ABSTRACT
Oral corticosteroids (OCS) are often prescribed to patients with asthma that remains uncontrolled with maintenance therapy. We performed a real-world analysis to describe the geographic distributions of patients with asthma and OCS dispensed in Nordic countries. This observational, retrospective study examined patient-level data from nationally prescribed drug registries from January to December 2018 for individuals aged ≥12 years in Denmark, Finland, and Sweden. Using an algorithm based on asthma treatment combinations defined by the Global Initiative for Asthma (GINA), we identified patients with asthma, those on GINA Step 4–5 treatments, and those being dispensed ≥2 courses of OCS and determined volumes of OCS dispensed to these patients over the 1-year analysis period. Data were plotted geographically within each country using colour-coded heat maps. The overall asthma prevalence rates were 7.4% in Denmark, 11.6% in Finland, and 8.1% in Sweden. In Denmark, Finland, and Sweden, respectively, the frequencies of patients on GINA Step 4–5 treatments were 19%, 15%, and 16%; among whom 10%, 23%, and 5% received ≥2 courses of OCS. The rates of patients on GINA Step 4–5 treatments who were dispensed OCS in each country were 23%, 30%, and 46%, of which 22%, 17%, and 10% were dispensed doses averaging ≥5 mg/day over the year. Heat maps revealed considerable heterogeneity in geographic densities of patients with asthma and OCS claims within each country. Taken together, these results demonstrate regional variations in estimated asthma severity, control, and OCS dispensed within and between countries. Patterns of medication use suggest that a high proportion of patients in Denmark, Finland, and Sweden are on GINA Step 4–5 treatments, many of whom are dispensed OCS; this poses a considerable corticosteroid burden to these patients. Geographic differences in medication use within and between Nordic countries may reflect variations in population characteristics and/or treatment approaches.
Introduction
Asthma is a common, non-communicable disease that varies in severity and responsiveness to treatment and can be difficult to control. Of the estimated 339 million people with asthma worldwide, the global prevalence of severe disease ranges from 4% to 10% [Citation1–4]. Increased asthma severity and poor disease control are associated with greater disease burden, health care resource utilization, and asthma-related mortality [Citation1,Citation2,Citation5–9], which highlights the need for more effective treatment strategies.
In Nordic countries, recent estimates suggest asthma prevalence ranges from 5.2% (Denmark) to 11.2% (Finland) [Citation10,Citation11], while greater variation exists in reported prevalence rates of severe and uncontrolled asthma. For example, in Sweden, approximately 4.2% of the patients with asthma have severe disease, more than half of whom (53.6%) have poorly controlled asthma [Citation12]; by comparison, an estimated 8.6% of the Finnish patients with asthma have severe disease, nearly half of which (46.6%) are likely uncontrolled [Citation13]. Of the estimated 8.1% of the patients with asthma in Denmark who have severe disease, 36.4% have been reported to have poor asthma control [Citation14].
Asthma severity can be categorised according to the Global Initiative for Asthma (GINA) treatment steps, which are defined by the types of medication necessary to maintain asthma control (Supplemental Figure S1). Steps 1 and 2 are the least severe based on effective management with low-dose inhaled corticosteroids (ICS), whereas more severe disease (steps 3 and 4) is indicated by requirements for increasing doses of ICS and add-on medications, such as long-acting beta agonist (LABA). Patients with asthma that remain severe and/or uncontrolled with high-dosage ICS and controller therapies are often prescribed add-on oral corticosteroids (OCS) included in GINA Step 5 [Citation15].
