ABSTRACT
Background: Precision medicine means linking the right patient to the right management strategy including best possible pharmacological therapy, considering the individual variability of the disease characteristics, type of inflammation, genes, environment, and lifestyle. For heterogenous diseases such as asthma, reliable biomarkers are needed to facilitate the best possible disease control and reduce the risk of side effects. The present review examines fractional exhaled nitric oxide (FeNO) as a guide for the management strategy of asthma and predictor of its clinical course.
Method: The literature included was identified by searching the PubMed database using specific key words and MeSH terms. Studies were not excluded based on their design alone. The search resulted in 212 hits, of which 35 articles were included in this review.
Results: Several studies support a potential role for high FeNO levels as a prognostic biomarker for accelerated lung function decline in adults with newly diagnosed asthma. Furthermore, studies report an association between high FeNO levels and excess decline in FEV1 in adults with long-standing moderate to severe asthma despite optimised therapy, whereas the findings for patients with less severe disease are conflicting. Applying a FeNO-based management algorithm reduces the exacerbation rate in adults with asthma. Similar observations are seen in children, though based on fewer studies. The available studies provide evidence that the level of FeNO may be useful as a predictor of subsequent loss of asthma control in adults, though the evidence is somewhat conflicting in children and young adults.
Conclusion: The present review provides evidence of the prognostic value of FeNO as a surrogate biomarker for type 2 inflammation in the airways. FeNO is likely to emerge as an important biomarker in monitoring and tailoring modern asthma treatment, either alone or in combination with other biomarkers.
Introduction
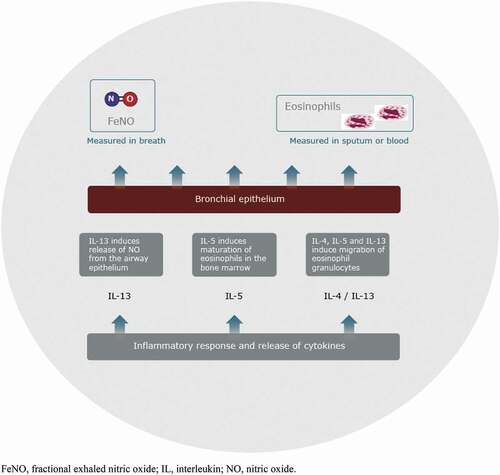
Asthma is a chronic heterogenous inflammatory disease of the airways affecting more than 300 million individuals globally [Citation1] and 5–10% of the population in Europe [Citation2]. Most asthma patients can be successfully treated with standard therapy. However, some individuals have difficult-to-treat or severe asthma, which remains partially controlled or uncontrolled, even with intensive treatment. Asthma is characterised by the type of predominant inflammatory cells that are increased (eosinophils, neutrophils, or both), or indeed a paucity of such cells [Citation3,Citation4]. Asthma immunological phenotypes are complex with differing clinical and inflammatory characteristics [Citation5,Citation6]. Thus, there is an increasing need for biomarkers with predictive and prognostic value for the progression of asthma, and their association with available treatments [Citation7]. One phenotype of severe asthma is related to type 2 inflammation, found in approximately half the people with severe asthma [Citation8]. Type 2 inflammation is characterised by the release of cytokines such as interleukin (IL)-4, IL-5 and IL-13 from cells of both the innate and adaptive immune systems acting on respiratory epithelium and other stromal cells, often on recognition of allergens [Citation3,Citation9]. Type 2 inflammation is also characterised by the presence of eosinophils [Citation10]. Biomarkers that reflect the pathophysiological mechanisms involved in type 2 inflammation-driven asthma include fractional exhaled nitric oxide (FeNO), serum IgE, serum periostin, and blood and sputum eosinophils [Citation3].
The production of nitric oxide (NO) in bronchial epithelial cells helps to regulate pulmonary blood flow, ciliary activity, mucus secretion and mucosal inflammation [Citation11]. In asthma, levels of NO in exhaled breath (FeNO) are increased due to the activation of inducible NO synthase (iNOS) by inflammatory cytokines in the airway epithelial cells, suggesting a role for NO in asthma pathogenesis [Citation12,Citation13]. NO can be measured in exhaled air as FeNO () that is used to support the diagnosis and management of asthma. The level of FeNO appears to increase in proportion to the severity of bronchial wall inflammation [Citation14].
Figure 1. Type 2 inflammation. FeNO, fractional exhaled nitric oxide; IL, interleukin; NO, nitric oxide
FeNO is measured from a single breath exhalation, usually using online techniques [Citation15]. The standard measurement is at an exhalation flow-rate of 50 mL/s. Exhalation against positive pressure excludes the possibility of contamination from nasal NO, which can affect the FeNO value. Other factors influencing FeNO values include the exhalation flow rate and the individual’s age, sex, height, smoking habits, allergies and anti-inflammatory medications used [Citation16–18].
FeNO is probably the most widely used biomarker in clinical practice today for the assessment of airway inflammation [Citation7,Citation16]. FeNO can facilitate the identification of patients with type 2 inflammation [Citation19], and has the potential to identify those patients who will respond to anti-inflammatory treatment, particularly inhaled corticosteroids (ICS). Identifying patients at the greatest risk of future exacerbations and the ability to control inflammation and thereby prevent exacerbations and decline in lung function is key in achieving good asthma control [Citation19].
In this review, we aim to address the following clinical question: Does the level of FeNO have prognostic value in the clinical course of asthma, in terms of (a) decline in lung function, (b) risk of exacerbations and (c) loss of control in adults and children with mild, moderate or severe asthma?
