult-to-treat asthma’ rather than severe asthma [Citation8]. These findings emphasize a substantial room for improvement in the systematic assessment and management of severe asthma patients.
Biological therapies that target specific inflammatory pathways have emerged as promising personalized medicines in the treatment of severe asthma [Citation9,Citation10]. Currently approved biologics include three anti-interleukin (IL)-5 drugs (mepolizumab, reslizumab, benralizumab), one anti-IgE (omalizumab) drug, and one anti-IL-4/IL-13 drug (dupilumab). Potential new targets for drug development are being tested, such as thymic stromal lymphopoietin (TLSP), and IL-33, and are at variable stages of development [Citation9]. The approved drugs have shown to be very effective in reducing the risk of asthma exacerbations and with few side effects. However, they are expensive and there is substantial treatment response variability [Citation11]. Appropriate selection of biologics for each individual patient requires knowledge about reliable biomarkers and precise characterization of asthma phenotypes and this knowledge is therefore becoming increasingly important for specialists managing patients with severe asthma.
Taken together, a confirmation of the severe asthma diagnosis and the following best possible selection of treatment (personalized medicine) require collection and evaluation of comprehensive information. Hence, there is a need for systematic registration of severe asthma patients and of tools that can facilitate this process.
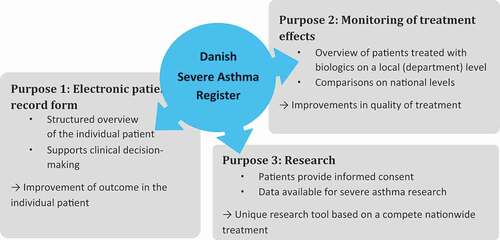
The Danish Severe Asthma Register (DSAR) is a new nationwide treatment register for severe asthma that has been developed with a threefold purpose: (1) An electronic journal to capture operational data and provide clinical overview, (2) to monitor the effect of biological treatment in Danish patients with severe asthma, and (3) to provide research possibilities.
In this paper we describe DSAR and its organizational structure, the patient population, the variables collected, the time points of data collection, the model for data entry and the expected benefits of DSAR. We also provide a brief characterization of the study population, as well as discuss some strengths and limitations of the collected data.
Cohort description
Study population
All Danish patients commenced on a biological asthma therapy must be entered into DSAR, with a baseline visit, prior to commencing the treatment. Outpatient clinics may also choose optionally to include all patients with severe asthma according to ERS/ATS guidelines [Citation1], but this is not a formal requirement.
Organizational structure
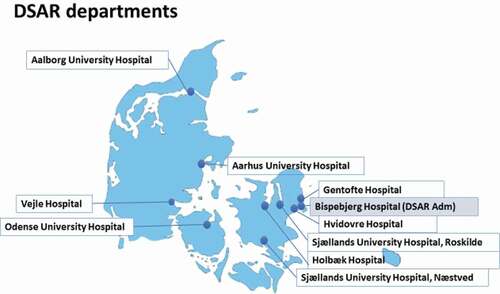
DSAR was initiated by the Danish Respiratory Society in 2017 and is governed by an independent steering committee, with representatives from each of the participating clinical departments. Experience from other fields has shown that the long-term sustainability of a clinical registry is dependent on a culture change at the every-day clinical practical level in order for clinicians to gain value from their participation in the registry [Citation12]. Therefore, it was deemed important to have representatives from all departments that administer biological drugs to treat severe asthma in Denmark to secure support for the register locally. Originally, this included eight respiratory departments, later, this has grown to include ten departments from all regions of Denmark (see ). It is the Danish National Board of Health that determines which departments are allowed to administer biological treatment for severe asthma, and it is a specialist task to prescribe the treatment and secure follow-up according to guidelines.
The steering committee is responsible for handling financial, regulatory, and scientific matters of DSAR. The daily administration is based at Bispebjerg Hospital in Copenhagen staffed with an academic secretary and a project coordinator, led by the chair of the steering committee.
