ABSTRACT
Background Difference in dyspnea mMRC ≥2 between Finnish speaking and Swedish-speaking populations in Finland has not been previously studied.
Methods In February 2016, a respiratory questionnaire was sent to 8000 randomly selected subjects aged 20–69 years in western Finland with a response rate of 52.3%. The registered native language of each subject determined whether questionnaire in Finnish or Swedish was applied. Multiple logistic regression was performed to calculate Odds Ratios (OR) with 95% CI for the simultaneous effects of independent variables on dyspnea mMRC ≥2.
Results Of all participants, 2780 (71.9%) were Finnish speakers and 1084 (28.1%) were Swedish speakers. Finnish speakers had a higher prevalence of dyspnea mMRC ≥2 (11.1% vs 6.5% p < 0.001) when compared to Swedish speakers. Finnish speakers smoked more often, had higher BMI, spent less time moving during the day, had more often occupational exposure to vapours, gases, dusts or fumes (VGDF), and had lower socioeconomic status based on occupation. Significant risk factors for dyspnea mMRC ≥2 were COPD (OR = 10.94), BMI >35 (OR = 9.74), asthma (OR = 4.78), female gender (OR = 2.38), older age (OR = 2.20), current smoking (OR = 1.59), and occupational exposure to VGDF (OR = 1.47).
Conclusions Swedish speakers had less dyspnea mMRC ≥2 which is explained by a healthier lifestyle. Smoking, obesity, and occupational exposures should be in focus to improve respiratory health.
Introduction
Significant differences in respiratory health have been described in different populations, e.g., between urban and rural populations [Citation1] and between different countries. For example, a major difference in the prevalence of asthma and allergies has been described between Finnish and Russian Karelia [Citation2]. However, the conclusions related to respiratory health and risk factors may be hampered by the fact that populations in these studies often are genetically different, people live in different areas and/or are being served by different health-care provider systems.
Finland is a bilingual country with 5.5 million habitants with Finnish and Swedish as the two official languages. Swedish speakers are a minority, less than 6% of the population, living mostly in coastal regions in southern and western Finland and having a tight protective community. There are several studies comparing health and socioeconomic, demographic and geographical circumstances between Swedish and Finnish-speaking Finns. Swedish-speaking school children are healthier in terms of objective measures of health [Citation3]. The Swedish speakers have a less harmful drinking pattern [Citation4] and lower rates of sickness allowance receipt and early retirement [Citation5,Citation6]. There is also a correlation between family origin and mortality [Citation7]: the relative death risk compared to Finnish speakers born in western Finland was 1.13 for Finnish speakers born in eastern Finland and only 0.60 for Swedish speakers [Citation8]. The language-group-related differences in mortality are highest for deaths related to alcohol, suicide, and other external causes [Citation9].
To our knowledge, no previous studies have compared respiratory symptoms between Swedish and Finnish speakers in Finland. The present study cohort can give further insights into factors behind respiratory symptoms in a unique study population living in the same geographical area, being genetically similar and being served by the same social and health-care services. The main objective of this population-based study is to estimate and compare the prevalence of dyspnea mMRC ≥2 between Finnish and Swedish-speaking persons in Western Finland and to define risk factors for dyspnea.
Materials and methods
The present study population is a part of the latest FinEsS survey (Finland-Estonia-Sweden) conducted in Western Finland in February 2016. Respiratory questionnaires were sent to 8,000 randomly selected recipients aged 20–69 years in the Western Finland hospital districts of South Ostrobothnia and Vaasa. The sample was identified from the Finnish Population Register and reflected the language, age, and sex distribution of the population in the study area. Two reminders were sent to those not responding. The two main official languages of Finland are Finnish and Swedish and the registered native language was obtained from the Finnish Population Register. The registered native language of each subject determined whether questionnaire in Finnish or Swedish was applied. The basic characteristics of the cohort and the non-responder analysis have been published elsewhere [Citation10]. Non-responders were more often males (47.4% vs. 55.5%, p < 0.001) and younger (50 vs 42 years, p < 0.001) than responders.
