ABSTRACT
Objective: Lung cancer is the leading cause of cancer-related death worldwide. This population-based longitudinal study investigates survival rates and the burden of comorbidity before and after being diagnosed with lung cancer in Denmark.
Methods: From the Danish National Patient Registry (NPR) and the Danish Civil Registration System (CPR), 53,749 patients with lung cancer were identified and matched with 214,304 controls on age, gender, region of residence and marital status in the period 1998–2010. From the NPR, data on survival and comorbidity, registered as ICD-10 diagnoses, were extracted. Comorbidity was assessed using the Deyo-Charlson comorbidity score (DCcs) and mortality using Kaplan-Meier survival curves.
Results: 1-year survival rate for Danish lung cancer patients was 51.7 % (CI 51.3-52.1) and 5-year survival rate was 14.7 % (CI 14.3-15.0) compared to 96.8 % (CI 96.7-96.8) and 84.0 % (CI 83.9-84.2) for controls respectively. Overall, cases had significantly more comorbidity compared to controls before being diagnosed with lung cancer. Prior to being diagnosed with lung cancer, more cases than controls had been diagnosed with other malignancies (11.4 % vs 6.0 % p<0.005), diseases of the circulatory system (16.4 % vs 13.0 % p<0.005) and respiratory diseases (12.2 % vs 4.8 % p<0.005). Among lung cancer patients 21.8 % had a DCcs ≥ 1 compared to 13.3 % among controls (P<0.005). The 1-year survival for DCcs =0 was 54.8 % (CI 54.3-55.3) for lung cancer patients and 97.8 % (CI 97.7-97.9) for controls. Decreasing survival with increasing DCcs was found in both groups.
Conclusion: This study provides unique nationwide comorbidity data on patients before and after being diagnosed with lung cancer. We found increased mortality with increasing comorbidity, however more pronounced among controls compared to patients with lung cancer.
Background
To be diagnosed with cancer has multiple and significant consequences for the patient, relatives and society. Lung cancer constitutes 13% of the total cancer incidence in Denmark, and Danish patients with lung cancer have a lower survival rate compared to the other Nordic countries [Citation1]. The incidence of lung cancer has increased rapidly since the beginning of the 20th century and is currently the leading cause of cancer death not only in Denmark, but globally. Moreover, lung cancer is currently the most frequent cancer type in men, and is second only to breast cancer in women [Citation1].
In addition to the direct negative impact on survival, comorbidity can delay the staging phase and limit treatment options, including reduced probability of resection [Citation2], resulting in a lower 1-year survival rate [Citation3–5]. Comorbidities such as cardiovascular diseases, diabetes, cerebrovascular diseases and chronic obstructive pulmonary disease (COPD) all contribute to a lower survival rate [Citation6]. The Deyo-Charlson comorbidity score (DCcs) is an established tool to assess the burden of comorbidity and the impact on mortality [Citation7,Citation8].
Inequality in health regarding time to diagnosis, treatment and survival rate for patients with lung cancer has previously been established. Level of education, disposable income and co-habitation status have all been found to influence short-term survival [Citation9,Citation10]. Thus, shorter education and living alone were associated with a more advanced cancer stage at the time of diagnosis as well as an increased time between referral and diagnosis [Citation11]. Differences in survival can partly be explained by social inequality regarding disease stage at diagnosis, treatment options and comorbidity status [Citation12].
The comprehensive Danish registries provide a unique possibility to extract data from all lung cancer patients, holding data on comorbidity, medication use and socio-economic factors. In this study, we aimed to portray the comorbidity of Danish lung cancer patients both before and after being diagnosed with lung cancer as well as assess the impact of DCcs on overall survival. Comparing comorbidity of lung cancer patients to matched controls is novel, and we hypothesized that patients with lung cancer have a higher burden of comorbidity than controls, both before and after the lung cancer diagnosis.