DSAR is sponsored by the Danish Respiratory Society as well as pharmaceutical companies (Glaxo Smith Kline, Novartis, AstraZeneca, Teva, and Sanofi). The sponsors have no influence on the management of the registry, or the results that will be published based on data from the registry. Each company has signed a contract with the registry that has been approved by the legal department in the Capital Region.
IT solution
DSAR is a customized electronic database that is accessed at www.dsar.dk. Health care professionals have a personal log-in and their activity is logged. DSAR is placed on a private virtual service running Ubuntu-Linux with separation between frontend server (visible from the Internet) and backend-server where the data are stored. The DSAR system consists of two interconnected systems for health care professionals and patients. The system is developed and maintained by ZiteLab ApS with use of an Opensource IT-platform called Plone (plone.org) known for its outstanding security-record.
ZiteLab has implemented similar systems in several medical fields using the same IT-platform including DANBIO, which is a large nationwide registry including approximately 60.000 patients with rheumatologic diseases. The use of research data from the DANBIO registry has led to more than 60 peer-reviewed articles [Citation13–15].
Variables collected
Variables included in DSAR are coordinated with the variables collected in the two existing international severe asthma databases, which are currently being set up: The International Severe Asthma Register (ISAR) [Citation16] and the pan-European database under ERS, the Severe Heterogenous Asthma Research Collaboration, Patient-centered (SHARP) ERS Clinical Research Collaboration [Citation17]. This will provide opportunities for comparing data, but also for integrating data for international research projects and publications. Furthermore, DSAR includes additional variables decided upon in the Nordic Severe Asthma Network as well as variables decided by the DSAR steering committee to ensure that all needed clinical information was collected to support the use of DSAR as a decision-supporting patient record form.
The content of DSAR is presented in . A full list of the variables collected in DSAR can be seen in Appendix 1.
Table 1. Overview of core variables collected in the Danish Severe Asthma Register
Data entry
Data are entered by patients, nurses, and doctors. The collection of real-life data in a busy clinical setting is associated with some logistic challenges. The data entry performed by health care professionals must be feasible and possible to integrate in normal routine care to secure that only very limited extra time is spent on the data entry [Citation18]. When designing DSAR, a key priority has been to develop a user-friendly web-based solution that can be used ‘real-time’ during the consultation with no post-registration needed. Importantly, this solution furthermore ensures that summaries of the collected data are immediately available to the clinicians, providing an overview of the patient’s situation and response to treatment. This overview can also be used in real-time to discuss treatment decisions and evaluations with the patient. The clinical overview is described in further detail below.
Patients
Patients are asked to provide their input by answering electronic Patient Reported Outcome Measures (PROMs) on tablets handed out upon registration in the outpatient clinics. The PROMs have been selected by the steering committee and include internationally approved questionnaires to assess asthma control, quality of life, and identification and control of co-morbidities. For a list of the PROMs currently collected in DSAR, see . The validity of electronic questionnaires compared to paper forms has not yet been investigated in this patient group, but experience from other medical fields has shown good performance of the electronic questionnaires [Citation19]. After patients have completed the PROMs, their answers can immediately be imported into DSAR and made available for the doctors’ evaluation during the consultation.
Professionals
Nurses and doctors enter clinical information into DSAR during the consultation with direct data entry into the electronic journal. The local departments decide who is responsible for entering which variables depending on the local organization and staff responsibilities. Variables are collected in a standardized format, using tick boxes or free text fields. For variables entered as values, there is an automatic data range validation implemented in the system with pre-defined meaningful ranges for each variable (if applicable). If an unrealistic value is entered, a pop-up warning appears.
Follow-up visits
Patients treated with biologics are followed-up according to predefined time points to secure comparable information from all patients. provides an overview of the follow-up and the visit types in DSAR. The extent of data entry varies according to these visits.