The current study was approved by the ethical committee of Helsinki University Hospital. Concurrently with this study a similar FinEsS-study was conducted in Helsinki with an identical questionnaire and corresponding protocols.
Questionnaire and definitions
The Finnish FinEsS questionnaire contains features from the ATS and Tucson questionnaires [Citation11,Citation12] and is developed from the OLIN questionnaire [Citation13]. The questionnaire consists of questions on symptoms, respiratory diseases, medication and comorbidities, risk factors, and occupational factors considered relevant to respiratory epidemiology like exposure to vapours, gases, dusts or fumes (VGDF) [Citation14] and occupations. Occupations were classified according to the International Standard Classification of Occupations 2008 (ISCO-08) that provides a system for classifying and aggregating occupational information in a four-level hierarchically structured classification where ISCO-08 skill level 1 is the primary level of education and level 4 is usually obtained as a result of higher education [Citation15]. Median Body Mass Index (BMI) categories for analysis were normal weight <24.9 kg/m2, overweight 25.0–29.9 kg/m2, obesity category I 30.0–34.9 kg/m2 and obesity category II >35.0 kg/m2.
Dyspnea mMRC ≥2 was defined by an answer ‘yes’ to the question ‘Do you have to walk slower than other people of your age on level ground because of breathlessness?’ This question is comparable to the Modified Medical Research Council Dyspnea (mMRC) scale grade 2 and higher dyspnea [Citation16] that shows a limitation in daily life due to exercise-induced dyspnea.
Attacks of breathlessness were defined by an answer ‘yes’ to the question ‘Have you had intermittent breathlessness or attacks of breathlessness, with or without simultaneously appearing cough or wheezing during the last 12 months?’
Wheeze was defined by an answer ‘yes’ to the question “Have you had wheezing or whistling in your chest at any time during the last 12 months?”
Longstanding cough was defined by an answer ‘yes’ to the question “Have you had longstanding cough during the last 12 months?“
Translation process
Independent translation of the questionnaire from Swedish to Finnish was produced by a bilingual translator that was aware of the objective of the study and had an expertise in the study topic. Back-translations were done by two independent bilingual translators, one of them was Finnish, and the other was Swedish. A committee of professionals consisting of seven physicians, four of them bilinguals, compared translated versions and corrected errors in the first translations and improved cross-cultural adaptation. The quality and limitations of the final translation were estimated by an expert translator blinded to objectives of the study. The translation and cross-cultural adaptation process was performed according to guidelines [Citation17].
Statistical analysis
Statistical analyses were performed using SPSS software version 24 (IBM SPSS, Armonk, NY, USA). Mann–Whitney U-test was used for continuous and Pearson chi-square – test for categorical variables. A p-value <0.05 was considered significant and 95% confidence intervals (CI) were calculated. Multiple logistic regression was performed to calculate Odds Ratios (OR) with 95% CI for the simultaneous effects of independent variables on different respiratory symptoms.
Results
Characteristics of the study subjects
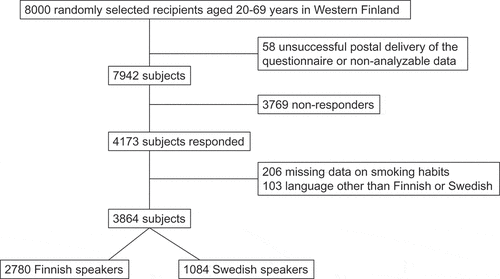
The corrected sample size was 7942 subjects after exclusion of subjects with unsuccessful postal delivery of the questionnaire or non-analysable data as shown in . In total, 4173 subjects of the 8000 invited responded yielding a participation rate of 52.3%. Of the responders, 206 were excluded because of missing data on smoking and 103 were excluded from the present study because of native language being other than Finnish or Swedish. Altogether 3864 subjects (48.3%) were included in the present study population, of which 2780 (71.9%) were Finnish speaking and 1084 (28.1%) were Swedish speaking (). The participation rate for Finnish speakers was 51.4% (2932 out of 5704) and 60.0% (1132 out of 1886) for Swedish speakers. The median age of responders was 54 years for Finnish speakers and 50 years for Swedish speakers (p < 0.001) and a slight dominance of women over men was observed in both groups (). BMI based on self-reported height and weight were 26.3 for Finnish speakers and 25.4 for Swedish speakers (p < 0.001).