Methods
In Denmark, data on all hospital contacts are registered in the National Patient Registry (NPR) [Citation13]. The NPR includes information on diagnoses and treatments in accordance with the International Classification of Diseases (ICD-10) as well as administrative information. For the time period 1998–2010, we extracted data from the NPR on first occurrence of the following primary or secondary diagnoses: ‘C34 Malignant neoplasm of bronchus and lung’ and ‘C33 Malignant Neoplasm of Trachea’. Data on disease stage were not available. Data on comorbidity were also extracted from the NPR as ICD-10 diagnoses given in the secondary health-care sector for all patients and controls. DCcs were calculated for each patient using ICD-10 diagnostic codes.
The Danish Civil Registration System (CPR) contains data on all Danish citizens including social factors, employment status, income, marital status, etc., by linkage to the Danish Income Statistics [Citation14]. Using the CPR system, each lung cancer patient was matched with four controls of same gender, age and residents in the same postal code area at the year of diagnosis. Cases and matched controls that could not be identified in the Danish Income Statistics database were excluded from the sample. Successfully matched observations were obtained for more than 99% of patients. If a person was not present in the registry on the first of January each year due to death, immigration or imprisonment, the corresponding control or patient was excluded. Smoking status was not available in any of the national databases.
Lung cancer patients and matched controls were followed from 1998 to 2010 or until they died. Patients diagnosed in 1998 were followed for 11 years forward in time making it possible to observe what happened after diagnosis. Patients diagnosed in 2010 were followed 11 years backwards in time making it possible to observe what happened before diagnosis. A patient diagnosed between the first and last year, e.g. in 2005 was traced backwards 6 years and forwards 5 years in time in the period of 1998 to 2010. Controls were equally followed forwards and backwards in time from the matched lung cancer patient diagnosis and this method has previously been described [Citation15–17].
The study was approved by the Danish Data Protection Agency. Data were anonymized and neither individual consent nor ethical approval was required.
Statistical analyses were performed using SAS 9.1.3 (SAS, Inc., Cary, NC). Statistical significance of the cost estimates was assessed by nonparametric bootstrap analysis. A significance level of 0.05 was assumed for all tests. Comorbidity data were analyzed in a conditional logistic regression model, yielding odds ratios (ORs) with 95% confidence intervals (CIs). Data reported as percentages were compared using Pearson’s chi-square test. Survival data were reported using the Kaplan-Meier survival function. Relative survival was expressed as hazard ratios (HRs) derived from a Cox proportional hazards model.
Results
Demography
Fifty-three thousand seven hundred and forty-nine patients were diagnosed with lung cancer from 1998 to 2010 and extracted from the databases. Age distribution of the lung cancer patients and matched controls are shown in . The majority of patients were aged 60–79 years (68.8%). Gender and co-habitation status are also shown in ; more males than females were diagnosed with lung cancer. More than half of the patients were married or lived with a partner.
Table 1. Age and gender distribution of all cases and controls in number and per cent. Per cent of married or co-habiting cases and controls
Survival and comorbidity
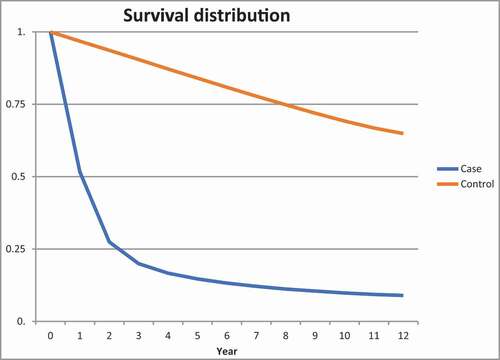
shows survival distribution of all lung cancer patients and matched controls. Survival of lung cancer patients decreased rapidly in the first two years after diagnosis whereas the survival curve of controls shows a linear descent. The 1-year survival rate for lung cancer patients was 51.7% (CI 51.3–52.1) and the 5-year survival rate was 14.7% (CI 14.3–15.0) compared to 96.8% (CI 96.7–96.8) and 84.0% (CI 83.9–84.2) for controls, respectively. Four years after diagnosis, survival rates for cases descend linearly similarly to controls.