Figure 2. Overview of DSAR visits and collected data. One model for data entry with patients completing PROMs and nurses and doctors responsible for part of the clinical data entry
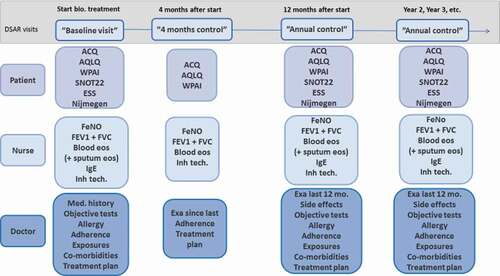
Patients are included in DSAR when biological treatment is initiated. Information from the systematic assessment that precedes the decision to start the patient in biological treatment is entered into DSAR as a ‘baseline visit’. The specific indications for starting treatment are recorded as well as the type of drug and start date. Currently prescribed medication for each individual patient is registered.
A ‘four months control visits’ follows the baseline visit and includes the first mandatory assessment of the treatment effect. At this visit, symptom control is assessed by the means of the Asthma Control Questionnaire (ACQ), the number of exacerbations since start of treatment are registered, as well as changes in medication (OCS use).
After one year of treatment, an ‘annual control visit’ is completed with a thorough assessment of symptom control, medication status, objective tests, allergy tests, inflammatory markers, treatment barriers, exposures and triggers, co-morbidities and treatment plan. Changes in medication are registered as well as information on potential severe side effects from biological treatment.
After the first year of treatment, patients are followed longitudinally with at least one annual doctor visit. ‘Extra doctor visits’ can be entered as needed if departments wish to use DSAR for all doctor visits. If a patient switches to another biological drug, a new baseline visit should be entered, followed by a 4 months control, and an annual control and so on.
Administration of biological treatment can also be recorded at each injection, and if clinical measures are obtained, these can also be recorded. This registration is voluntary but allows electronic registration of treatment administration which has obvious advantages compared to registration on paper forms.
App for home treatment
DSAR’s IT provider has developed an app that can be downloaded on smartphones that eventually will allow patients to complete PROMs from home or in connection with home treatment that are expected to grow in numbers in the coming years. Patients can log in from home on their smartphones or tablets and answer questionnaires about their self-reported health and symptom control. The app will also enable completion of questionnaires at home prior to visiting the outpatient clinic. The app is currently being expanded to allow for monitoring of home treatments: This will include reminders to the patients to take their medications, registration of dates of administration and side effects, as well as an action plan in case of exacerbations. The app is expected to be ready for clinical use in 2020.
The app has been approved by authorities in the Capital Region of Denmark overseeing IT-technical and legal aspects of General Data Protection Regulation (GDPR).
The contribution of DSAR to severe asthma management, monitoring of treatment efficacy, and research
DSAR is unique in that it will both serve as an important real-life decision-making tool in the clinic, it will provide a solid foundation for nationwide monitoring of treatment efficacy of biologics for severe asthma, and it will serve as an important research database. The main benefits of DSAR are summarized in and described in detail below.
DSAR purpose 1: clinical overview
DSAR will provide health care providers with a well-presented longitudinal electronic patient record of the severe asthma patient treated with biologics that ultimately has the potential to improve outcome in the individual patient.
The clinical overview is designed into three main outputs: a patient summary table, a biological treatment table, and a patient scoreboard ().
Figure 4. Clinical overview in DSAR: patient summary, biologic drug table, and scoreboard. Information is expanded when clicking on the hyperlinks
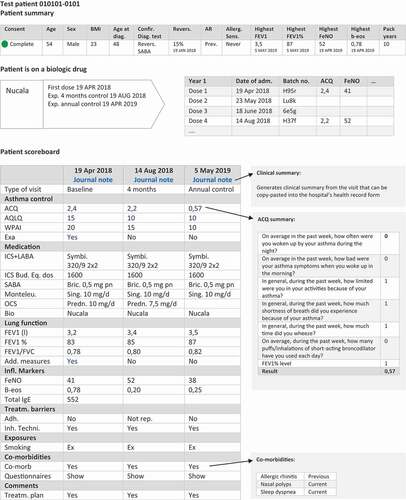
The patient summary includes demographic and phenotypic characteristics of the patient, information about which diagnostic tests confirmed the asthma diagnosis, the presence of allergy, values of the highest ever recorded FeNO, FEV1 and blood eosinophils, and smoking history. The values are directly extracted from the database and are automatically updated when new values that exceed previous are entered. The biological treatment table includes type of biologics, start date, and number of doses administered. The main part of the patient overview is comprised of the patient scoreboard that presents longitudinal information about the disease course. The scoreboard is a table with each column corresponding to a visit on a given date in the outpatient clinic. Displayed variables include information on ACQ, exacerbations, medication status including OCS use, lung function, inflammatory markers, presence of co-morbidities, smoking status, and comments to treatment plan. FEV1% of predicted value is auto-calculated based on the entered value of FEV1 held together with reference values for lung function and core data about the patient, including height, ethnicity, and sex.