Methods
This is a review of the current literature on the prognostic and predictive value of FeNO in the clinical course of asthma in adults and children. A systematic literature search of the PubMed database was done on 2 April 2020 using the following key words or MeSH terms: ‘FeNO [Title/Abstract] OR fractionated exhaled nitric oxide [Title/Abstract] OR (FeNO OR fractionated exhaled nitric oxide [MeSH Terms]) AND prognosis [Title/Abstract] OR prognosis [MeSH Terms] AND asthma [Title/Abstract] OR asthma [MeSH Terms] OR airway inflammation [Title/Abstract] OR airway inflammation [MeSH Terms] OR lung function decline [Title/Abstract] OR exacerbation [Title/Abstract] OR chronic airway disease [Title/Abstract] OR chronic airway disease [MeSH Terms]’. We did not exclude any studies based on the study design, i.e. we included randomised, controlled trials as well as prospective and retrospective observational studies.
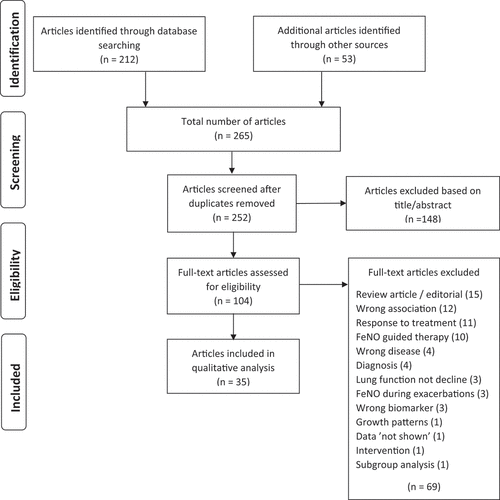
The search resulted in 212 articles (). A further 53 articles were found by other means (known by the authors or mentioned in the retrieved articles). Of the 265 total articles, 252 were screened after duplicates were removed. Of these, 148 were excluded based on the abstract due to the following reasons: the article did not answer the clinical question, concerned a different disease than asthma (for example, allergic rhinitis or chronic obstructive pulmonary disease), was related to asthma diagnosis, was a methodology article or was not in the English language. Of the remaining 104 articles, 69 were excluded based on read of the full text for the following reasons: the article was a review article or editorial and contained no new information (15), concerned prediction of asthma, asthma phenotype or blood eosinophilia (12), concerned response to a type of treatment rather than prognosis for long-term asthma outcome (11), evaluated FeNO guided treatment versus conventional or symptom-based therapy (10), concerned a different disease or condition than asthma per se (bronchial wall thickening, lung cancer, nasal polyps, irreversible airway obstruction) (4), was related to asthma diagnosis (4), assessed lung function not decline in lung function (3), assessed FeNO before and after an exacerbation (3), focussed on a different biomarker(s) (3), examined the association between childhood growth patterns and FeNO (1), the FeNO data were ‘not shown’ (1), concerned an intervention (1), or was a subgroup analysis of an original article (1). The remaining 35 articles were included in this review.
Results
There is increasing evidence potentially supporting FeNO as a prognostic biomarker for disease progression in asthma. Results from studies investigating the association between FeNO and long-term asthma clinical course and outcome, that is, decline in lung function, exacerbations, and loss of disease control are presented in the following.
Decline in lung function
In the general adult asthma population, factors known to contribute to accelerated lung function decline include smoking [Citation20], recurrent exacerbations [Citation21] and low forced expiratory volume in 1 s (FEV1) [Citation22]. Another important contributor to excess lung function decline may be the severity and persistence of airway inflammation [Citation23–25], possibly by facilitating airway remodelling [Citation26]. Accelerated decline in FEV1 has also been shown to be associated with severe asthma exacerbations [Citation21] and the presence of CD8-positive T-cells in the bronchial epithelium [Citation27]. In a recent study, adults with severe asthma had a decrease of 50 mL in FEV1 for each severe exacerbation [Citation28]. However, another study showed no association between exacerbations and accelerated decline in lung function [Citation29].
High FeNO levels have been shown to be associated with an excess decline in lung function both in long-standing disease and in adults with newly-diagnosed asthma (). Adult-onset asthma accounts for approximately half of new asthma diagnoses [Citation30,Citation31]. A prospective 5-year study in 200 individuals with adult-onset asthma measured several potential predictors for a decline in lung function at baseline and subsequent visits [Citation29]. The study found that higher FeNO levels and lower body mass index (BMI) were independently associated with lung function decline, as defined by a decline of >54.2 mL/year in post-bronchodilator FEV1, and all patients with a combination of FeNO ≥57 ppb (p = 0.015) and BMI ≤23.05 kg/m2 (p = 0.002) showed an accelerated decline in FEV1. The results suggest that, for new-onset asthma, individuals of normal weight and with relatively severe airway inflammation are at highest risk of subsequent lung function decline.
Table 1. Summary of studies investigating the association between FeNO and lung function decline in adults
Other studies have reported an association between high FeNO levels and accelerated lung function decline in patients with long-term asthma. In a prospective 3-year study in 140 patients with controlled long-standing (~10 years) asthma, a persistently high FeNO level of ≥40 ppb was independently associated with an accelerated decline in FEV1 (p < 0.05), whereas there was no association between lung function decline and age, BMI, blood eosinophil numbers, Asthma Control Test (ACT) score or airflow limitation [Citation32]. High levels of FeNO were also associated with a decrease in bronchodilator response.