Table 1. Basic demographics of Finnish and Swedish-speaking responders
Respiratory symptoms
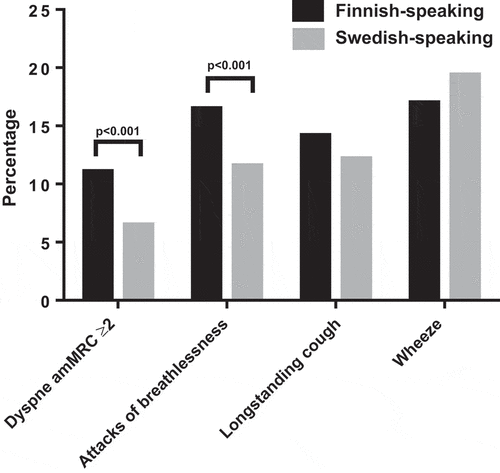
A higher proportion of Finnish speakers (11.1%) had dyspnea mMRC ≥2 as compared with Swedish speakers (6.5%, p < 0.001) (). In addition, 16.5% of the Finnish speakers and 11.6% of the Swedish speakers had attacks of breathlessness during the last 12 months (p < 0.001). There was no difference between Finnish and Swedish speakers in the prevalence of longstanding cough (14.2% vs. 12.2% p = 0.106) or wheeze during the last 12 months (17.0% vs. 19.4% p = 0.082).
Respiratory diagnoses
There were no significant differences in the prevalence of allergies or physician-diagnosed asthma between Finnish and Swedish speakers (). However, physician-diagnosed chronic bronchitis, COPD, and emphysema were more prevalent (3.0%) in Finnish speakers than in Swedish speakers (1.3%, p = 0.002).
Life-style and socioeconomic factors
Smoking was more common in Finnish speakers, 23.5% vs 16.1% (p < 0.001), as well as heavy smoking (). On average, Finnish speakers spent 3 h and Swedish speakers 4 h moving during the day (p < 0.001); thus, Finnish speakers were more physically inactive than Swedish speakers. However, no difference in exercise (‘sports’) frequency was found between language groups. During the first 5 years of life, Finnish speakers had lived less often in a rural area (p < 0.001), but had more often families that were farmers (p < 0.024). There was a difference in exposure to VGDF in the working environment: 40.8% of the Finnish speakers and 27.8% of the Swedish speakers were exposed (p < 0.001). According to ISCO-08 skill level, Finnish speakers had lower socioeconomic status based on occupation than Swedish speakers (p = 0.007).
Table 2. Social and lifestyle factors among Finnish and Swedish-speaking responders
Risk factors for dyspnea
Multiple logistic regression analysis was performed to define Odd Ratios for risk factors of having dyspnea mMRC ≥2. Most significant risk factors were diagnosis of COPD (OR = 10.9, p < 0.001) and BMI >35 (OR = 9.7, p < 0.001). Other statistically significant risk factors were diagnosis of asthma, older age, female gender, smoking, occupational exposure to VGDF, overweight, and grade 1 obesity. Native language, skill level, time spent moving or sitting were not found to be significant risk factors (). The same risk factors were found to be associated with attacks of breathlessness during the last 12 months, except for age. Asthma (OR 19.9, p < 0.001) and COPD (OR 14.7, p < 0.001) were the most significant risk factors for attacks of breathlessness ().
