ABSTRACT
Background
Oncological treatment of primary pulmonary adenocarcinoma (AC) includes drugs targeting the pathways involving programmed death-ligand 1 (PD-L1), epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK). The aim of the study was to report the prevalence of these tumour markers in pleural fluid with cytology positive for pulmonary AC and the potential influence of volume pleural fluid tested.
Methods
We retrospectively reviewed all thoracenteses performed in a two-year period at our interventional unit at Department of Respiratory Medicine at Zealand University Hospital Naestved, Denmark. ALK and PD-L1 testing was done using immunohistochemistry and EGFR testing using next-generation sequencing. We included pleural fluid specimens containing malignant cells originating from primary pulmonary AC and with at least one tumour marker requested by the clinicians.
Results
When screening 927 pleural fluid specimens, we identified 57 in accordance with the inclusion criteria. PD-L1, ALK and EGFR were obtained in 35/55 (64%), 38/57 (67%) and 26/47 (55%), respectively. The prevalence did not increase when analysing volumes > 50 mL (p = 0.21–0.58)
Conclusion
Tumour markers in pleural fluid specimens containing cells from pulmonary AC can be demonstrated in more than half of the cases. Therefore, supplementary invasive procedures than thoracentesis could potentially await these analyses.
Introduction
Lung cancer is globally the most common cause of cancer-related death [Citation1]. Non-small cell lung cancer (NSCLC) constitutes the majority of cases, and is at the time of diagnosis locally advanced or metastasized in more than 70% of patients [Citation2]. Thus, curative treatment is not an option in the majority of NSCLC patients, who may be candidates to palliative systemic treatment. The optimal choice of the latter depends on stage, histological subtype and the demonstration of tumour markers such as programmed death-ligand 1 (PD-L1) expression [Citation3], epidermal growth factor receptor (EGFR) mutation [Citation4–6] and anaplastic lymphoma kinase (ALK) gene-rearrangement [Citation7,Citation8]. Tumour markers are not solely important for optimal treatment response but are also associated with metastatic spread [Citation9] and survival [Citation10–13].
Even though tumour markers are mostly demonstrated in tissue samples, they can also be found in fluids, such as pleural effusion, pericardial effusion or ascites [Citation14]. Malignant pleural effusion (MPE) is present in 15% of NSCLC patients at the time of diagnosis and denotes stage M1a classification and thereby stage IVA disease [Citation15]. The sensitivity of pleural fluid cytology for primary pulmonary AC is estimated to be 82%, while only 14.3% for primary pulmonary squamous cell carcinomas (SCC) [Citation16]. Thus, it is possibly much more likely to obtain tumour markers for AC than for SCC in MPE. Recently, a systematic review indicated that detection of ALK and EGFR in pleural effusion can be used as a substitute for tumour tissue, though there was some heterogeneity among the included studies [Citation17]. A good correlation has been shown between PD-L1 status in pleural fluid specimens and matched surgical biopsies [Citation18,Citation19]. The probability of demonstrating tumour marker status in MPEs has been investigated in previous studies [Citation18,Citation20–28]. However, the majority of these studies included only few cases of pleural effusions in selected patients [Citation21,Citation24,Citation27] or pooled results from pleural and pericardial effusions [Citation21,Citation22,Citation26,Citation27].
On this background, we decided to investigate the prevalence of the tumour markers PD-L1, ALK and EGFR in MPE with cytology positive for primary pulmonary AC in unselected and consecutive patients. Secondly, we wanted to evaluate the importance of the volume of the analysed fluid on the prevalence.
Methods
Study design and data collection
We conducted a retrospective, observational single-centre study at the Department of Respiratory Medicine at Zealand University Hospital Naestved, Naestved, Denmark. The unit serves in-patients from the respiratory ward and the oncology ward, as well as referred patients for invasive workup of pleural effusion, suspected lung cancer and metastasis without known origin. The unit performs bronchoscopies, endobronchial ultrasound (EBUS), and/or endoscopic ultrasound (EUS) as outpatient procedures in conscious sedation (intravenous midazolam and fentanyl). Furthermore, it performs ultrasound-, X-ray-, or CT-guided transthoracic needle aspiration biopsies (TTNABs); pleurocenteses; and non-endoscopic, non-thoracic, ultrasound-guided biopsies (fine-needle aspiration [FNA] biopsies from liver, spleen, skin tumours, bonelesions, or superficial lymph nodes) in local analgesia. Yearly approximately 2500 patients are referred for invasive work-up. All invasive procedures are consequently labelled with a procedure-specific code.