Despite the effectiveness of OCS in achieving or maintaining asthma control, the addition of OCS is associated with an increase in both treatment costs [Citation16,Citation17] and risk of serious adverse events [Citation18–21]. A recent assessment determined that lifetime exposure to as little as 0.5–1 g OCS is associated with significantly increased risk for certain adverse outcomes [Citation18,Citation22]. As a result, in 2019 GINA amended its guidelines to recommend that health care providers consider side-effects before prescribing OCS. Recent data from Nordic countries suggest that nearly 25% of the Swedish patients with asthma and 47% of the Finnish patients with severe asthma use OCS [Citation13,Citation23]. Effective alternatives to OCS, to achieve and maintain asthma control, have emerged in the past 20 years, including the development of biologic therapies like monoclonal antibodies against inflammatory mediators or interleukin receptors. Real-world data suggest these treatments are effective for achieving asthma control, have favourable safety and tolerability profiles, and are a preferred option for patients with asthma that is uncontrolled with high-dose ICS/LABA [Citation24–28].
The PRECISION program is a global initiative to improve treatment for patients with severe asthma, in part, by implementing targeted activities to increase access, quality, and speed of care. Recent PRECISION studies include characterization of regional distributions of severe and/or uncontrolled asthma prevalence in different countries and regions to identify areas with the greatest need for improved access to care [Citation29–32]. As part of this initiative, we examined pharmacy claims data from Denmark, Finland, and Sweden to identify patients with asthma, those on GINA Step 4–5 treatments, and the amount of OCS dispensed. These data were plotted geographically to characterize the regional variation in the intensity of inhaled asthma medication and the use of OCS.
Materials and methods
Study design and data source
This was an observational, retrospective study of longitudinal patient-level data from national prescription registries to determine frequencies of patients on GINA Step 4–5 treatments, patients on GINA Step 4–5 treatments receiving ≥2 courses of OCS, and OCS dispensed to patients aged ≥12 years from January to December 2018 in Denmark, Finland, and Sweden. Longitudinal patient-level data originating from electronic dispensing systems at pharmacies were obtained as aggregated patient counts by region from the Danish National Prescription Registry (Statistics Denmark), the Finnish Prescription Register (KELA), and the Swedish National Prescribed Drug Register (Socialstyrelsen). Ethics approval was not required as only deidentified aggregated patient data were used.
Asthma prevalence estimates and OCS claims
A high-level algorithm was developed to identify patients with asthma and categorise the intensity of inhaled asthma medication and OCS use based on prescription claims data (). Patients were classified as having asthma if they received Anatomical Therapeutic Chemical (ATC) classification system R03 medications, excluding long-acting muscarinic agonists except for tiotropium. To estimate treatment intensity, one group included patients on at least GINA Step 4 treatment (Supplemental Figure S1), as outlined in the 2018 Global Strategy for Asthma Management and Prevention report, and included patients on medium to high dose ICS + LABA [Citation33]. Daily ICS dose was calculated by dividing cumulative ICS dispensed over the study year by 365. Among patients on GINA Step 4 or 5 medication, we also assessed treatment intensity for those with claims for OCS considered sufficient to cover at least two exacerbations (two courses of 40 mg daily prednisolone equivalent for 7 days) during the analysis period. Among patients on GINA Step 4–5 treatments, total OCS dispensed was determined and quantified by any OCS use (≥1 prescription) or cumulative OCS exposure of ≥912.5 mg or ≥1825 mg (equivalent to 2.5 mg/day or 5 mg/day, respectively, over the course of 1 year).
Figure 1. High-level algorithmic approach to identifying patients with asthma, by treatment intensity.
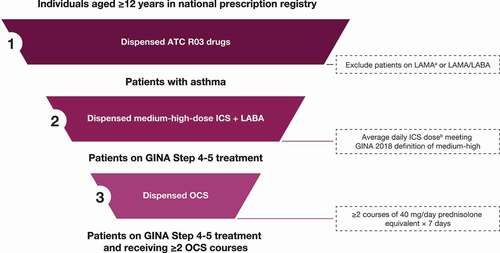
Regional analyses
Prescription claims data were analysed by administrative regions within each country to determine geographic differences in estimated asthma severity and control and OCS dispensed. Color-coded heat maps were generated using four colours representing statistical ranges corresponding to approximate normal distributions within each map.