Table 3. Multiple logistic regressions for dyspnea mMRC ≥2
Table 4. Multiple logistic regressions for Attacks of breathlessness during the last 12 months
Discussion
In this study we report for the first time that prevalence of dyspnea mMRC ≥2 and attacks of breathlessness was higher in Finnish speakers when compared to Swedish speakers from the same population, the difference having significance for public health. Even though there was a clear difference in symptom prevalence between the language groups, belonging to a language population per se was not a risk factor for dyspnea or attacks of breathlessness. Differences in lifestyle was observed between Finnish and Swedish speakers and significant risk factors for these respiratory symptoms were COPD, asthma, obesity (especially grade II), current smoking, occupational exposure, and female gender.
Overall the prevalence of different respiratory symptoms found in this study was similar to what has been previously reported in comparable populations [Citation18–21]. For example, the prevalence of dyspnea mMRC ≥2 was 11.1% for Finnish speakers and 6.5% for Swedish speakers in the present study. In a previous study, there was a considerable geographical variation in dyspnea in populations from 15 countries: the prevalence of dyspnea mMRC ≥2 was 13% in the combined cohort [Citation18]. Prevalence of attacks of breathlessness has been reported to be approximately 14–15% in a previous study from Scandinavia [Citation19], and in this study the prevalence of attacks of breathlessness was between 11.6% and 16.5%. Prevalence of longstanding cough has been reported to be between 10% and 12% in Sweden [Citation20], while in this study it was 14.2 vs 12.2% in Finnish and Swedish speakers, respectively. In previous study prevalence of wheeze has been around 23% in Nordic countries and Australia [Citation19,Citation22,Citation23], but 13% in US population [Citation21], and in this current study it was 17.0% for Finnish speakers and 19.4% Swedish speakers. Thus, the reported prevalence of respiratory symptoms in the present study falls into the previously reported range.
Differences in the prevalence of respiratory symptoms between Swedish and Finnish speakers are unlikely explained by the prevalence of asthma or allergy as there was no difference in the prevalence of these diseases between the two language groups. COPD was more common in Finnish speakers (3.0% versus 1.3%), but the prevalence of COPD was low, and explains the differences in symptom prevalence only partly. Although the symptoms were more frequent in the Finnish speakers, the spoken language was not an independent risk factor for respiratory symptoms in multiple logistic regression analysis. This suggests that the other culture-related factors affecting respiratory health may explain the differences in symptom prevalence between the language groups. In previous studies, Swedish speakers had a higher sense of mastery and the association was mediated by social support [Citation24], and they possessed more structural and cognitive social capital [Citation25], while the Finnish speakers were more often migrated, mistrusting and less active in community events [Citation26]. These cultural differences might lead to healthier habits for the Swedish-speaking Finns. Interestingly, significant differences were found in many health-related habits between Finnish and Swedish speakers and these were also found to be independent risk factors for respiratory symptoms. In addition to diseases such as asthma and COPD or non-modifiable factors like age and sex, also clearly modifiable factors like high BMI, current smoking, and occupational exposure were significant risk factors for having respiratory symptoms. BMI >35 was associated with dyspnea mMRC ≥2 with OR 9.74 that was almost as high as the OR for COPD and 2 times higher than the OR for asthma. Overweight and obesity gr I were also risk factors for dyspnea and therefore, we consider that difference in BMI between the two language groups is an important factor explaining the higher prevalence of respiratory symptoms in Finnish speakers. Previous studies have suggested that dyspnea is very frequent in obese subjects and mMRC scale can be used in the assessment of dyspnea in obese patients [Citation27,Citation28]. The significant risk factors for dyspnea mMRC≥2 were similar to those for attacks of breathlessness, but dyspnea mMRC≥2 was more often associated with COPD, obesity, and old age, whereas attacks of breathlessness with asthma and not with age.