Figure 1. Kaplan-Meier survival curves of patients with lung cancer (blue) and controls (red) with differences estimated using the Cox proportional hazard model. Number and percentage of lung cancer patients and controls that died during follow-up and HR for death distributed by age groups
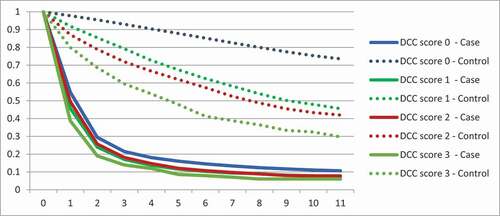
The risk of death among lung cancer patients was increased for all age groups compared to controls, but the HR decreased by age (); the HR for the 20–29 years age group was 94.1 compared to 10.4 for the 70–79 years age group.
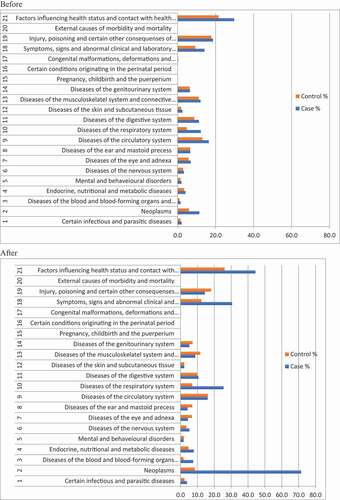
The distribution of comorbidity identified by ICD-10 is shown in displaying the registered comorbidities before and after the patients were diagnosed with lung cancer. Overall, patients had more diagnoses than controls both before and after being diagnosed with lung cancer. Prior to being diagnosed with lung cancer more cases than controls had been diagnosed with other malignant diseases (11.4% vs 6.0% p < 0.005), cardiovascular diseases (16.4% vs 13.0% p < 0.005), respiratory diseases (12.2% vs 4.8% p < 0.005) and mental and behavioral disorders (2.0% vs 1.5% p = 0.01). After being diagnosed with lung cancer, other neoplasms were registered more frequently in patients with lung cancer (71.5%) than in controls (8.4%) and more lung cancer patients as well as controls, although to a lesser extent, had been diagnosed with respiratory diseases (25.5% vs 6.9% p < 0.005), compared to before (12.2% vs 4.8% p < 0.005). However, for cardiovascular diseases, fewer cases had a diagnosis after being registered with lung cancer (from 16.4% to 16.1% p < 0.005), whereas there was a significant increase in controls diagnosed with cardiovascular diseases (13.0% before vs 16.2% after p < 0.005).
Figure 2. ICD-10 diagnoses for cases and controls before and after being diagnosed with lung cancer in each disease chapter. The x-axis displays the percentages of cases (in blue) and controls (in red) with a diagnosis within the chapter
Prior to being diagnosed with lung cancer, significantly more cases than controls had been diagnosed with angina pectoris, atherosclerosis in the heart and extremities, atrial fibrillation, heart failure and stroke (data in supplementary file).
Seventy-eight percent of lung cancer patients had no registered comorbidity included in the DCcs at the time of lung cancer diagnosis, compared to 87% of controls. As shown in , any DCcs of 1 or higher were more frequent in the lung cancer group.
Table 2. Distribution of Deyo-Charlson comorbidity score (DCcs) for lung cancer patients and controls
Decreasing 1- and 5-year survival rates with increasing DCcs were seen in both groups (). shows Kaplan-Meier survival curves for all lung cancer patients and controls for each DCcs group. Decreasing survival with increasing DCcs was found in both groups.
Discussion
This epidemiological study presents data on all Danish lung cancer patients over a 12-year period and provides a unique insight into the incremental comorbidities of lung cancer. Age is closely related to comorbidity and is also a strong predictor of mortality in cancer patients. By comparing the lung cancer patients to a gender- and age-matched control group, we limited the need for adjustment for these prognostic baseline variables seen in previous studies in this area. We found a higher burden of comorbidities in patients with lung cancer compared to controls, and comorbidities significantly affected survival rates of patients with lung cancer as well as controls.