Results
Asthma prevalence and treatment intensity by country
In Denmark, a total of 374,167 residents were classified as having asthma (prevalence of 7.4%) (). Among these patients, 19% were on GINA Step 4–5 treatments, of which 10% were classified as receiving ≥2 OCS courses. At least 1 OCS prescription was dispensed to 23% of the Danish patients on GINA Step 4–5 treatments during the analysis period. Among all patients on GINA Step 4–5 who were prescribed ≥1 course of OCS, 35% and 22% were dispensed cumulative doses of ≥912.5 mg and ≥1825 mg, respectively.
Table 1. Estimated asthma prevalence and treatment intensity by country
In Finland, 560,534 residents were classified as having asthma (prevalence of 11.6%) (). A total of 15% of the patients with asthma were on GINA Step 4–5 treatments, among which 23% received ≥2 OCS courses. At least 1 OCS prescription was dispensed to 30% of the patients on GINA Step 4–5 treatments during the analysis period. Of patients on GINA Step 4–5 treatments who were prescribed ≥1 course of OCS, 43% and 17% were dispensed cumulative doses of ≥912.5 mg and ≥1825 mg, respectively.
In Sweden, a total of 711,012 residents were identified as having asthma (prevalence of 8.1%) (). A total of 16% of the patients with asthma were on GINA Step 4–5 treatments, 5% of whom received ≥2 OCS courses. OCS were dispensed to 46% of the patients on GINA Step 4–5 treatments. Of patients on GINA Step 4–5 treatments who were prescribed ≥1 course of OCS, 21% and 10% had cumulative doses of ≥912.5 mg and ≥1825 mg, respectively.
Regional variations in disease severity and OCS claims
Heat maps revealed regional distributions of treatment intensity of asthma in all three countries. In Denmark, the prevalence of patients on GINA Step 4–5 treatments was generally consistent between regions, ranging from 17% to 20%, and was highest in the Region of Southern Denmark ()). By contrast, the highest rates of patients on GINA Step 4–5 treatments receiving ≥2 OCS courses, which ranged from 8% to 13% within regions, were observed in the North and Central Denmark Regions ()). Geographic distributions of high cumulative OCS dispensed among patients in GINA Step 4–5 treatments are shown in Figure (c) and (d). Patients with the highest OCS claims were mainly located in the North Denmark Region.
Figure 2. Estimated regional prevalence of patients on GINA Step 4–5 treatments, those on Step 4–5 treatments receiving ≥2 OCS courses, and OCS claims in Denmark. (a) Regional prevalence of patients on GINA Step 4–5 treatments among all patients with asthma; (b) frequency of patients receiving ≥2 OCS courses among patients on GINA Step 4–5 treatments, and frequencies of patients on GINA Step 4–5 treatments dispensed (c) ≥912.5 mg and (d) ≥1825 mg OCS during the 1-year observation period.
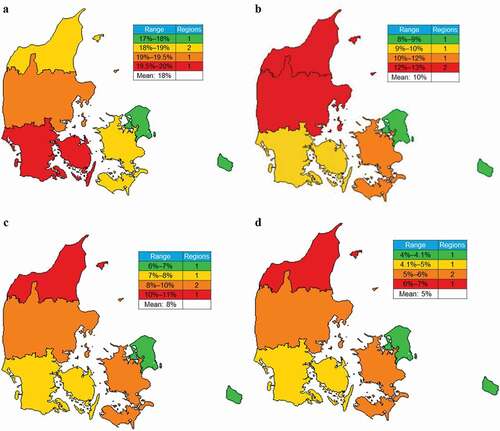
OCS, oral corticosteriods.
In Finland, the prevalence of patients on GINA Step 4–5 treatments between regions ranged from 13% to 22%; the highest rates were observed in the northern and eastern parts of the country ()), while the highest rates of these patients receiving ≥2 OCS courses were observed in the east-central region comprising areas around Kuopio and Joensuu ()). Patients on GINA Step 4–5 treatments with the highest amounts of OCS dispensed were most prevalent in the southern half of the country () and (d)).