Much of the information in the patient scoreboard is displayed as collapsible content that can further be expanded by simple clicks. Additional information can be obtained by clicking on a value that opens a pop-up window with more information about a given variable. This function ensures that the scoreboard is simple and provides a quick overview without too much information but at the same time it can be expanded to show detailed information if needed.
DSAR will not initially be linked directly with the hospital’s electronic patient records and therefore some double data-entry is needed. Therefore, an important functionality in DSAR is the automated clinical summary that can be generated and copy-pasted directly into the hospital’s electronic patient record. When a visit is completed in DSAR, the automatic clinical summary extracts values from the entered variables and presents the output in a structured text that fulfills the standard for a sufficient clinical output. This has minimized the amount of double data entry.
DSAR purpose 2: monitoring of treatment effects
With the introduction of DSAR, there will be a possibility to monitor the treatment of severe asthma with biologics in Denmark on several levels. DSAR will allow systematic evaluation on a national level of the number of patients treated and the use of different types of treatment. Biological treatment for severe allergic asthma has been available for several years, but the spread and follow-up geographically seem to be heterogeneous in Denmark. New anti-IL-5 treatments have been approved and in the upcoming years additional biological treatments will be available. Recently, notification duty has become mandatory in Denmark when it comes to the use of IL-5 biologics [Citation20]. DSAR will therefore enable departments to fulfill this juridical requirement.
Health care providers will have an overview of all patients treated in their department with biologics and some summary statistics such as number of patients treated stratified by type of treatment, and they will be able to compare their patients with national data.
DSAR purpose 3: a research database
Data from clinical registries provide an ideal platform for medical research and enable studies on real-life efficacy [Citation18]. DSAR is therefore expected to provide unique research opportunities for conducting observational studies.
The use of data from DSAR for research purposes relies on patients providing informed consent. Consent can then be given on the tablets, with a signature on the screen. A clear distinction between DSAR as an electronic journal and a research database is made; informed consent is only provided with respect to research. The informed consent is automatically requested annually, in accordance with Danish laws.
Researchers can apply for access to data from DSAR by contacting the steering committee which approves applications. Projects must be approved by the relevant authorities before approval from the steering committee can be sought and anonymous data ultimately delivered to the researchers.
There will be opportunities to link data from DSAR with data from many Danish nationwide registers such as the Danish National Patient Register (hospital admissions, therapies, diagnoses) and the Registry of Medicinal Product Statistics (prescriptions redeemed in Danish pharmacies) or the Danish General Health Database (education, socioeconomic, demographics). Data linkage is conducted through the personal identification number given to all citizens in Denmark and enables coupling of information on an individual level [Citation21].
Data reports
Annually, a report presenting information on a national level will be publicly available on the homepage www.dsar.dk. These reports will include key data on characteristics of severe asthma patients treated with biological and the efficacy of treatment. Regular reports will be created based on national DSAR data to the steering committee to secure high data quality and allow monitoring of the performance of each department involved in DSAR.
Collaboration
The Danish Severe Asthma Register collaborates with the International Severe Asthma Registry, the Severe Heterogeneous Asthma Research collaboration, Patient-centred (SHARP), and the Nordic Severe Asthma Network. It is the goal of DSAR that with the necessary permission processes in place, collaborators can get access to data for research. More information is available at www.dsar.dk.