Among lung cancer patients, 21.8% had a DCcs ≥1 compared to 13.3% among controls. Other studies have shown a higher rate of comorbidity using the Charlson Comorbidity Index (43% [Citation18] and 50% [Citation4]) as opposed to DCcs. We found decreasing survival rates with increasing DCcs in both groups; however, comorbidity had a greater impact on survival rates for controls compared to lung cancer patients. For the matched control group, comorbidity was associated with more significant changes in overall survival compared to lung cancer patients. Comorbidity significantly reduced survival for lung cancer patients; however, their survival was already greatly diminished by the lung cancer diagnosis. Reviewing the literature on comorbidity and lung cancer survival, data have been contradicting and comparison is difficult due to different study setups (i.e. self-reported versus register-based comorbidities [Citation19]). A few studies have not found comorbidities to significantly impact survival [Citation20,Citation21] or merely to introduce a minor impact due to the poor prognosis of lung cancer [Citation22]. However, in accordance with the present results, several reports have shown comorbidity to impact lung cancer survival [Citation2–5,Citation17,Citation19,Citation23–25]. In other cancers, comorbidity has been reported to be a negative prognostic factor for cancer survival. A review of 18 breast cancer studies demonstrated that presence of comorbidity at diagnosis was an important prognostic factor in early breast cancer, irrespective of age and stage [Citation26]. A systematic review and meta-analysis in patients with colorectal cancer has shown that frailty and comorbidity were associated with poor short- and long-term survival and that the effects of comorbidity on overall mortality appeared to decrease with advancement in cancer stage [Citation27]. Due to the poor prognosis of lung cancer, it seems that comorbidity affects survival less than for cancer with better long-term prognosis [Citation28].
We found a 5-year survival rate of 14.7%, which is remarkably better than the NORDCAN report for the same time period (9% for men and 11% for women) [Citation1]. Prior to 2003, patients with lung cancer in advanced stages were often not registered with a lung cancer diagnosis in the NPR (personal communication with leader of Danish Lung Cancer Registry (DLCR)). Accordingly, the stage distribution of our patients was probably shifted towards patients with a lower disease stage and thus a better survival rate. For lung cancer patients, the survival rates decreased rapidly in the first few years following the lung cancer diagnosis compared to controls. However, after 5 years the survival rates for patients mimicked those of the controls. Thus, our findings suggest a possible healthy survivor effect. Previous studies have not compared survival of lung cancer patients to a matched control group [Citation2–4,Citation6,Citation19–25]. Additional research is required to further enlighten this area.
Cardiovascular diseases, diabetes mellitus, cerebrovascular diseases and COPD are known to decrease the survival rate of patients with lung cancer [Citation5], but are they also more common in lung cancer patients compared to controls? We discovered that many patients with lung cancer had several comorbid conditions prior to being diagnosed with lung cancer; this was significantly different from the control group. The most frequently reported comorbidities were cardiovascular diseases, respiratory diseases and other malignancies. A great number of these comorbidities can be explained by smoking as a common contributory factor for lung cancer as well as the comorbidities. Unfortunately, it was not possible to adjust for smoking status, because it was not registered in the databases. Previous studies have found larger percentages of lung cancer patients with COPD (43% [Citation5], 28% [Citation23]); however, these studies were small and without control groups.
Cardiovascular diseases such as atrial fibrillation and heart failure increased significantly for the control group after their corresponding cases had been diagnosed with lung cancer. This can be explained by the group of controls getting older. In general, a significant portion of the control group was diagnosed with different age-related diseases over time, whereas the majority of patients with lung cancer died within a timespan of a few years.