Figure 3. Estimated regional prevalence of patients on GINA Step 4–5 treatments, those on Step 4–5 treatments receiving ≥2 OCS courses, and OCS claims in Finland. (a) Regional prevalence of patients on GINA Step 4–5 treatments among all patients with asthma; (b) frequency of patients receiving ≥2 OCS courses among patients on GINA Step 4–5 treatments; and frequencies of patients on GINA Step 4–5 treatments dispensed (c) ≥912.5 mg and (d) ≥1825 mg OCS during the 1-year observation period.
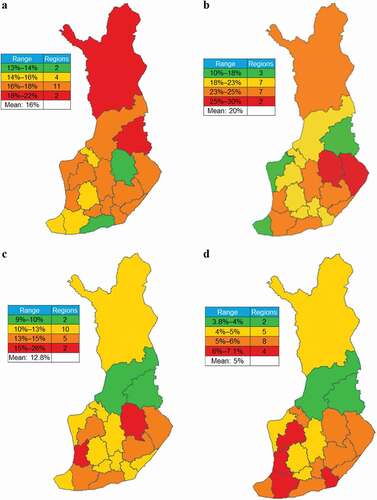
OCS, oral corticosteriods.
In Sweden, the regional distribution of patients on GINA Step 4–5 treatments ranged from 14% to 19% ()), and the regions with the highest percentages of these patients (18% to 19%) were generally located in the southern half of the country. Rates of patients on GINA Step 4–5 treatments receiving ≥2 OCS courses were the most prevalent in east-central Sweden (specifically Västernorrland) ()), and patients on GINA Step 4–5 treatments dispensed cumulative OCS of ≥912.5 mg were most densely concentrated in the south ()), while hot spots of higher claims (≥1825 mg) were also found in the north ()).
Figure 4. Estimated regional prevalence of patients on GINA Step 4–5 treatment, those on Step 4–5 treatments receiving ≥2 OCS courses, and OCS claims in Sweden. (a) Regional prevalence of patients on GINA Step 4–5 treatments among all patients with asthma; (b) frequency of patients receiving ≥2 OCS courses among patients on GINA Step 4–5 treatments; and frequencies of patients on GINA Step 4–5 treatments dispensed (c) ≥912.5 mg and (d) ≥1825 mg OCS during the 1-year observation period.
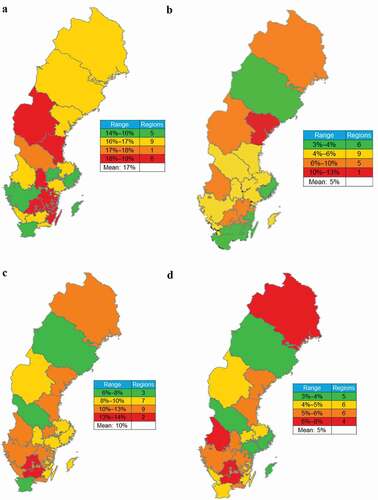
OCS, oral corticosteriods
Discussion
In this study, we used a prescription-based algorithm and national prescription registries to estimate the prevalence of asthma, patients on GINA Step 4–5 treatments, and those receiving ≥2 OCS courses on top of GINA Step 4–5 treatments in three Nordic countries. We found asthma prevalence rates ranging from 8.5% to 11.6% across countries, while also showing variation in the prevalence of patients on GINA Step 4–5 treatments and OCS claims, both overall and geographically within each country.