Similarly, in a 5-year prospective follow-up of 136 adults with difficult-to-treat long-standing (18.5 years) asthma, high baseline FeNO levels predicted accelerated lung function decline, with no influence of other potential predicting factors, apart from baseline FEV1 [Citation33]. Individuals with a FeNO level ≥20 ppb, despite high doses of ICS with or without oral corticosteroids (OCS), had an increased risk of accelerated lung function decline (defined as an annual decline of ≥25 mL/year) over 5 years compared to those with FeNO <20 ppb (relative risk [RR] 1.9 [95% confidence interval (CI), 1.1; 2.6]). In individuals with both FeNO ≥20 ppb and FEV1 within normal levels (≥80% of predicted values), the risk of accelerated lung function decline was even greater (RR 3.1 [95% CI, 1.7; 3.4]).
In contrast, in a 5-year longitudinal observational study of 30 patients with well-controlled, stable asthma (duration ~10 years), only the severity of airway hyperresponsiveness and not FeNO was associated with a decline in FEV1 over 5 years () [Citation34]. In this study, FeNO was measured every 6 months during the first 2 years of the study. Furthermore, in a follow-up study of 212 patients from two previous cohorts in a broad asthma population (cohort 1 enrolled patients with intermittent mild to moderate asthma; cohort 2 enrolled those with stable moderate to severe asthma), no association between baseline FeNO level and lung function decline was noted [Citation35]. Instead an association between lung function decline and baseline blood eosinophils was observed (p < 0.001). The authors suggested that the lack of an association between FeNO and decline in lung function could have been due to the population assessed, consisting predominantly of patients with mild to moderate asthma of whom only 74% were on ICS therapy.
In general, the above studies tend to support the use of FeNO as a prognostic biomarker for accelerated lung function decline in adults with newly diagnosed asthma, uncontrolled asthma or difficult-to-treat asthma, though the evidence for an association in patients with mild to moderate disease is less compelling. FeNO-guided therapy is currently recommended for individuals with frequent exacerbations only [Citation36]. However, the guidelines are based on studies that have not taken the rate of lung function decline into account [Citation29]. It might be useful to include FeNO measurements in the clinical assessment of asthma patients, in order to identify those who are at risk of poor asthma outcome and who might be eligible for novel asthma treatments or personalised treatment strategies [Citation33,Citation37].
Data are not sufficient to determine the prognostic value of FeNO for the course of lung function in children [Citation38]. However, a 5-year follow-up study in 193 Chinese children with asthma (mean age 9.7 years) showed that a high baseline FeNO value was associated with decreased lung function development [Citation39]. In a longitudinal cohort study of 42 infants and toddlers with wheezing (mean age 15.6 months), high baseline FeNO values were associated with a decline in lung function by 3 years of age and also bronchodilator response [Citation40].
Risk of exacerbations
Exacerbations in adults
Several studies support a role for FeNO as a prognostic biomarker for the risk of future exacerbations (). In a 3-year observational study of 105 adults with severe long-standing (~20 years) asthma on high-dose ICS with or without OCS, the patients were split into 3 groups: those who did not experience exacerbations, those with ≥2 exacerbations over each of the 3 years, and those who did not fulfil either of these criteria. A multivariate analysis was conducted with several type 2-related biomarkers measured annually over 3 years, including blood/sputum eosinophil levels, total IgE, serum periostin and FeNO, as well as exacerbation history [Citation41]. Of the biomarkers assessed, only FeNO was associated with exacerbation status (p = 0.013), and this association remained significant even when considering past exacerbation status, which is usually the strongest predictor of future exacerbations. In a 1-year study of 93 patients with severe asthma and 76 with mild to moderate asthma (asthma duration ~20 years), biomarkers including FeNO were measured at baseline and at subsequent visits [Citation42]. Patients in the severe group had 104 exacerbations and those in the mild to moderate group had 18 exacerbations. FeNO >45 ppb (odds ratio 4.32, p = 0.047) and a history of smoking (odds ratio 2.90, p = 0.025) were associated with an increased risk of 2 or more exacerbations per year ().
Table 2. Summary of studies investigating the association between FeNO and exacerbation risk in adults
Similar associations between FeNO and asthma exacerbations were observed in other observational studies of adults with severe, moderate or mild asthma (). A prospective 22-week study in 1800 adults with severe asthma prescribed high-dose ICS plus a second controller demonstrated that FeNO (measured regularly over the 22 weeks) was a valuable prognostic biomarker for exacerbations, together with FEV1 [Citation43]. Similarly, a strong correlation with asthma exacerbations was shown for FeNO measured longitudinally in a real-life retrospective study (duration not stated) of 115 adults with severe asthma on high-dose ICS plus OCS [Citation44]. The correlation between FeNO and exacerbations (r = 0.42, p = 0.0008) was stronger than for peripheral blood eosinophils or periostin. Similar results were shown in a prospective study of 44 non-smoking adults with stable mild to severe asthma who had received treatment for at least 3 years [Citation45]. Independent of baseline FEV1, baseline FeNO values of ≥28 ppb were associated with an increased relative risk for exacerbations of 3.4 (95% CI, 1.3; 9.1; Mantel-Haenszel, p = 0.007). Combining baseline FeNO of ≥28 ppb and FEV1 of ≤76% identified 13 stable adults with asthma with 85% probability for future exacerbations, whereas 9 adults with FeNO <28 ppb and FEV1 > 76% had a 0% probability of exacerbations. In the follow-up study of 212 patients from two previous cohorts in a broad asthma population (mild, moderate or severe) in which no association between baseline FeNO and lung function decline was noted (mentioned above), a significant association between baseline log FeNO and time to severe exacerbation was observed (hazard ratio 0.65 [95% CI, 0.52; 0.81] per 0.693 log FeNO increase), p < 0.001 [Citation35].