Ethics and approvals
Approval to DSAR was sought simultaneously as the European Union GDPR was implemented, which resulted in some challenges in getting the sufficient approvals. DSAR has been approved as a treatment database by the Danish Data Protection Agency meaning that it can be used as a patient record form in the department without seeking direct approval from the patients regarding the use for clinical purposes (approval number VD-2018-116). A separate approval for research has furthermore been obtained, and this approval is conditioned on patients providing informed consent (approval number VD-2018-31). Data processing agreements are made between the data controller and the data processor that sets out the rights and obligations that apply to data processor’s handling of data on behalf of the data controller. The agreements comply with the regulations by the European Parliament.
Results
DSAR currently includes data from 621 biological treatment courses. Of these, 440 are treatments with anti-IL 5 drugs (71%), and 181 (29%) are treatment with the anti-IgE drug, omalizumab. The anti-IL 5 drugs used are mepolizumab (n = 311), benralizumab (n = 87), and reslizumab (n = 42).
To date, 311 patients have provided informed consent that their data can be used for research purposes. Baseline characteristics of these patients are presented in .
Table 2. Baseline characteristics of patients with severe asthma treated with biologics in the Danish Severe Asthma Register. Only patients who have provided informed consent that their data can be used for research purposes are included
The average patient starting on biologics in Denmark is 54 years (interquartile range (IQR): 18) old with adult onset asthma (90%) and is most often never-smoker (56%). There is almost an equal amount of men and women starting on biologic treatment (48% women). With respect to the demographic characteristics, there are few differences between patients starting on the four types of biologics drugs. However, patients starting on omalizumab are younger when they start treatment (46 years, IQR: 18), more often female (61%), and a higher proportion of patients had onset of asthma in childhood or adolescence (14%) compared to patients starting on anti-IL-5 drugs (0–10%).
At start of treatment, patients starting on biologics have experienced a median number of 3 (IQR: 4) exacerbations in the previous year. The median number of exacerbations varies between the four different types of drugs with numbers of 3 (IQR: 3), 1 (IQR: 2), 3 (IQR: 6), and 6 (IQR: 4) for mepolizumab, omalizumab, benralizumab, and reslizumab, respectively. The average patient has a FEV1 of 2.4 (1.7–3.0) l with few differences in lung function measures between the patients starting on the four biologic drugs. Patients starting on anti-IL5 drugs have on average higher FeNO and blood eosinophil counts compared to patients starting on omalizumab, whereas patients starting on omalizumab and benralizumab have higher total IgE compared to patients starting on mepolizumab and reslizumab.
Discussion
DSAR is a nationwide registry and includes all patients in Denmark with severe asthma who are treated with biologics. To our knowledge, it is the first severe asthma register to include all patients in an entire country. Currently, there are data from 621 biologic treatment courses included in DSAR.
Although efforts have been made to make DSAR easy to use, the implementation process has revealed that it is time consuming for clinicians to get used to a new patient record form in a busy outpatient clinic. Doctors and nurses have been taught how to use the system during training sessions, but real-life use of DSAR has been challenging for some departments due to heavy work load. Post-registration of patients has therefore been necessary, which is not how DSAR is intended to be used and which has added a data entry burden on the clinician. In line with this, we have observed that departments with many clinicians overseeing few patients on biological treatments have perceived DSAR more difficult to use due to long time gaps between the use of the system for the individual doctor and nurse. In contrast, departments organized around fewer nurses and doctors overseeing many patients on biologics have shown a stronger commitment to use the system and have built up routines faster in the clinic that has allowed for real-time use of DSAR. In the initial phase, prioritizing dedicated time along with frequent use of the system is therefore warranted.