To better understand the treatment needs of patients, research on the epidemiology of severe and uncontrolled asthma continues to evolve; however, consistent findings have been difficult to achieve. Reasons for this may include variations in sampling (i.e. general population vs hospitalized patients), data source and quality (i.e., self-report vs medical records or registry data), and lack of standard definitions of severe and/or uncontrolled asthma. For example, compared with our observation that 16% of the patients with asthma in Sweden were on GINA Step 4–5 treatments, which includes patients with severe disease (5% of which received ≥2 OCS courses that could likely indicate uncontrolled asthma), a previous study reported that 4.2% of the patients with asthma had severe disease [Citation12]. Those patients who receive frequent courses of OCS can be labelled as having an uncontrolled disease. Among patients on GINA Steps 4–5 treatments in our study, 5% received ≥2 OCS courses. In the previous study, 53.6% were labelled as having an uncontrolled disease based on reliever use and exacerbation [Citation12]. Our other finding that 19% of the patients with asthma in Denmark were on GINA Step 4–5 treatments, 10% of which received ≥2 OCS courses, also diverges considerably from previously reported rates of severe and uncontrolled asthma as 8.1% and 36.4%, respectively, based on a national prescription database, albeit with different definitions of asthma severity and control [Citation14]. Standardization of study methods and definitions of disease characteristics would surely improve consistency and provide better consensus between studies, but this is different because of limitations in different data sources.
GINA updated its recommendations in 2019 regarding OCS use to suggest OCS should be avoided when possible, in favour of more preferrable add-on therapies [Citation15]; however, use of these medications are also likely influenced greatly by physician preference, treatment success, and national guidelines. Although we observed similar rates of patients on GINA Step 4–5 treatments between countries (15–19%), rates of these patients receiving ≥2 OCS courses varied widely from 5% (Sweden) to 23% (Finland). OCS use is not a comprehensive measure in comparison to assessing symptoms and lung function and could contribute to the low proportion of potential uncontrolled asthma (estimated by the prevalence of patients receiving ≥2 OCS courses) reported here. Interestingly, Sweden also had the highest rate of OCS dispensed to patients on GINA Step 4–5 treatments (46%). If the high rate of asthma control in Sweden is related to the high rate of OCS use, it would occur at the cost of an increased risk of adverse health consequences [Citation18,Citation22]. Notably, a recent study that characterized high OCS prescription rates to patients with asthma in Sweden revealed a trend for increased adverse health outcomes among patients prescribed ≥5 mg/day/year OCS vs those prescribed lower dosages, although OCS dosage increased with age, which also could have influenced adverse health outcomes [Citation23]. Moreover, Finland, which also had a high rate of OCS claims among patients on GINA Step 4–5 treatments in our study (30%), had the highest rate of patients receiving ≥2 OCS courses (23%). Nonetheless, evidence from these and other studies reveal high rates of OCS prescriptions, highlighting the need for safe and effective alternatives to achieve and maintain asthma control. Notably, a policy change that occurred in Finland in May 2020 shifted administration and reimbursement of biologics from being limited to the hospital setting to being reimbursed by KELA (the Social Insurance Institution of Finland), thus allowing patients to self-administer biologics at home. Over time, this will likely result in an overall shift toward more biologic use and less OCS use.
Our geographic analysis also shows considerable heterogeneity in treatment intensity within countries. Geographic differences in estimated severity and control could be related to variables such as climate, population density and age distribution, industrialization, and/or treatment access. A detailed analysis of all factors contributing to asthma severity and control in the Nordics would be an interesting topic for future research; however, such a detailed analysis was beyond the scope of this investigation.