From the hospital´s electronic patient file system, we identified all patients having thoracentesis performed in a two-year period. The pathological results of the patients were systematically reviewed and all pleural fluid specimens positive for primary pulmonary AC were identified. Specimens with at least one tumour marker requested was included in the study. If a patient had repeated pleural fluid specimens fulfilling the inclusion criteria within the same work-up, only the first specimen was included. Additional pleural fluid specimens from the same patient were included in case of clinical progression and the request of new tumour marker status. In case of bilateral pleural fluid specimens analysed at the same time, both were included. We recorded the number of thoracentesis screened, number of pleural fluid specimens positive for primary pulmonary AC, volume pleural fluid sent for cytological analysis and tumour markers requested and obtained.
Endpoints
Primary endpoint was the prevalence of tumour markers demonstrated in the pleural fluid specimens with cytology positive for primary pulmonary AC when requested. Secondary endpoint was the diagnostic yield in pleural fluid specimens ≤ 50 mL versus > 50 mL.
Pleural fluid preparation
Whenever possible, 50 mL of pleural fluid were sent for cytology [Citation29]. Cell-blocks were prepared by using plasma thrombin cell block preparation. The specimen was centrifuged for 5 minutes at 1,650 rpm. After centrifugation, the supernatant was decanted. Human plasma and two drops of three percent aqueous eosin to the sediment were added and vortexed briefly. Next, 0.25–0.5 mL of reconstituted thrombin was added and the solution quickly agitated to obtain a clot within 30–60 seconds. The clot was placed into a labelled cassette containing formalin. The specimens were subsequently processed in the histopathology laboratory [Citation30].
PD-L1 analysis
PD-L1 test was performed on cell-blocks using PD-L1 antibodies 22C3 and staining platform Dako Omnis (Agilent, Glostrup, Denmark). Sample adequacy was defined as presence of at least 100 tumour cells and absence of excessive necrosis or inflammatory cells. PD-L1 expression was scored by evaluating any perceptible membranous staining (>1+) of tumour cells and by quantifying the percentage of viable PD‐L1‐expressing tumour cells in the cytology samples as previously described [Citation31].
ALK analysis
ALK test was performed on cell-blocks using staining platform Dako Omnis (Agilent, Glostrup, Denmark) and ALK antibodies ‘Origene’ clone OT1A4. Sample adequacy was assessed as for PD-L1 (see above). Cells were considered positive if presence of a strong continuous membranous staining (3+). ALK expression was presented dichotomously as either positive or negative expression [Citation32].
EGFR analysis
Following tumour content evaluation of hematoxylin and eosin stained slides, relevant regions were macrodissected and subjected to a standard genomic DNA extraction procedure using the GeneRead DNA FFPE Kit (Qiagen, Düsseldorf, Germany) according to manufacturer’s specifications. Extracted DNA was analysed by next-generation sequencing using the GeneRead QIAact Actionable Insights Tumour Panel (Qiagen) containing areas of 12 genes including EGFR (coverage > 500x in > 99% of all reads, sensitivity ~ 5%). Analysis was carried out in accordance with the manufacturer’s specifications with at least 20% neoplastic content (estimated by pathologist) in each tissue sample. Data interpretation was performed using QCI Analyze for GeneReader version 1.5.1 [Citation33].
Ethics and approvals
Approval from the Ethics Committee system was not warranted in this retrospective, non-interventional study on anonymized biological material. The study was approved by the Danish Data Protection Agency (approval number REG-021-2019).
Statistics
Statistical analyses were performed using STATA16.0 (StataCorp®, Texas, US). Categorical data were described as number (n) and percentage (%), and continuous variables as mean and standard deviation or, where appropriate, as median and range. Intergroup differences in categorical variables were analysed with Chi2-test or Fisher´s Exact test. A p-value <0.05 was considered statistically significant.
Results
A total of 927 pleural fluid specimens from the time period 1 January 2019 to 31 December 2020 were collected. Study flow chart is presented in and patient characteristics in . We identified 89(10%) pleural fluid specimens positive for primary pulmonary AC, of those 57(64%) with at least one tumour marker requested. The included specimens represented 51 patients, since six patients participated with two specimens; one due to bilateral thoracentesis and five due to clinical progression and repeated work-up within the two-year period.