In contrast to the above studies, a 12-month study of different prediction models (history of previous exacerbations; history plus spirometry; and history plus spirometry plus FeNO, assessed at baseline and every 3 months) in 611 adults diagnosed with asthma (82% on ICS with or without a LABA or short-acting beta agonist) found that the model of exacerbation history plus spirometry identified those adults prone to severe exacerbations and the additional prognostic value of FeNO was modest () [Citation46]. Furthermore, in a recent subgroup analysis of a 1-year open-label randomised, controlled trial in 675 patients with mild asthma, elevated FeNO (measured at baseline, week 12 and week 52) showed no value as a prognostic biomarker for exacerbation rate [Citation47].
In general, the above studies tend to support the clinical use of FeNO for the prediction of future exacerbation risk in adults with moderate to severe asthma, in addition to the individual’s exacerbation history, though the evidence in those with mild asthma is less conclusive. Monitoring of airway inflammation as reflected by FeNO may enable early detection of an exacerbation in a subclinical form and the potential to adjust anti-inflammatory treatment to reduce the risk of future exacerbations.
Exacerbations in children and young adults
There is also evidence to support the use of FeNO as a prognostic biomarker for exacerbations in children. In a prospective 1-year cohort study of 70 Thai children and young adults (median age 12.6 years (range 7.2 to 19.8 years)) and atopic asthma, median baseline FeNO levels were significantly higher in individuals having an exacerbation compared to those who did not (35.6 vs. 16.5 ppb; p = 0.012), and previous 12-month exacerbations were also predictive [Citation48]. In a single-centre longitudinal study of 45 infants and toddlers with wheezing (mean age 15.7 months), high baseline FeNO values had a prognostic value for future exacerbations and were superior to both bronchodilator responsiveness and a positive asthma predictive index [Citation40].
Overall, the monitoring of FeNO levels in children holds some promise as a prognostic biomarker for exacerbations in children. Assessing asthma status at an early age using an objective test such as FeNO may facilitate more targeted use of asthma therapies, prevent under- and over-treatment with ICS, and reduce preventable emergency department visits and hospitalisation for asthma exacerbations.
Loss of asthma control
Asthma control is usually evaluated by symptoms and lung function with the aim to reduce future risk [Citation49,Citation50].
Loss of control in adults
Several studies have shown that increased FeNO levels are associated with a deterioration in asthma control in adults (). In a prospective 2-year study in 90 adults with moderate or severe asthma who were on ICS and without clinical symptoms for ≥6 months, patients had their ICS dose halved while remaining on other asthma medication (theophylline, LABA, leukotriene receptor antagonists, and/or anticholinergic agents) [Citation51]. Asthma control was defined by a reduction in ICS dose with no increase in symptoms. In a multivariate logistic regression model, baseline FeNO level was predictive of successfully maintaining control over the second year of the study (p = 0.028), and 39 of 50 patients continued to maintain asthma control for ≥2 years after ICS reduction. In a prospective study of 78 patients with mild to moderate asthma on ICS for ≥6 months, ICS therapy was stopped and patients followed weekly for up to 6 weeks or until loss of control occurred [Citation52]. Sixty patients (77.9%) experienced loss of control. Different FeNO measurements, in terms of varying cut-off values, were all associated with a positive predictive value of between 80 and 90% for predicting loss of asthma control. In a prospective observational study of 250 patients with stable asthma on ICS with or without LABA and/or other therapies, poor asthma control was defined as an ACT score <20, or FEV1 < 80% or peak expiratory flow variability <80% [Citation53]. After 12-weeks, 229 patients who maintained high or low FeNO were selected and the study found that a FeNO level >39.5 ppb gave 67% sensitivity and 76% specificity for identifying the patients with poorly controlled asthma.
Table 3. Summary of studies investigating the association between FeNO and loss of asthma control in adults
The prognostic value of FeNO was assessed in a regular clinic setting in a prospective longitudinal 3-month study of 341 patients with mild, moderate or severe asthma [Citation54]. The patients were either newly diagnosed and ICS naïve or had chronic asthma and were on ICS with or without LABA and/or other therapies. FeNO was measured once or on several visits for each patient. In the whole population, FeNO >45 ppb was associated with asthma that was not well controlled in terms of Asthma Control Questionnaire (ACQ) >0.75 (negative predictive value 88%), p < 0.001. FeNO values >45 ppb were less effective for predicting asthma control as ICS dose increased: negative predictive values of 92%, 76% and 85% with ICS naïve, medium-dose or high-dose ICS, respectively. The study also suggested that sequential FeNO measurements may be beneficial for predicting improvement in asthma control over time.
In a prospective 6-month study of 90 patients with severe or non-severe asthma, asthma control was evaluated according to the ACQ (ACQ ≥1.5 denoted uncontrolled asthma) [Citation55]. FeNO (the number of measurements not stated) was significantly increased in uncontrolled as compared to controlled asthmatics using both a chemiluminescent device (the ‘gold standard’ recommended technique) and a portable electrochemical device for the measurement. FeNO levels were able to predict maintenance of control in well-controlled asthma patients, and with cut-off values of 31 and 40 ppb, the negative predictive values were 95 and 97% for the two devices. In this study, the authors were able to predict loss of control from a single FeNO measurement using the electrochemical device, which could be helpful in clinical practice.