Our study had several strengths, including the use of representative samples based on registries covering 100% of outpatient prescriptions dispensed within each country. However, there were also several limitations. As pharmacy claims data do not provide diagnostic information, we used an algorithm to identify patients with asthma; consequently, patients who received R03 medications (including OCS) for other conditions may have been included, or patients with asthma who were not receiving such medications during the analysis period may have been excluded. If OCS were used for other reasons besides asthma, this discrepancy would be the same across all countries and regions in the analysis; therefore, the findings still add valuable insights to similarities and differences in OCS use between Nordic countries. Another limitation of prescription claims data is that they do not indicate whether the medications were actually used; this is of particular concern for patient populations such as those with asthma, for which treatment nonadherence is common [Citation34,Citation35]. Determination of OCS use for the full asthma patient population was limited by the algorithm, which only determined OCS use among patients on GINA Step 4–5 treatments, excluding patients with mild or moderate disease who are also prescribed OCS for exacerbations [Citation36]. Second, limited OCS package size options may not correspond with individual patient needs. For example, the smallest available package of prednisolone 40 mg in Finland is 30 tablets (1200 mg total), which would result in a considerable overestimate of OCS used by a patient who requires only a 7-day course (280 mg). Variations in package sizes between countries could create false differences in estimates of OCS used between countries. Our study was also limited by the size of geographic regions selected, as city-/town-level data would provide additional details and better inform local initiatives to improve access and quality of care; this is particularly relevant in the case of Denmark, where only five regions were analysed. Finally, our study could have been improved by including data from Norway, which were not readily available for the analysis period. Nonetheless, the real-world analysis we present here is an important step toward generating detailed local information on asthma disease characteristics, treatment patterns, and areas of focus when considering future initiatives to improve asthma management practices.
Conclusion
In summary, our analysis of national prescription claims data reveals that the prevalence of patients with asthma on GINA Step 4–5 treatments is similar in Denmark, Finland, and Sweden, while the rates of these patients receiving ≥2 OCS courses vary more widely between countries. In all countries, relatively substantial proportions of patients continue to be prescribed OCS despite the associations with significant health risks. Our study is an important step toward improving our understanding of regional differences in asthma management. These insights will help improve asthma care in Nordic countries and will support future initiatives to improve long-term outcomes for patients with asthma.
Supplemental Material
Download MS Word (14 KB)Acknowledgments
Medical writing support was provided by Nate Connors, PhD, CMPP™, of Citrus Health Group, which was in accordance with Good Publication Practice (GPP3) guidelines and funded by AstraZeneca (Gaithersburg, Maryland).
Disclosure statement
Lauri Lehtimäki has received personal fees for lectures and consultations from AstraZeneca, BoehringerIngelheim, Chiesi, Circassia, GSK, Mundipharma, Novartis, OrionPharma, and Sanofi. Gunilla Telg is an employee of AstraZeneca. Cassandra Nan is an employee of AstraZeneca and a shareholder of GlaxoSmithKline and AstraZeneca. Bora Erdemli, Aditya Samant, and Tra-My Nguyen are employees of ZS Associates, whose work was funded by AstraZeneca.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Chastek B, Korrer S, Nagar SP, et al. Economic burden of illness among patients with severe asthma in a managed care setting. J Manag Care Spec Pharm. 2016;22:848–9.
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373.
- Hankin CS, Bronstone A, Wang Z, et al. Estimated prevalence and economic burden of severe, uncontrolled asthma in the USA. J Allergy Clin Immunol. 2013;131:AB126.
- Hekking PP, Wener RR, Amelink M, et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135:896–902.
- GBD. DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2016;390(2017): 1260–1344.
- GBD. Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2016;390(2017): 1211–1259.
- Omachi TA, Iribarren C, Sarkar U, et al ., Risk factors for death in adults with severe asthma. Ann Allergy Asthma Immunol. 2008;101:130–136.
- Suruki RY, Daugherty JB, Boudiaf N, et al. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17:74.
- Zeiger RS, Schatz M, Dalal AA, et al, Utilization and costs of severe uncontrolled asthma in a managed-care setting. J Allergy Clin Immunol Pract. 2016;4:120–129 e123.
- Honkamaki J, Hisinger-Molkanen H, Ilmarinen P, et al. Age- and gender-specific incidence of new asthma diagnosis from childhood to late adulthood. Respir Med. 2019;154:56–62.
- Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1204–1222.
- Larsson K, Ställberg B, Lisspers K, et al. Prevalence and management of severe asthma in primary care: an observational cohort study in Sweden (PACEHR). Respir Res. 2018;19:12.
- Viinanen A, Lassenius MI, Toppila I, et al. The burden of adult asthma in Finland: impact of disease severity and eosinophil count on health care resource utilization. J Asthma. 2020;57:1092–1102.