Unfortunately, some double data entry is inevitable, since DSAR is not currently integrated with the existing hospital records. Hospitals in Denmark use different electronic record forms and therefore it has been impossible to make data integrations between these systems and DSAR. However, prescription medications are registered in the same nationwide system, the Shared Medication Record, and there are currently efforts being made to integrate DSAR with this central database, which would have obvious advantages. The double data entry issue has, however, been minimized by the possibility of creating a journal summary in DSAR that can be copy-pasted into the hospital record. Feedback from users has indicated that this is a helpful feature of DSAR.
Although the registry includes all patients on biological treatment for severe asthma in Denmark, some patients were already on treatment before DSAR was initiated. A retrospective data entry was completed for existing users of biologics starting from 2015 and onwards to include all patients being treated with anti-IL-5 biologics. However, for patients treated with omalizumab which has been on the market for almost two decades, it was not feasible to make retrospective journal reviews going all the way back to start of treatment, which has resulted in some incomplete baseline data. For other omalizumab patients, ‘baseline’ visits have been entered with clinical values from 2015 that could have been obtained after several years of treatment. This may explain why patients starting on omalizumab experience fewer exacerbations in the year leading up to treatment compared to the number of exacerbations in patients starting on anti-IL-5 treatment. The retrospective data, and in particular for omalizumab patients treated for a long duration may therefore be of varying quality.
Some patients may switch biologics, for instance if there is a lack of sufficient treatment response, and in DSAR, a new baseline visit should be created at time of switching to the new drug. These new baseline visits include clinical values that are not independent of the previous treatment. This could perhaps explain the high number of exacerbations in patients starting on reslizumab which is often not the first choice of treatment with anti-IL-5 biologics. On the other hand, switching biologics to benralizumab may also be done in patients who are well controlled at time of switching, since benralizumab is administered every 8 weeks compared to every 4 weeks for the other anti-IL-5 drugs. This may explain why blood eosinophils in patients starting on benralizumab are lower than that of patients starting on mepolizumab and reslizumab. These above examples clearly indicate that careful considerations of the potential pitfalls of real-life data are important to keep in mind when using data from DSAR. Analyses of bionaive patients may be one way of tackling these specific issues.
Currently, eight departments are using DSAR in the clinic, and two more departments are set to start using the system in 2020. The registration of patients has so far solely been done for patients on biological treatment, and data from DSAR are therefore currently restricted to this specific severe asthma patient population rather than patients with severe asthma in general. Future use of DSAR may include registration of all severe asthma patients, which would allow for interesting comparisons between patients with severe asthma treated with and without biologics.
Conclusion
DSAR is a new nationwide treatment- and research register over patients with severe asthma treated with biologics in Denmark. DSAR serves as an electronic patient record form on the individual level and provides real-life data to the clinician to improve decision making through several well-presented outputs. This feature ensures that value is being provided back to the clinician, rather than being a ‘one-way’ data entry into a registry. DSAR also provides opportunities to monitor treatment effects nationally, and it will be a valuable research platform that will aid in answering important research questions on severe asthma in the future.
Acknowledgments
The authors would like to acknowledge Marcus Ross Christensen and Olivia Nielsson for entering the retrospective data; Pia Høiagaard-Sørensen for daily management of DSAR; secretaries, nurses, and doctors from the participating departments for taking active part in DSAR.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
Notes on contributors
Celeste Porsbjerg
Professor, consultant, Ph.D Celeste Porsbjerg is a professor in severe asthma at the Respiratory Research Unit at Bispebjerg Hospital, Copenhagen, Denmark. Her research is focused on answering important clinical problems in severe asthma patients through a better understanding of disease mechanisms and to develop targeted treatment. Celeste Porsbjerg is head of the Danish Severe Asthma Register (DSAR) and the Nordic Severe Asthma Network. Additionally, she is the national representative in the Severe Heterogenous Asthma Research Collaboration, Patient-centred (SHARP) consortium and in the International Severe Asthma Registry (ISAR).
References
- Chung KF, Wenzel SE, Brozek JL, et al. m.fl. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014 Feb 1;43(2):343–13.
- Chanez P, Wenzel SE, Anderson GP, et al. m.fl. Severe asthma in adults: what are the important questions? J Allergy Clin Immunol. 2007 Jun;119(6):1337–1348.