Current smoking and occupational exposures which are known risk factors for many respiratory symptoms [Citation22,Citation29–34] were more common in the Finnish speakers. In a previous study in northern Sweden, there was a parallel trend of decreasing respiratory symptoms and a lower prevalence of smoking [Citation20]. Association between respiratory symptoms and increasing age has been shown before [Citation35] and was observed also in this study. In the present study, females reported more often dyspnea when compared to males and this finding is supported by results from previous studies [Citation18,Citation29]. In a previous study low socioeconomic status was a risk factor for respiratory symptoms [Citation30]. In contrast, socioeconomic status based on occupation was not associated with respiratory symptoms in our study. The discrepancy between the previous study [Citation30] and our study may be explained by the additional adjustment for lifestyle factors performed in our study. As these are associated with both low socioeconomic status and symptoms, they may explain the increased prevalence of respiratory symptoms in the studied Finnish-speaking population.
Significant disparities in respiratory health have been described in ethnically different populations and with uneven access to healthcare [Citation1,Citation2,Citation36,Citation37]. Most studies on ethnic minority-group inequalities are comparing genetically and socioeconomically different populations [Citation38]. This study compared populations living in the same geographic area and having the same access to public healthcare public schools without fees in their respective native languages, using same shops, gyms, swimming halls, and sport clubs, but that still are prone to social gatherings within the language group. Populations in hospital districts of South Ostrobothnia and Vaasa are part of the same Finnish Western genetic subpopulation [Citation39]. Swedish-speaking population and Finnish-speaking population show a high degree of admixture from Sweden which most probably occurred at an early period after immigration over a thousand years ago [Citation40]. It is unclear how much saturation of genes has happened in language groups and therefore language was included in the regression analysis. Finnish and Swedish speakers have been later culturally divided by the language. Language and culture are even more tightly bound together than blood bonds and this makes a significant difference to daily habits and socioeconomic factors. In this study population, we can study how daily habits affect the risk for symptoms.
Major strengths of this study include the random sample reflecting the general population, large sample size, established structured questionnaire and validity of the translation. A limitation of this study is that the response rate could have been higher, but it corresponds well with similar recent surveys [Citation41]. The participation rate was lower for Finnish speakers, 51.4% compared to 60.0% for Swedish speakers. Still, both language groups are well represented. Further studies with clinical data, e.g., on the exercise capacity differences might strengthen the results found in this survey. Furthermore, it should be noted that that exercise and physical activity are two different concepts. Physical exercise is high or moderate-intensity movement conducted with a purpose for shorter periods of time (i.e., ‘sports’) whereas physical activity includes all human bodily movement [Citation42].
The clinical implication of this study is that smoking, high BMI, and occupational exposures should be the main considerations for respiratory health in the general public. Association between dyspnea and obesity might be underestimated in clinical practice. In medicine, we strive to diagnose and treat diseases and often have limited resources and knowledge to treat obesity as a cause of dyspnea.
We found that in genetically similar populations with different languages and culture the Swedish speakers had less respiratory symptoms and lower prevalence of COPD as they smoked less, had lower BMI, and had less occupational exposures compared to the Finnish speakers from the same region. Further behavioural, clinical, and even genetic studies are needed in order to find differences in respiratory disease phenotypes due to habits in this worldwide unique population.
Authors contribution
HA conceptualized and designed the study, performed analyses together with PI, drafted the initial manuscript, and approved the final manuscript as submitted. PI, LL, HK conceptualized and designed the study, provided statistical input and critical revision of the manuscript, and approved the final manuscript as submitted; JH, LET, PP, HHM, AS, HB, BL, and ER conceptualized and designed the study, provided critical revision of the manuscript, and approved the final manuscript as submitted. We affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of the Department of Medicine of Helsinki University Central Hospital (199/13/03/00/15) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study. The general Data Protection Regulation (EU) 2016/679 was followed.