Figure 1. Flowchart showing inclusion of pleural fluid specimens (†small-cell lung cancer, ‡squamous cell carcinoma, §adenocarcinoma)
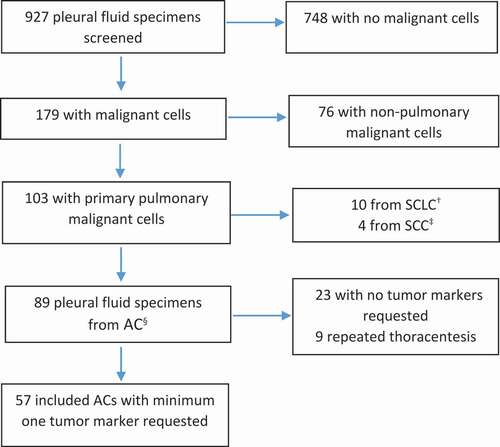
Table 1. Basic characteristics of the patients in the study cohort (patients with pleural fluid cytology positive for primary pulmonary adenocarcinoma and minimum one tumor marker requested)
The prevalence of tumour markers in pleural fluid specimens with cytology positive for pulmonary AC are shown in . PD-L1, ALK and EGFR was successfully obtained in 35/55(64%), 38/57(67%) and 26/48(55%), respectively.
Table 2. PD-L1†, ALK‡ and EGFR§ measured in pleural fluid specimens positive for primary pulmonary adenocarcinoma and minimum one tumor marker requested
shows tumour markers obtained based on volume pleural fluid with 50 mL as cut-off. The volume of one sample was not registered. In the 15 samples containing less than 50 mL of pleural fluid PD-L1 was obtained in 10/15 (67%), ALK in11/15 (73%) and EGFR in 10/15 (67%). In the 41 specimens containing more than 50 mL PD-L1 was obtained in 24/41 (59%), ALK in 26/41 (63%) and EGFR in 15/32 (47%). All p-values were above 0.05, but the risk ratios (RR) all had broad 95% confidence intervals including one, thus implying that the sample size is too small to comment on the difference in the prevalence of tumour markers in pleural fluids specimens below 50 mL versus above 50 mL.
Table 3. Obtained tumor markers in pleural fluid specimens in relation to volume pleural fluid analysed
Discussion
In the present study of the prevalence of tumour markers in pleural fluid specimens with cytology positive for primary pulmonary AC, PD-L1, ALK and EGFR could be demonstrated in 35/55(64%), 38/57(67%) and 26/48(55%), respectively. Thus, tumour markers can be demonstrated in pleural fluid obtained by thoracentesis, which is an inexpensive and minimally invasive procedure. This could imply that the clinician, in some cases, could await the result of pleural fluid cytology before initiating endoscopic procedures, especially in frail patients where endoscopic procedures may constitute a risk. However, it should be taken into account that in MPEs caused by pulmonary AC, pleural fluid cytology is positive in 82% [Citation16].
This study only assessed the possibility of demonstration tumour markers in pleural fluid with cytology positive for primary pulmonary AC. The knowledge of tumour markers in pleural fluid specimens positive for pulmonary SCC is limited due to the low prevalence of SCC in pleural fluid specimens. In a study by Grosu et al. [Citation18] including 115 pleural fluid cytology positive for NSCLC only 2 were SCC. Hence, if SCC is suspected, e.g. central tumours or Pancoast tumours the clinician should not await the result of pleural fluid cytology before proceeding to collecting histological biopsies.
Previous studies have investigated the detection of PD-L1 in MPE caused by pulmonary AC [Citation18,Citation21,Citation26]; however, two of the studies do not report a diagnostic yield specific for MPEs [Citation21,Citation26]. The study by Grosu et al. [Citation18] retrospectively evaluated 115 MPEs caused by NSCLC using cell-block and immunohistochemistry. With a limit for adequate sample of ≥100 tumour cells, 71% of samples were successfully analysed for PD-L1-status. This corresponds to our 64%.
Previous studies on detection of ALK-rearrangement in MPE due to NSCLC report of ALK-status obtained in 82% [Citation20] and 70% of analysed samples [Citation25]. This is also in line with our result of 73%. In contrast to our procedure, both studies used florescence in situ hybridization (FISH). However, previous studies suggest that FISH is not superior to immunohistochemistry assays in detection of ALK [Citation28,Citation34]. The study by DeMaio et al. [Citation20] is very similar to our study, except for analysing analysed larger volumes. However, the study concluded that effusion volume do not impact diagnostic yield on ALK- and EGFR-status. DeMaio et al. investigated volumes larger and lower than 100 mL [Citation20]. We used a 50 mL cut-off, as the diagnostic yield for pleural fluid cytology does not increase with volumes >50 mL [Citation29,Citation35].