- Bel EH, Sousa A, Fleming L, et al. m.fl. Diagnosis and definition of severe refractory asthma: an international consensus statement from the Innovative Medicine Initiative (IMI). Thorax. 2011 okt 1;66(10):910–917.
- GINA report. Global strategy for asthma management and prevention 2019 [Internet]. Global Initiative for Asthma; Tilgængelig hos; https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf
- Gibson PG, McDonald VM. Management of severe asthma: targeting the airways, comorbidities and risk factors: management of severe asthma. Intern Med J. 2017 Jun;47(6):623–631.
- Porsbjerg C, Ulrik C, Skjold T, et al. m.fl. Nordic consensus statement on the systematic assessment and management of possible severe asthma in adults. Eur Clin Respir J. 2018 Jan;5(1):1440868.
- von Bülow A, Backer V, Bodtger U, et al. m.fl. The level of diagnostic assessment in severe asthma: a nationwide real-life study. Respir Med. 2017 mar;124:21–29.
- von Bülow A, Backer V, Bodtger U, et al. m.fl. Differentiation of adult severe asthma from difficult-to-treat asthma – outcomes of a systematic assessment protocol. Respir Med. 2018 Dec;145:41–47.
- Bel EH, Ten Brinke A. New anti-eosinophil drugs for asthma and COPD. Chest. 2017 Dec;152(6):1276–1282.
- Fajt ML, Wenzel SE. Development of new therapies for severe asthma. Allergy Asthma Immunol Res. 2017;9(1):3.
- Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012 maj;18(5):716–725. .
- Blumenthal S. The use of clinical registries in the USA: a landscape survey. EGEMs Gener Evid Methods Improve Patient Outcomes. 2017 Dec 7;5(1):26.
- Hetland ML. DANBIO–powerful research database and electronic patient record. Rheumatology. 2011 Jan 1;50(1):69–77.
- Hetland ML, Jensen DV, Krogh NS. Monitoring patients with rheumatoid arthritis in routine care: experiences from a treat-to-target strategy using the DANBIO registry. Clin Exp Rheumatol. 2014 okt;32(5Suppl 85):S-141-146.
- Ibfelt EH, Jensen D, Hetland ML. The Danish nationwide clinical register for patients with rheumatoid arthritis: DANBIO. Clin Epidemiol. 2016 okt;8:737–742. .
- Bulathsinhala L, Eleangovan N, Heaney LG, et al. m.fl. Development of the International Severe Asthma Registry (ISAR): a modified Delphi study. J Allergy Clin Immunol Pract. 2019 Feb;7(2):578–588.e2.
- Djukanovic R, Adcock IM, Anderson G, et al. m.fl. The Severe Heterogeneous Asthma Research collaboration, Patient-centred (SHARP) ERS clinical research collaboration: a new dawn in asthma research. Eur Respir J. 2018 Nov;52(5):1801671.
- Dixon WG, Carmona L, Finckh A, et al. m.fl. EULAR points to consider when establishing, analysing and reporting safety data of biologics registers in rheumatology. Ann Rheum Dis. 2010 Sept 1;69(9):1596–1602.
- Schefte DB, Hetland ML. An open-source, self-explanatory touch screen in routine care. Validity of filling in the bath measures on ankylosing spondylitis disease activity index, function index, the health assessment questionnaire and visual analogue scales in comparison with paper versions. Rheumatology. 2010 Jan;49(1):99–104.
- Medicinrådets fælles regionale behandlingsvejledning med lægemiddelrekommandation for biologiske lægemidler til svær astma - valg mellem lægemidler [Internet]. Danish Medicines Council; 2018. Tilgængelighos:https://medicinraadet.dk/media/9576/medicinraadets-faelles-regionale-behandlingsvejledning-med-laegemiddelrekommandation-for-biologiske-laegemidler-til-svaer-astma-valg-mellem-laegemidler.pdf
- Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014 Aug;29(8):541–549.