Disclosure statement
The authors declare no conflict of interest related to this study. Outside this study, HA reports personal fees from Boehringer Ingelheim, MSD and Roche. Outside this study, PI reports personal fees from MundiPharma, Orion, Astra Zeneca, and GlaxoSmithKline. LET reports non-financial support from Chiesi, non-financial support from Boehringer-Ingelheim, personal fees from Astra Zeneca, non-financial support from Orion Pharma, non-financial support from TEVA and other from Novartis, outside the submitted work. HB reports personal fees from Boehringer–Ingelheim and AstraZeneca outside the submitted work. HHM reports employment at GSK. LL reports personal fees from ALK, AstraZeneca, Boehringer Ingelheim, Chiesi, Novartis, Orion Pharma, GSK, Teva, Mundipharma, SanofiGenzyme, outside the submitted work. HK report grants, personal fees, and non-financial support from AstraZeneca, personal fees from Chiesi Pharma AB, personal fees, and non-financial support from Boehringer-Ingelheim, personal fees from Novartis, personal fees from Mundipharma, personal fees from Roche, personal fees, and non-financial support from Orion Pharma, personal fees from Sanofi-Genzyme outside the submitted work.
Additional information
Funding
Notes on contributors
Heidi Andersén
Heidi Andersén is a researcher at the FinEsS studies, Tampere University, and a respiratory physician working at Karolinska University Hospital. Her research interests are obstructive airway diseases and lung cancer.
Pinja Ilmarinen
Pinja Ilmarinen is a researcher at the FinEsS and SAAS studies, Seinäjoki Central Hospital, and her research focus on phenotypes and mediators of adult-onset asthma. She is an Associate Professor of Experimental Pulmonology at Tampere University.
Jasmin Honkamäki
Jasmin Honkamäki is a medical student at the University of Tampere and a researcher at the FinEsS studies. Her research focuses on the age of asthma onset.
Leena E Tuomisto
Leena E Tuomisto is an Associate Professor of Respiratory Medicine at Tampere University and head of respiratory medicine at Seinäjoki Central Hospital. Her research interest includes adult-onset asthma from diagnosis to prognosis.
Päivi Piirilä
Päivi Piirilä is a Professor of Clinical Physiology and Functional Imaging at Helsinki University and head physician of the unit of clinical physiology of Meilahti unit of Helsinki University Central Hospital, and her research interests are clinical physiology, occupational pulmonary diseases, and epidemiology of obstructive airway diseases.
Hanna Hisinger-Mölkänen
Hanna Hisinger-Mölkänen is a respiratory physician and researcher at the FinEsS studies. Her research interest is chronic airway diseases.
Anssi Sovijärvi
Anssi Sovijärvi is a senior Professor of Clinical Physiology at Helsinki University; his research interest covers chronic airway diseases and clinical physiology.
Helena Backman
Helena Backman is a statistician and senior researcher at the OLIN studies, Norrbotten County Council. She has a PhD in public health and is affiliated with the Department of Public Health and Clinical Medicine, Section of Sustainable Health, Umeå University. Her research interest is the epidemiology of obstructive airway diseases.
Bo Lundbäck
Bo Lundbäck is a senior professor at Krefting Research Centre, University of Gothenburg. He is a member of the college of experts of the European Respiratory Society, ERS, and former member of the Executive Committee and the Science Council of the ERS, and former head of ERS’ Assembly of Epidemiology and Occupation.
Eva Rönmark
Eva Rönmark is the head of the OLIN studies, Norrbotten County Council, and professor at the Department of Public Health and Clinical Medicine, Division of Sustainable Health, Umeå University. Her research interest is the clinical epidemiology of obstructive airway diseases and allergy.
Lauri Lehtimäki
Lauri Lehtimäki is a consultant in respiratory medicine at Allergy Centre, Tampere University Hospital. and works as an Associate Professor oat Faculty of Medicine and Life Sciences at the University of Tampere. His research interest covers chronic airway diseases, including severe asthma and inflammatory lung diseases.
Hannu Kankaanranta
Hannu Kankaanranta is the head of the FinEsS Western Finland and SAAS studies. He works as a Professor of Asthma and Allergy Research in the Krefting Research Centre, Gothenburg University, and as a Professor of Respiratory Medicine at Tampere University. His main research interest is adult-onset asthma.