The detection-rate of EGFR-mutations in previous studies varies considerably from 54% [Citation25], 78% [Citation20] and 96% [Citation22] of pleural fluid specimens; however, the latter study only analysed 27 specimens, including pericardial fluid. DeMaio et al. [Citation20] finds considerably higher diagnostic yield than our 55%, which cannot be explained by small differences in use of analysis methods. Both studies retrospectively assessed unselected consecutive patients suspected of lung cancer. The strength of our study is the valid electronic patient file system and thorough screening process. Nevertheless, it is possible that a thoracentesis was not labelled with a procedure-specific code and thus not included in the study. However, if at least one of the patient’s thoracenteses were correctly labelled, pathological result of all the patient’s pleural fluid specimens was accessible. Our study is limited by the retrospective design and the relatively small sample size, enabling us to comment on the influence of the volume fluid analysed on the prevalence of tumour markers. In addition, we are unable to comment on the concordance between tumour markers measured in pleural fluid specimens and histological biopsies, since only 10 positive tumour marker analyses, were repeated in histological biopsies. In patients not achieving the requested analysis, we did not assess the prevalence of tumour markers in the following biopsies. Thus, we cannot compare the prevalence of tumour markers in pleural fluid to cytological/histological biopsies.
For the future, there is a need for prospective studies to estimate the clinical impact of obtaining tumour markers in pleural fluid specimens and the concordance with histological biopsies.
Conclusion
Tumour markers in pleural fluid specimens containing cells from pulmonary AC can be demonstrated in more than half of the cases. Therefore, the planning of more burdensome invasive procedures than thoracentesis could await these analyses, especially in fragile patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ferlay J, Lam F, Colombet M, et al. Global cancer observatory: cancer today. Lyon: Fr Int Agency Res Cancer; Published online 2018 [cited 2020 May 11]. Available from: https//gco.iarc.fr/today.
- Ridge CA, McErlean AM, Ginsberg MS. Epidemiology of lung cancer. Semin Interv Radiol. 2013;30(2):93–6.
- Lantuejoul S, Damotte D, Hofman V, et al. Programmed death ligand 1 immunohistochemistry in non-small cell lung carcinoma. J Thorac Dis. 2019;11(Suppl1):S89–S101.
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388.
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742.
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–222.
- Zhou C, Kim SW, Reungwetwattana T, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med. 2019;7(5):437–446.
- Shaw AT, Solomon BJ, Besse B, et al. ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol. 2019;37(16):1370–1379.
- Doebele RC, Lu X, Sumey C, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer. 2012;118(18):4502–4511.
- Kerr KM, Thunnissen E, Dafni U, et al. A retrospective cohort study of PD-L1 prevalence, molecular associations and clinical outcomes in patients with NSCLC: results from the European thoracic oncology platform (ETOP) lungscape project. Lung Cancer. 2019;131:95–103.
- Sepesi B, Nelson DB, Mitchell KG, et al. Prognostic value of PD-L1 mRNA sequencing expression profile in non-small cell lung cancer. Ann Thorac Surg. 2018;105(6):1621–1626.
- Brody R, Zhang Y, Ballas M, et al. PD-L1 expression in advanced NSCLC: insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer. 2017;112:200–215.
- Terry L Ng; , Yiwei Liu, and Anastasios, Dimou et al. Predictive value of oncogenic driver subtyde, programmed death-1 ligand (PD-L1) score, and smoking status on the efficacy og PD-1/PD-L1 inhibitors in patients with oncogene-driven non-small cell lung cancer. Cancer 125 (7) . 1038–1049 doi:https://doi.org/10.002/cncr.31871. Published online 2019. Published online 2019
- Zhang P, Wu X, Tang M, et al. Detection of EGFR gene mutation status from pleural effusions and other body fluid specimens in patients with lung adenocarcinoma. Thorac Cancer. 2019;10(12):2218–2224.