A retrospective 1-year study of 71 adults with mild newly-diagnosed asthma not on ICS found that full asthma control based on the ACT score was associated with lower FeNO levels (p < 0.01), shorter duration until first clinic visit (p < 0.01), lower sputum eosinophil count (p < 0.05) and lower initial ICS dose (p < 0.05) () [Citation56]. In a multivariate logistic regression analysis comparing findings in patients with controlled versus uncontrolled asthma, inclusion of FeNO levels at the first visit in the model significantly improved the prediction of asthma control (odds ratio [OR] 0.9459 (95% CI, 0.9024; 0.9915); p = 0.021). A cohort study of 170 patients with mild to moderate (72%) or severe (28%) asthma on ICS and/or other treatments found that sputum IL-13 was superior to both FeNO and sputum eosinophils with respect to predicting well-controlled asthma at 6 months [Citation57]. Nevertheless, FeNO levels differed significantly between patients with well-controlled asthma (ACT ≥20) and those with uncontrolled asthma: median (interquartile range [IQR]) 21 (14–28) vs. 45 (19–67), p < 0.001. A baseline FeNO value <43 ppb was a modest predictor of well-controlled asthma for the whole population (negative predictive value 50%) compared to an IL-13 value <156 pg/mL (negative predictive value 76%), indicating the superiority of IL-13, though the methodology for this biomarker can be semi-invasive and time-consuming.
In contrast to the above studies, a cross-sectional outpatient study of 81 women, 41 of whom were obese, with mild (84%), moderate (12%) or severe (4%) stable asthma and on at least one asthma controller for at least 6 months, found that only high BMI was associated with poor asthma control (in terms of ACT score <20) [Citation58]. In the obese group, no significant difference was observed with respect to median FeNO levels between those with uncontrolled asthma (n = 24; 21.0 [range 15–51] ppb FeNO) and those with controlled asthma (n = 17; 20.5 [Citation11–52] ppb), p = 0.799. Similar results were observed for the nonobese group, with median FeNO 26.0 (10.0–297.0) ppb for those with uncontrolled asthma (n = 15) and 19.5 (6.0–171.0) ppb for those with controlled asthma (n = 25), p = 0.194.
Loss of control in children and young adults
In children and young adults, some evidence supporting the prognostic value of FeNO to predict loss of asthma control is available; however, the evidence is somewhat conflicting. A study of the association between the loss of asthma control and longitudinal FeNO measurements in 178 children and adolescents (aged between 8 and 16 years) with atopic asthma found that only a high mean FeNO value and the frequency of FeNO values above 21 ppb (participants had FeNO measured at least 10 times) were independently associated with a loss of control (p = 0.001 and 0.021, respectively) [Citation59]. When different cut-off values of each were investigated, the best combination of sensitivity and specificity for predicting a future loss of asthma control was observed with a mean FeNO value >47 ppb (70% sensitivity and 96% specificity) as well as FeNO levels >21 ppb achieved at a frequency of >41% (72% sensitivity and 88% specificity). Also, in a study of 201 children aged 8–16 years with atopic intermittent or mild persistent asthma, pulmonary function tests including FeNO and bronchodilator response were performed ≥10 times over 2 years and loss of control was assessed by the ACQ (ACQ ≥1.5 denoted uncontrolled asthma) after an additional year [Citation60]. The study showed that the risk for loss of control in the FeNO >35 ppb group was 2.58 times that in the FeNO ≤35 ppb group (95% CI 1.60; 4.15; p < 0.001), and the prognostic value of both FeNO >35 ppb and bronchodilator response ≥12% was greater than including each measurement separately (hazard ratio 7.08; 95% CI 2.57; 19.49; p < 0.001).
With regard to older children and young adults, a large population-based cohort study of FeNO levels and blood eosinophil counts in 406 children and young adults aged 10–35 years (~80% of patients had atopic asthma) showed that a combination of increased baseline FeNO levels (≥20-25 ppb) and blood eosinophil counts (≥300 cells/µL) was able to predict uncontrolled asthma (ACT score <20) at 3 months better than either biomarker alone [Citation7]. A prospective non-interventional study in 304 children and adults aged 12–56 years with persistent asthma found that high baseline FeNO levels predicted future uncontrolled asthma at 1 year [Citation61]. In a study of 30 children aged 7–17 years with mild (n = 8), moderate (n = 17) or severe (n = 5) asthma, baseline FeNO was significantly correlated with asthma control (r = −0.51, p = 0.001), which was based on overall evaluation of symptoms, FEV1 measurements, and the frequency of beta-agonist use [Citation62].
Other studies have shown that elevated FeNO may predict asthma relapse after cessation of ICS treatment. In a study of 40 children with asymptomatic asthma (mean age 12.2 years) who had discontinued use of ICS, FeNO was measured at baseline and at 2, 4, 12 and 24 weeks after discontinuation of ICS use. Geometric mean FeNO values in children who were about to relapse were higher than in those who did not relapse: 35.3 ppb vs. 15.7 ppb at 2 weeks (ratio 2.3; 95% CI 1.2; 4.1; p = 0.01) and 40.8 ppb vs. 15.9 ppb at 4 weeks (ratio 2.6; 95% CI 1.3; 5.1; p = 0.009) [Citation63]. Similarly, in a study of 40 children aged 6–17 years with stable asthma whose ICS dose was halved if clinically indicated, increased FeNO (OR 6.3; CI, 3.75; 10.58) and percentage of sputum eosinophils (OR 1.38; CI, 1.06; 1.81) were significant predictors of a failed reduction in terms of loss of asthma control [Citation64]. In this study, biomarkers were assessed every 8 weeks.