References
- Von Ehrenstein OS, Von Mutius E, Illi S, et al. Reduced risk of hay fever and asthma among children of farmers. Clin Exp Allergy. 2000;30(2):187–9.
- Pekkarinen PT, Laatikainen T, Jousilahti P, et al. A disparity in the association of asthma, rhinitis, and eczema with allergen-specific IgE between Finnish and Russian Karelia. Allergy. 2007;62(3):281–287.
- Saarela J, Finnäs F. The health of Swedish-speaking and Finnish-speaking schoolchildren in Finland. Child Care, Heal Dev. 2004;30(1):51–58.
- Paljärvi T, Suominen S, Koskenvuo M, et al. The differences in drinking patterns between Finnish-speaking majority and Swedish-speaking minority in Finland. Eur J Public Health. 2009;19(3):278–284.
- Reini K, Saarela J. Differences in sickness allowance receipt between Swedish speakers and Finnish speakers in Finland. Finnish Yearb Popul Res. 2017;52:43–58.
- Saarela J, Finnäs F. Language-group differences in very early retirement in Finland. Demogr Res. 2002;7:49–66.
- Saarela J, Finnäs F. Mortality inequality in two native population groups. Popul Stud (NY). 2005;59(3):313–320.
- Saarela J, Finnäs F. Family origin and mortality: prospective Finnish cohort study. BMC Public Health. 2011;11(1):385.
- Sipilä P, Martikainen P. Language-group mortality differentials in Finland: the effects of local language composition. Health Place. 2010;16(3):446–451.
- Honkamäki J, Hisinger-Mölkänen H, Ilmarinen P, et al. Age- and gender-specific incidence of new asthma diagnosis from childhood to late adulthood. Respir Med. 2019;154:56–62.
- Juniper EF, O’Byrne PM, Guyatt GH, et al. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907.
- Lebowitz MD, Burrows B. Comparison of questionnaires: the BMRC and NHLI respiratory questionnaires and a new self-completion questionnaire. Am Rev Respir Dis. 1976;113(5):627–635.
- Lundbäck B, Nyström L, Rosenhall L, et al. Obstructive lung disease in northern Sweden: respiratory symptoms assessed in a postal survey. Eur Respir J. 1991;4(3):257–266.
- Blanc PD, Eisner MD, Balmes JR, et al. Exposure to vapors, gas, dust, or fumes: assessment by a single survey item compared to a detailed exposure battery and a job exposure matrix. Am J Ind Med. 2005;48(4):110–117.
- International Labour Organization. Classification of Occupations. Int Labour Organ. 2012;1:1–420.
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586.
- Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol. 1993;46(12):1417–1432.
- Grønseth R, Vollmer WM, Hardie JA, et al. Predictors of dyspnoea prevalence: results from the BOLD study. Eur Respir J. 2014;43(6):1610–1620.
- Axelsson M, Lindberg A, Kainu A, et al. Respiratory symptoms increase health care consumption and affect everyday life – a cross-sectional population-based study from Finland, Estonia, and Sweden. Eur Clin Respir J. 2016;3(1):31024.
- Backman H, Hedman L, Jansson SA, et al. Prevalence trends in respiratory symptoms and asthma in relation to smoking - Two cross-sectional studies ten years apart among adults in northern Sweden. World Allergy Organ J. 2014;7:1.
- Wheaton AG, Ford ES, Thompson WW, et al. Pulmonary function, chronic respiratory symptoms, and health-related quality of life among adults in the USA–National Health and Nutrition Examination Survey 2007-2010. BMC Public Health. 2013;13(1):854.
- James AL, Knuiman MW, Divitini ML, et al. Risk factors for respiratory symptoms in adults: the Busselton Health Study. Respirology. 2013;18(8):1256–1260.