- Morgensztern D, Waqar S, Subramanian J, et al. Prognostic impact of malignant pleural effusion at presentation in patients with metastatic non-small-cell lung cancer. J Thorac Oncol. 2012;7(10):1485–1489.
- Arnold DT, De Fonseka D, Perry S, et al. Investigating unilateral pleural effusions: the role of cytology. Eur Respir J. 2018;52:5.
- Pang C, Ma H, Qin J, et al. Pleural effusion as a substitute for tumour tissue in detecting EGFR/ALK mutations in non-small cell lung cancer: a systematic review and meta-analysis. Med (USA). 2019;98:18.
- Grosu HB, Arriola A, Stewart J, et al. PD-L1 detection in histology specimens and matched pleural fluid cell blocks of patients with NSCLC. Respirology. 2019;24(12):1198–1203.
- Song Z, Cheng G, Zhang Y. PD-L1 expression in malignant pleural effusion samples and its correlation with oncogene mutations in non-small cell lung cancer. J Thorac Dis. 2020;12(4):1385–1392.
- DeMaio A, Clarke JM, Dash R, et al. Yield of malignant pleural effusion for detection of oncogenic driver mutations in lung adenocarcinoma. J Bronchol Interv Pulmonol. 2019;26(2):96–101.
- Heymann JJ, Bulman WA, Swinarski D, et al. PD-L1 expression in non-small cell lung carcinoma: comparison among cytology, small biopsy, and surgical resection specimens. Cancer Cytopathol. 2017;125(12):896–907.
- Billah S, Stewart J, Staerkel G, et al. EGFR and KRAS mutations in lung carcinoma: molecular testing by using cytology specimens. Cancer Cytopathol. 2011;119(2):111–117.
- Betz BL, Dixon CA, Weigelin HC, et al. The use of stained cytologic direct smears for ALK gene rearrangement analysis of lung adenocarcinoma. Cancer Cytopathol. 2013;121(9):489–499.
- Rosenblum F, Hutchinson LM, Garver J, et al. Cytology specimens offer an effective alternative to formalin-fixed tissue as demonstrated by novel automated detection for ALK break-apart FISH testing and immunohistochemistry in lung adenocarcinoma. Cancer Cytopathol. 2014;122(11):810–821.
- Carter J, Miller JA, Feller-Kopman D, et al. Molecular profiling of malignant pleural effusion in metastatic non-small-cell lung carcinoma. the effect of preanalytical factors. Ann Am Thorac Soc. 2017;14(7):1169–1176.
- Wang H, Agulnik J, Kasymjanova G, et al. Cytology cell blocks are suitable for immunohistochemical testing for PD-L1 in lung cancer. Ann Oncol. 2018;29(6):1417–1422.
- Wang X, Chen S, Emerson RE, et al. Molecular testing for EGFR mutations and ALK rearrangements in the cytological specimens from the patients with non-small cell lung cancer. Appl Immunohistochem Mol Morphol. 2019;27(2):119–124.
- Wang W, Tang Y, Li J, et al. Detection of ALK rearrangements in malignant pleural effusion cell blocks from patients with advanced non-small cell lung cancer: a comparison of Ventana immunohistochemistry and fluorescence in situ hybridization. Cancer Cytopathol. 2015;123(2):117–122.
- Hooper C, Lee YC, Maskell N; Group BTSPG. Investigation of a unilateral pleural effusion in adults: British thoracic society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii4–17.
- Thunnissen E, Kerr KM, Herth FJF, et al. The challenge of NSCLC diagnosis and predictive analysis on small samples. Practical approach of a working group. Lung Cancer. 2012;76(1):1–18.
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028.
- Shen Q, Wang X, Yu B, et al. Comparing four different ALK antibodies with manual immunohistochemistry (IHC) to screen for ALK-rearranged non-small cell lung cancer (NSCLC). Lung Cancer. 2015;90(3):492–498.
- Koitzsch U, Heydt C, Attig H, et al. Use of the GeneReader NGS system in a clinical pathology laboratory: a comparative study. J Clin Pathol. 2017;70(8):725–728.
- Liu L, Zhan P, Zhou X, et al. Detection of EML4-ALK in lung adenocarcinoma using pleural effusion with FISH, IHC, and RT-PCR methods. PLoS One. 2015;10(3):1–9.
- Abouzgheib W, Bartter T, Dagher H, et al. A prospective study of the volume of pleural fluid required for accurate diagnosis of malignant pleural effusion. Chest. 2009;135(4):999–1001.