- von Bülow A, Kriegbaum M, Backer V, et al. The prevalence of severe asthma and low asthma control among Danish adults. J Allergy Clin Immunol Pract. 2014;2:759–767.
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention, 2020, <https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf>
- Jansson SA, Backman H, Andersson M, et al. Severe asthma is related to high societal costs and decreased health related quality of life. Respir Med. 2020;162:105860.
- Janson C, Lisspers K, Stallberg B, et al. Health care resource utilization and cost for asthma patients regularly treated with oral corticosteroids - a Swedish observational cohort study (PACEHR). Respir Res. 2018;19:168.
- Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204.
- Bleecker ER, Menzies-Gow AN, Price DB, et al. Systematic literature review of systemic corticosteroid use for asthma management. Am J Respir Crit Care Med. 2020;201:276–293.
- Volmer T, Effenberger T, Trautner C, et al. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018;52:1800703.
- Lefebvre P, Duh MS, Lafeuille MH. et al, Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136:1488–1495.
- Bloechliger M, Reinau D, Spoendlin J, et al. Adverse events profile of oral corticosteroids among asthma patients in the UK: cohort study with a nested case-control analysis. Respir Res. 2018;19:75.
- Ekstrom M, Nwaru BI, Hasvold P, et al. Oral corticosteroid use, morbidity and mortality in asthma: a nationwide prospective cohort study in Sweden. Allergy. 2019;74:2181–2190.
- Maselli DJ, Rogers L, Peters JI. Benralizumab, an add-on treatment for severe eosinophilic asthma: evaluation of exacerbations, emergency department visits, lung function, and oral corticosteroid use. Ther Clin Risk Manag. 2018;14:2059–2068.
- Leung E, Al Efraij K, FitzGerald JM. The safety of mepolizumab for the treatment of asthma. Expert Opin Drug Saf. 2017;16:397–404.
- Bourdin A, Shaw D, Menzies-Gow A, et al. Two-year integrated steroid-sparing analysis and safety of benralizumab for severe asthma. J Asthma. 2019;1–9. DOI:10.1080/02770903.2019.1705333.
- Menzies-Gow A, Corren J, Bel EH, et al. Corticosteroid tapering with benralizumab treatment for eosinophilic asthma: PONENTE trial. ERJ Open Res. 2019;5. DOI:10.1183/23120541.00009-2019.
- Kavanagh JE, Hearn AP, Dhariwal J, et al. Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest. 2020. DOI:10.1016/j.chest.2020.08.2083.
- Menzies-Gow A, Haslam T, Morris T, et al. P144 regional variation in OCS use for UK patients with asthma: heat map analysis. Thorax. 2019;74:A169–A169.
- Chapman KR, Remtulla A, Gendron A, et al. Regional variation in asthma prevalence and oral corticosteroid use for Canadian patients: heat map analysis. Am J Respir Crit Care Med. 2020;201:A1835–A1835.
- Tran TN, King E, Sarkar R, et al, Oral corticosteroid prescription patterns for asthma in France, Germany, Italy and the UK. Eur Respir J. 2020;55. DOI:10.1183/13993003.02363-2019.
- Bleecker ER, Gandhi H, Gilbert I, et al. Mapping geographic variability of severe uncontrolled asthma in the USA: management implications. Ann Allergy Asthma Immunol. 2022;128:78–88.
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention, <http://www.ginasthma.org> (2018).
- Hew M, McDonald VM, Bardin PG, et al. Cumulative dispensing of high oral corticosteroid doses for treating asthma in Australia. Med J Aust. 2020;213:316–320.
- Reddel HK, Beckert L, Moran A, et al, Is higher population-level use of ICS/LABA combination associated with better asthma outcomes? Cross-sectional surveys of nationally representative populations in New Zealand and Australia. Respirology. 2017;22:1570–1578.
- FitzGerald JM, Barnes PJ, Chipps BE, et al, The burden of exacerbations in mild asthma: a systematic review. ERJ Open Res. 2020;6:00359–02019.