In contrast to the above results, a prospective observational study of 28 children aged 6–14 years with controlled asthma found no association between baseline FeNO levels (≥49 ppb or <49 ppb) and loss of control, in terms of recurrence of asthma attacks, at 6 months [Citation65]. In fact, 5 patients in the low FeNO group experienced loss of control, as did only 1 in the high FeNO group. A 1-year observational study of 96 children aged 6–18 years with persistent asthma found no association between exhaled inflammatory markers measured every 2 months, including FeNO, and asthma control in children, as assessed by the ACQ [Citation66]. Another study investigated associations between inflammatory biomarkers and asthma control in a prospective cohort study of 40 children and adolescents aged between 6 and 18 years with severe asthma (all on ICS and LABA) [Citation67]. No significant differences in FeNO levels between the two groups (those who achieved control and those who did not) were observed, either at baseline or after a 3-month follow-up period. In a cross-sectional clinical study in 114 children aged ≥7 years (mean age 12.1 years) with atopic asthma, the median baseline FeNO level in the uncontrolled group of 39.15 (range 2.4–192.3) ppb was higher than in the partly controlled group (24.9 [2.2–85.7] ppb), and the controlled group (19.2 [5.1–108.9] ppb), though the differences were not statistically significant (p = 0.238) [Citation68].
Overall, the above results indicate the potential benefit of using FeNO as a prognostic marker of asthma control and relapse in adults, although the evidence for a role of FeNO as a robust prognostic indicator of asthma control in children and young adults is less compelling. There is some indication that combining the FeNO assessment with another biomarker, such as blood eosinophil counts [Citation7] or bronchodilator response [Citation60], may help to fully obtain the status of a patient with asthma and to optimise the type and intensity of treatment. Also, sequential FeNO measurements may be better than a single assessment [Citation54].
Conclusions
The present review has clearly demonstrated the prognostic value of measurement of FeNO as a surrogate biomarker for type 2 inflammation in the airways. High FeNO levels measured at the time of asthma diagnosis may reflect ongoing airway inflammation and indicate a poorer prognosis, with long-term impairment of lung function and increased exacerbation risk and loss of control. In order to determine individual disease characteristics and optimise treatment, a combination of the FeNO assessment with other biomarkers could be useful.
Acknowledgments
The authors thank Angela Stocks, PhD and Irene Vejgaard Sørensen, PhD (Larix A/S, Copenhagen, Denmark) for editorial and medical writing services, which were funded by Sanofi Genzyme, Copenhagen, Denmark.
Disclosure statement
CSU has received personal fees from Sanofi, AstraZeneca, Boehringer-Ingelheim, Chiesi, GSK, Mundipharma, TEVA, Novartis, Orion Pharma, Mylan, and ALK-Abello. P Lange has received personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, and GSK and research grants from Boehringer Ingelheim and GSK. OH has received personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis and Sanofi, and research grants from Boehringer Ingelheim, Chiesi, Novartis, AstraZeneca, and GSK.
Additional information
Funding
Notes on contributors
Charlotte Suppli Ulrik
Charlotte Suppli Ulrik is professor in pulmonary medicine and Head of the Respiratory Research Unit and Severe Asthma Clinic, Hvidovre Hospital. Her research interest are in obstructive lung diseases (asthma, COPD and the overlap between asthma and COPD) since her years as medical student with a special interest in early detection, management and – not least – outcome. In recent years also with a growing interest in the association between life style factors, including obesity and leisure time activity, and obstructive lung disease together with the possible impact of early life factors and subsequent development of obstructive lung disease.
Peter Lange
Peter Lange is consultant at section of respiratory diseases at Herlev Hospital and professor at Department of Public Health at University of Copenhagen. His main research interest is in epidemiology of obstructive lung diseases. Since 1987, he has been involved in the running of the Copenhagen City Heart Study and is the pulmonary member of the Steering Committee of the Copenhagen General Population Study.
Ole Hilberg
Ole Hilberg is clinical professor in pulmonology at Southern Danish University. His research areas are within various lung diseases, lung infections - especially tuberculosis and allergies. In the last few years, there has been a focus on lung cancer Ole Hilberg has been employed as chief physician at the Department of Medicine since 2016, and previously as chief physician in Aarhus for 11 years and for 14 years as an associate professor at Aarhus University. Ole Hilberg has been a specialist in pulmonary medicine since 2004 and internal medicine since 2000.
References
- The Global Asthma Report 2018. cited 2019 Jun 22. Accessed at http://www.globalasthmareport.org/Global%20Asthma%20Report%202018%20EMBARGOED.pdf
- Sears MR. Trends in the prevalence of asthma. Chest. 2014;145(2):219–13.
- Robinson D, Humbert M, Buhl R, et al. Revisiting Type 2-high and Type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy. 2017;47(2):161–175.
- Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet. 2018;391(10118):350–400.
- Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–224.
- Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med. 2010;181(4):315–323.
- Malinovschi A, Janson C, Borres M, et al. Simultaneously increased fraction of exhaled nitric oxide levels and blood eosinophil counts relate to increased asthma morbidity. J Allergy Clin Immunol. 2016;138(5):1301–8 e2.
- Global Initiative for Asthma. 2019 GINA Report, Global strategy for asthma management and prevention. cited 2019 Jun 22. Accessed at https://ginasthma.org/wp-content/uploads/2019/04/GINA-2019-main-Pocket-Guide-wms.pdf
- Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–725.