This study has several limitations as it is a registry-based epidemiological study without clinical validation. A major limitation of this study is the lack of adjusting to disease stage, as disease stage highly affects survival. This could have been done by linking to the extensive DLCR [Citation29,Citation30]. DLCR contains information on staging, histology, performance and smoking status for most patients, which would have added valuable information to this study. However, the present study contains data on lung cancer patients prior to the establishment of the DLCR in 2001. Generally, the reported burden of comorbidities was lower than expected and lower compared to other studies [Citation2–6,Citation19–25]. One Danish study reported similar levels of comorbidities in lung cancer with data extracted from hospital records [Citation6]. In Denmark, ICD-10 classification is used only in the secondary health care sector (hospitals) and not in the primary health care sector (general practitioners). Whereas the majority of patients with lung cancer are diagnosed in the secondary sector, comorbidities are more commonly registered in the primary sector; however, the disorders included in the Deyo-Charlson Index are generally of such serious nature that if present, it would have led to hospital contact at some point in time. Our results do not reveal whether the underreporting is equally distributed among patients with lung cancer and controls. Further studies including review of medical records and/or including prescription data from The Danish National Prescription Registry could provide additional knowledge to this field and help determine the magnitude of under-reporting of comorbidities. Over the last decades, the treatment regiments and survival prognosis for lung cancer have improved significantly [Citation1,Citation18]. So, over the 12-year time period studied, prognosis has improved, which affects our results. Matching on income, educational level or other socioeconomic factors could have been relevant, as a previous Danish study found low income and shorter education to reduce the probability of first-line treatment for lung cancer independent of age, gender and comorbidity [Citation12]. In Denmark, fast-track cancer referral programs were introduced in 2008 diminishing diagnostic delay and initiation of treatment. To determine whether fast track cancer programs affect survival and comorbidities classified by ICD-10, studies including data after 2008 are required.
Conclusion
In this nationwide study, comorbidities among Danish lung cancer patients have been described and compared to the comorbidities of matched controls. We found significantly more patients with lung cancer than controls with comorbidities and moreover that comorbidities decreased survival for both groups. The study corroborated previous findings concerning the survival of patients with lung cancer and contributed with knowledge of age and marital status among Danish patients with lung cancer. Overall, patients with lung cancer had more comorbidities than the control group both prior to and after diagnosis; comorbidities were primarily related to the cardiovascular system, the respiratory system and other malignancies. Thus, comorbidity has a significant impact on survival rates for both lung cancer patients and controls, yet the burden is relatively higher for controls.
Supplemental Material
Download MS Word (28.2 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- NORDCAN. 2006. (www.dep.iarc.fr/NORDCAN/DK
- Iachina M, Green A, Jakobsen E. The direct and indirect impact of comorbidity on the survival of patients with non-small cell lung cancer: a combination of survival, staging and resection models with missing measurements in covariates. BMJ Open. 2014 Feb 12;4(2):e003846. doi:https://doi.org/10.1136/bmjopen-2013-003846. PMID: 24523421; PMCID: PMC3927932).
- Lüchtenborg M, Jakobsen E, Krasnik M, et al. The effect of comorbidity on stage-specific survival in resected non-small cell lung cancer patients. Eur J Cancer. 2012 Dec;48(18):3386–7.
- Deleuran T, Thomsen RW, Nørgaard M, et al. Comorbidity and survival of Danish lung cancer patients from 2000–2011: a population-based cohort study. Clin Epidemiol. 2013 Nov 1;5(Suppl 1):31–38.
- Grose D, Morrison DS, Devereux G, et al. Scottish lung cancer forum. Comorbidities in lung cancer: prevalence, severity and links with socioeconomic status and treatment. Postgrad Med J. 2014 Jun;90(1064):305–310. Epub 2014 Mar 27.
- Iachina M, Jakobsen E, Møller H, et al. The effect of different comorbidities on survival of non-small cells lung cancer patients. Lung. 2015 Apr;193(2):291–297.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619.
- Dalton SO, Steding-Jessen M, Engholm G, et al. Social inequality and incidence of and survival from lung cancer in a population-based study in Denmark, 1994–2003. Eur J Cancer. 2008 Sep;44(14):1989–1995.
- Berglund A, Holmberg L, Tishelman C, et al. Social inequalities in non-small cell lung cancer management and survival: a population-based study in central Sweden. Thorax. 2010 Apr;65(4):327–333.
- Dalton SO, Frederiksen BL, Jacobsen E, et al. Socioeconomic position, stage of lung cancer and time between referral and diagnosis in Denmark, 2001–2008. Br J Cancer. 2011 Sep 27;105(7):1042–1048.