- Voll-Aanerud M, Eagan TML, Wentzel-Larsen T, et al. Respiratory symptoms, COPD severity, and health related quality of life in a general population sample. Respir Med. 2008;102(3):399–406.
- Reini K, Nyqvist F. Sense of mastery differences between working-age Swedish- and Finnish-speaking Finns: a population-based study. Scand J Public Health. 2017;45(4):404–410.
- Nyqvist F, Finnäs F, Jakobsson G, et al. The effect of social capital on health: the case of two language groups in Finland. Health Place. 2008;14(2):347–360.
- Hyyppä MT, Mäki J. Individual-level relationships between social capital and self-rated health in a bilingual community. Prev Med. 2001;32(2):148–155.
- Launois C, Barbe C, Bertin E, et al. The modified Medical Research Council scale for the assessment of dyspnea in daily living in obesity: a pilot study. BMC Pulm Med. 2012;1;12(61. DOI:https://doi.org/10.1186/1471-2466-12-61.
- Sjöström L, Larsson B, Backman L, et al. Swedish obese subjects (SOS). Recruitment for an intervention study and a selected description of the obese state. Int J Obes Relat Metab Disord. 1992;16(6):465–479.
- Lundbäck B, Stjernberg N, Nyström L, et al. Epidemiology of respiratory symptoms, lung function and important determinants. Report from the Obstructive Lung Disease in Northern Sweden Project. Tuber Lung Dis. 1994;75(2):116–126.
- Pallasaho P, Lindström M, Põlluste J, et al. Low socio-economic status is a risk factor for respiratory symptoms: a comparison between Finland, Sweden and Estonia. Int J Tuberc Lung Dis. 2004;8(11):1292–1300.
- Lindström M, Kotaniemi J, Jönsson E, et al. Smoking, respiratory symptoms, and diseases: a comparative study between northern Sweden and northern Finland: report from the FinEsS study. Chest. 2001;119(3):852–861.
- Kim J-L, Torén K, Lohman S, et al. Respiratory symptoms and respiratory-related absence from work among health care workers in Sweden. J Asthma. 2013;50(2):174–179.
- Khan AW, Moshammer HM, Kundi M. Industrial hygiene, occupational safety and respiratory symptoms in the Pakistani cotton industry. BMJ Open. 2015;5(4):e007266.
- Ghosh T, Gangopadhyay S, Das B. Prevalence of respiratory symptoms and disorders among rice mill workers in India. Environ Health Prev Med. 2014;19(3):226–233.
- Viegi G, Pedreschi M, Baldacci S, et al. Prevalence rates of respiratory symptoms and diseases in general population samples of North and Central Italy. Int J Tuberc Lung Dis. 1999;3(11):1034–1042.
- Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the USA, 2001-2010. NCHS Data Brief. 2012;94:1.
- Gillies TD, Tomlin AM, Dovey SM, et al. Ethnic disparities in asthma treatment and outcomes in children aged under 15 years in New Zealand: analysis of national databases. Prim Care Respir J. 2013;22(3):312–318.
- Jannus-Pruljan L, Meren M, Põlluste J, et al. Postal survey on asthma, chronic bronchitis and respiratory symptoms among adult Estonians and non-Estonians (FinEsS-study). Eur J Public Health. 2004;14(2):114–119. .
- Kerminen S, Havulinna AS, Hellenthal G, et al. Fine-scale genetic structure in Finland. G3 (Bethesda). 2017;7(10):3459–3468.
- Virtaranta-Knowles K, Sistonen PNH. A population genetic study in Finland: comparison of the Finnish- and Swedish-speaking populations. Hum Hered. 1991;41(4):248–264.
- Tolonen H, Koponen P, Borodulin K, et al. Language as a determinant of participation rates in Finnish health examination surveys. Scand J Public Health. 2018;46(2):240–243.
- Loponen J, Ilmarinen P, Tuomisto LE, et al. Daily physical activity and lung function decline in adult-onset asthma: a 12-year follow-up study. Eur Clin Respir J. 2018;5(1):1533753.