- Israel E, Reddel HK, Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377(10):965–976. .
- Hoyte FCL, Gross LM, Katial RK. Exhaled Nitric Oxide: an Update. Immunol Allergy Clin North Am. 2018;38(4):573–585.
- Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J. 1993;6(9):1368–1370.
- Lane C, Knight D, Burgess S, et al. Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax. 2004;59(9):757–760.
- Smith AD, Cowan JO, Brassett KP, et al. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005;352(21):2163–2173.
- Maniscalco M, Vitale C, Vatrella A, et al. Fractional exhaled nitric oxide-measuring devices: technology update. Med Devices (Auckl). 2016;9:151–160.
- Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615.
- Schneider A, Wagenpfeil G, Jorres RA, et al. Influence of the practice setting on diagnostic prediction rules using FENO measurement in combination with clinical signs and symptoms of asthma. BMJ Open. 2015;5(11):e009676.
- Nerpin E, Olivieri M, Gislason T, et al. Determinants of fractional exhaled nitric oxide in healthy men and women from the European Community Respiratory Health Survey III. Clin Exp Allergy. 2019;49(7):969–979.
- Bjermer L, Alving K, Diamant Z, et al. Current evidence and future research needs for FeNO measurement in respiratory diseases. Respir Med. 2014;108(6):830–841. .
- Lange P, Parner J, Vestbo J, et al. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339(17):1194–1200.
- Bai TR, Vonk JM, Postma DS, et al. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J. 2007;30(3):452–456.
- Vonk JM, Jongepier H, Panhuysen CI, et al. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax. 2003;58(4):322–327.
- Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol. 2005;116(3):477–486.
- Miranda C, Busacker A, Balzar S, et al. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113(1):101–108.
- Shaaban R, Zureik M, Soussan D, et al. Rhinitis and onset of asthma: a longitudinal population-based study. Lancet. 2008;372(9643):1049–1057. .
- Salter B, Pray C, Radford K, et al. Regulation of human airway smooth muscle cell migration and relevance to asthma. Respir Res. 2017;18(1):156.
- van Rensen EL, Sont JK, Evertse CE, et al. Bronchial CD8 cell infiltrate and lung function decline in asthma. Am J Respir Crit Care Med. 2005;172(7):837–841. .
- Ortega H, Yancey SW, Keene ON, et al. Asthma exacerbations associated with lung function decline in patients with severe eosinophilic asthma. J Allergy Clin Immunol Pract. 2018;6(3):980–6.e1.
- Coumou H, Westerhof GA, de Nijs SB, et al. Predictors of accelerated decline in lung function in adult-onset asthma. Eur Respir J. 2018;51:2.
- Sood A, Qualls C, Schuyler M, et al. Adult-onset asthma becomes the dominant phenotype among women by age 40 years. the longitudinal CARDIA study. Ann Am Thorac Soc. 2013;10(3):188–197.
- Kankaanranta H, Tuomisto LE, Ilmarinen P. Age-specific incidence of new asthma diagnoses in Finland. J Allergy Clin Immunol Pract. 2017;5(1):189–91.e3.
- Matsunaga K, Hirano T, Oka A, et al. Persistently high exhaled nitric oxide and loss of lung function in controlled asthma. Allergol Int. 2016;65(3):266–271.
- van Veen IH, Ten Brinke A, Sterk PJ, van Veen IH, Ten Brinke A, Sterk PJ, Sont JK, Gauw SA, Rabe KF, et al. Exhaled nitric oxide predicts lung function decline in difficult-to-treat asthma. Eur Respir J. 2008;32(2):344–349.
- Ohkura N, Fujimura M, Tokuda A, et al. Evaluation of airway hyperresponsiveness and exhaled nitric oxide as risk factors for airway remodeling in patients with stable asthma. Allergy Asthma Proc. 2009;30(4):419–423.
- Semprini R, Williams M, Semprini A, et al. Type 2 biomarkers and prediction of future exacerbations and lung function decline in adult asthma. J Allergy Clin Immunol Pract. 2018;6(6):1982–8 e1.
- Petsky HL, Kew KM, Turner C, et al. Exhaled nitric oxide levels to guide treatment for adults with asthma. Cochrane Database Syst Rev. 2016(9):CD011440.
- Taylor DR, Pijnenburg MW, Smith AD, et al. Exhaled nitric oxide measurements: clinical application and interpretation. Thorax. 2006;61(9):817–827.
- Pijnenburg MW. The Role of FeNO in Predicting Asthma. Front Pediatr. 2019;7:41.
- Leung TF, Tang MF, Leung ASY, et al. Trajectory of spirometric and exhaled nitric oxide measurements in Chinese schoolchildren with asthma. Pediatr Allergy Immunol. 2018;29(2):166–173.
- Elliott M, Heltshe SL, Stamey DC, Elliott M, Heltshe SL, Stamey DC, Cochrane ES, Redding GJ, Debley JS. Exhaled nitric oxide predicts persistence of wheezing, exacerbations, and decline in lung function in wheezy infants and toddlers. Clin Exp Allergy. 2013;43(12):1351–1361.
- Kimura H, Konno S, Makita H, et al. Prospective predictors of exacerbation status in severe asthma over a 3-year follow-up. Clin Exp Allergy. 2018;48(9):1137–1146.