- Dalton SO, Steding-Jessen M, Jakobsen E, et al. Socioeconomic position and survival after lung cancer: influence of stage, treatment and comorbidity among Danish patients with lung cancer diagnosed in 2004–2010. Acta Oncol. 2015 May;54(5):797–804. Epub 2015 Mar 12.
- Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490.
- Pedersen CB. The Danish Civil Registration System. Scand J Pulic Health. 2011;32:183–190.
- Løkke A, Hilberg O, Tønnesen P, et al. Direct and indirect economic and health consequences of COPD in Denmark: a national register-based study: 1998–2010. BMJ Open. 2014 Jan 6;4(1):e004069.
- Rittig AH, Hilberg O, Ibsen R, et al. Incidence, comorbidity and survival rate of hypersensitivity pneumonitis: a national population-based study. ERJ Open Res. 2019 Oct 21;5(4):00259–2018.
- Gade Sikjær M, Hilberg O, Ibsen R, et al. Direct and indirect economic and health consequences related to sarcoidosis in Denmark: a national register-based study. Respir Med. 2019;152:7–13.
- Nilssen Y, Strand TE, Fjellbirkeland L, et al. Lung cancer survival in Norway, 1997–2011: from nihilism to optimism. Eur Respir J. 2016 Jan;47(1):275–287. Epub 2015 Nov 5. Erratum in: Eur Respir J. 2016 Apr;47(4):1297.
- Sandfeld-Paulsen B, Meldgaard P, Aggerholm-Pedersen N. Comorbidity in lung cancer: a prospective cohort study of self-reported versus register-based comorbidity. J Thorac Oncol. 2018 Jan;13(1):54–62. Epub 2017 Oct 19. PMID: 29056534.
- Grønberg BH, Sundstrøm S, Kaasa S, et al. Influence of comorbidity on survival, toxicity and health-related quality of life in patients with advanced non-small-cell lung cancer receiving platinum-doublet chemotherapy. Eur J Cancer. 2010 Aug 12;46:2225–2234. Epub 2010 May 12.
- Ganti AK, Siedlik E, Marr AS, et al. Predictive ability of Charlson comorbidity index on outcomes from lung cancer. Am J Clin Oncol. 2011 Dec;34(6):593–596.
- Read WL, Tierney RM, Page NC, et al. Differential prognostic impact of comorbidity. J Clin Oncol. 2004 Aug 1;22(15):3099–3103.
- Tammemagi CM, Neslund-Dudas C, Simoff M, et al. Impact of comorbidity on lung cancer survival. Int J Cancer. 2003 Mar 1;103(6):792–802.
- Firat S, Byhardt RW, Gore E. The effects of comorbidity and age on RTOG study enrollment in Stage III non-small cell lung cancer patients who are eligible for RTOG studies. Int J Radiat Oncol Biol Phys. 2010 Dec 1;78(5):1394–1399. Epub 2010 Jun 18.
- Janssen-Heijnen ML, Smulders S, Lemmens V, et al. Effect of comorbidity on the treatment and prognosis of elderly patients with non-small cell lung cancer. Thorax. 2004 Jul;59(7):602–607.
- Land LH, Dalton SO, Jørgensen TL, et al. Comorbidity and survival after early breast cancer. A review. Crit Rev Oncol Hematol. 2012 Feb;81(2):196–205. Epub 2011 May 4. PMID: 21536452.
- Boakye D, Rillmann B, Walter V, et al. Impact of comorbidity and frailty on prognosis in colorectal cancer patients: a systematic review and meta-analysis. Cancer Treat Rev. 2018 Mar;64:30–39. Epub 2018 Feb 10. PMID: 29459248.
- Søgaard M, Thomsen RW, Bossen KS, et al. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013 Nov 1;5(Suppl 1):3–29. PMID: 24227920; PMCID: PMC3820483.
- Jakobsen E, Green A, Oesterlind K, et al. Nationwide quality improvement in lung cancer care: the role of the Danish lung cancer group and registry. J Thorac Oncol. 2013;8(10):1238–1247.
- Jakobsen E, Rasmussen TR. The Danish lung cancer registry. Clin Epidemiol. 2016;8:537–541. [Published 2016 Oct 25].