- Kupczyk M, Ten Brinke A, Sterk PJ, Kupczyk M, ten Brinke A, Sterk PJ, Bel EH, Papi A, Chanez P, et al. Frequent exacerbators–a distinct phenotype of severe asthma. Clin Exp Allergy. 2014;44(2):212–221.
- Abdelrahman M, Iesa M, Awooda HA, et al. Fractional exhaled nitric oxide along with a reduced force expiratory volume are conclusive prognostic biomarkers to alert for asthma exacerbation. Sudan Med Monitor. 2017;12(1):1–7.
- Mansur AH, Srivastava S, Sahal A. Disconnect of type 2 biomarkers in severe asthma; dominated by FeNO as a predictor of exacerbations and periostin as predictor of reduced lung function. Respir Med. 2018;143:31–38.
- Gelb AF, Flynn Taylor C, Shinar CM, et al. Role of spirometry and exhaled nitric oxide to predict exacerbations in treated asthmatics. Chest. 2006;129(6):1492–1499.
- Loymans RJ, Honkoop PJ, Termeer EH, et al. Identifying patients at risk for severe exacerbations of asthma: development and external validation of a multivariable prediction model. Thorax. 2016;71(9):838–846.
- Pavord ID, Holliday M, Reddel HK, et al. Predictive value of blood eosinophils and exhaled nitric oxide in adults with mild asthma: a prespecified subgroup analysis of an open-label, parallel-group, randomised controlled trial. Lancet Respir Med. 2020;8(7):671–680.
- Visitsunthorn N, Mahawichit N, Maneechotesuwan K. Association between levels of fractional exhaled nitric oxide and asthma exacerbations in Thai children. Respirology. 2017;22(1):71–77.
- Malerba M, Radaeli A, Olivini A, et al. The combined impact of exhaled nitric oxide and sputum eosinophils monitoring in asthma treatment: a prospective cohort study. Curr Pharm Des. 2015;21(32):4752–4762.
- Expert Panel Report 3 (EPR-3): guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138.
- Tsurikisawa N, Oshikata C, Tsuburai T, et al. Markers for step-down of inhaled corticosteroid therapy in adult asthmatics. Allergol Int. 2012;61(3):419–429.
- Jones SL, Kittelson J, Cowan JO, et al. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am J Respir Crit Care Med. 2001;164(5):738–743.
- Matsunaga K, Yanagisawa S, Hirano T, et al. Associated demographics of persistent exhaled nitric oxide elevation in treated asthmatics. Clin Exp Allergy. 2012;42(5):775–781.
- Michils A, Baldassarre S, Van Muylem A. Exhaled nitric oxide and asthma control: a longitudinal study in unselected patients. Eur Respir J. 2008;31(3):539–546.
- Ozier A, Girodet PO, Bara I. Tunon de Lara JM, Marthan R, Berger P. Control maintenance can be predicted by exhaled NO monitoring in asthmatic patients. Respir Med. 2011;105(7):989–996.
- Yamashita M, Shibanai M, Sekimura K, et al. Fractional exhaled nitric oxide levels as a predictor of long-term prognoses in patients with mild asthma. Respir Investig. 2016;54(3):139–147.
- Tsilogianni Z, Hillas G, Bakakos P, et al. Sputum interleukin-13 as a biomarker for the evaluation of asthma control. Clin Exp Allergy. 2016;46(7):923–931.
- Kilic H, Oguzulgen IK, Bakir F, et al. Asthma in obese women: outcomes and factors involved. J Investig Allergol Clin Immunol. 2011;21(4):290–296.
- Yang S, Park J, Lee YK, et al. Association of longitudinal fractional exhaled nitric oxide measurements with asthma control in atopic children. Respir Med. 2015;109(5):572–579.
- Kim JK, Jung JY, Kim H, et al. Combined use of fractional exhaled nitric oxide and bronchodilator response in predicting future loss of asthma control among children with atopic asthma. Respirology. 2017;22(3):466–472.
- Zeiger RS, Schatz M, Zhang F, et al. Elevated exhaled nitric oxide is a clinical indicator of future uncontrolled asthma in asthmatic patients on inhaled corticosteroids. J Allergy Clin Immunol. 2011;128(2):412–414.
- Delgado-Corcoran C, Kissoon N, Murphy SP, et al. Exhaled nitric oxide reflects asthma severity and asthma control. Pediatr Crit Care Med. 2004;5(1):48–52.
- Pijnenburg MW, Hofhuis W, Hop WC, et al. Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax. 2005;60(3):215–218.
- Zacharasiewicz A, Wilson N, Lex C, et al. Clinical use of noninvasive measurements of airway inflammation in steroid reduction in children. Am J Respir Crit Care Med. 2005;171(10):1077–1082.
- Ferrer M, Jarque A, Tosca R, et al. Is it necessary to treat all asthmatic children with raised levels of exhaled nitric oxide?: treating the patient or the data. Allergol Immunopathol (Madr). 2011;39(5):280–283.
- Van Vliet D, Smolinska A, Jobsis Q, et al. Association between exhaled inflammatory markers and asthma control in children. J Breath Res. 2016;10(1):016014.
- Eller MCN, Vergani KP, Saraiva-Romanholo BM, et al. Can inflammatory markers in induced sputum be used to detect phenotypes and endotypes of pediatric severe therapy-resistant asthma? Pediatr Pulmonol. 2018;53(9):1208–1217.
- Visitsunthorn N, Prottasan P, Jirapongsananuruk O, et al. Is fractional exhaled nitric oxide (FeNO) associated with asthma control in children? Asian Pac J Allergy Immunol. 2014;32(3):218–225.