ABSTRACT
BACKGROUND: Although exercise and daily physical activity (PA) have long been known to benefit patients with chronic disorders, knowledge is limited regarding asthma.
OBJECTIVE: In a Danish setting, our aim was to measure physical activity, sedentary behavior, and physical capacity among patients with asthma. We hypothesized that people with severe asthma would be less active and more sedentary than their mild-moderate counterparts.
METHODS: Adults with asthma were recruited through respiratory outpatient clinics and subsequently examined twice, 4 weeks apart. At each visit, participants underwent a series of lung function tests, questionnaires, and maximum oxygen uptake testing (VO2max). Between the visits, participants wore an accelerometer continuously for 4 weeks, measuring sedentary time and daily steps. Sixty patients, 27 with mild-moderate asthma (GINA 1–3) and 33 with severe asthma (GINA 4–5), completed both visits and had valid accelerometer measurements.
RESULTS: No significant differences between the two groups were found in sedentary time, number of steps or VO2max. VO2max was significantly correlated with FeNO (r = −0.30, p < 0.05), Short Form-12 Mental Health (r = 0.37, p < 0.05), Asthma Control Questionnaire (r = −0.35, p < 0.05), and Mini Asthma Quality of Life Questionnaire (r = 0.36, p < 0.05).
CONCLUSION: No differences were observed between patients with mild-moderate and severe asthma regarding sedentary behavior, daily steps or level of cardiopulmonary fitness. Furthermore, patients with the highest VO2max had the higher quality of life scores.
Abbreviations: VO2max: Maximal Oxygen Uptake; CPET: Cardiopulmonary Exercise Testing; BMI: Body Mass Index; FEV1: Forced Expired Volume in the First Second; FVC: Forced Vital Capacity; PEF: Peak Expiratory Flow; EIB: Exercise-Induced Bronchoconstriction; COPD: Chronic Obstructive Pulmonary Disease; ACQ: Asthma Control Questionnaire; Mini-AQLQ: Mini Asthma Quality of Life Questionnaire; SF-12: Short Form 12 Health Survey; SNOT-22: Sino-Nasal Outcome Test 22; GINA: The Global Initiative for Asthma; CRP: C-reactive Protein; Hgb:Hemoglobin count; EOS: Eosinophil count; EVH: Eucapnic Voluntary Hyperventilation; FeNO: Fractional Exhaled Nitric Oxide; PA: Physical Activity ERS: European Respiratory Society; ATS: American Thoracic Society; CRS: Chronic Rhinosinusitis; AHR: Airway Hyperresponsiveness
Introduction
Asthma is characterized by variable airflow limitation and airway inflammation, causing symptoms such as breathlessness, cough, and wheeze during both rest and exercise [Citation1]. Exercise training and physical activity (PA) have long been known to benefit individuals with chronic illnesses [Citation2]. In asthma, it has been shown that patients participating in systematic exercise training achieve better asthma control [Citation3,Citation4] and may have the potential to reduce their use of controller medication [Citation5] and improve their lung function [Citation4]. The few studies conducted regarding PA and asthma suggest that patients with severe asthma are less active. The findings showed that those with severe asthma took 31% fewer daily steps than did healthy control persons [Citation6,Citation7] and 21% fewer steps than did patients with mild to moderate asthma [Citation7].
A higher level of PA among patients with asthma is likely to improve their health-related quality of life, reported number of ‘asthma-free days’, symptoms of anxiety and depression [Citation8,Citation9] as well as inducing weight loss – all of which points toward exercise training improving asthma control. Furthermore, exercise training has been suggested to reduce the frequency of exacerbations and reduce the level of airway hyperresponsiveness (AHR) [Citation10,Citation11], although reduction in AHR is partly explained by the improvement in exercise capacity. França-Pinto et al. found exercise training prompted a reduction in two serum proinflammatory cytokines, interleukin 6 and monocyte chemoattractant protein 1, but not a reduction in fractional exhaled nitric oxide (FeNO) in asthma patients.
Independent of the level of PA, sedentary behavior has been associated with higher rates of chronic disease, lower health-related quality of life, and higher prevalence of depressive symptoms and mood disorders [Citation12–14].
A meta-analysis of 21 randomized control trials, with 772 participants with asthma aged 8 years or older, showed that exercise training was well tolerated across all studies and that patients experienced an improvement in the maximal oxygen uptake (VO2max) – thus concluding that those with stable asthma should always be encouraged to perform regular exercise [Citation15]. Nevertheless, no improvement in forced expiratory volume in 1 second (FEV1) or peak expiratory flow rate (PEF) was found as an effect of exercise training [Citation15]. Another more recent review including 11 studies and 543 adult participants with asthma found a significant improvement in FEV1 induced by exercise training [Citation4]. Exercise-induced bronchospasm (EIB) has long been thought to be part of the explanation of the lower level of PA among patients with asthma [Citation16], but two systemic reviews suggest that exercise training in patients with stable asthma would not cause EIB limitations [Citation15,Citation17].
Compared with chronic obstructive pulmonary disease (COPD), in which correlations between PA, sedentary time, medication use, and disease control have been well studied [Citation18–20], guidelines or strategies for handling inactivity in asthma are sparse [Citation3,Citation21].
Philips et al. conclude that the use of wearable activity monitors in research is an efficient tool for collecting data on day-to-day PA [Citation22], and the current technology calculating sedentary time is considered a good measure, although accuracy declines when measuring the quality of PA [Citation23].
The aim of the present study was to measure PA, sedentary behavior, and cardiopulmonary capacity among patients with asthma across all GINA classifications, and to describe difference between patients with severe asthma and patients with mild-moderate asthma.
We also aim to describe the association of these behaviors with clinical measures of asthma control, lung function, quality of life, comorbidity with chronic rhinosinusitis and markers of airway inflammation.
We hypothesize that patients with severe asthma are less active and more sedentary than patients with mild-moderate asthma, and that mild-moderate asthma is associated with higher level of physical capacity. Furthermore, we hypothesize that higher level of physical capacity, higher level of PA, and lower level of sedentary behavior are all associated with better quality of life, better asthma control, and lower level of airway inflammation.
Material and methods
Design
The present study is a prospective follow-up study with two visits scheduled 4 weeks apart at the Center for Physical Activity Research (CFAS), Rigshospitalet, Copenhagen, Denmark. At both visits, participants underwent a series of questionnaires, FeNO, spirometry including bronchodilator reversibility test, blood samples, Cardiopulmonary Exercise Testing (CPET) including measurements of VO2max, and use of medication including self-assessed compliance.
The main objective was to measure the level of PA (estimated by and number of steps per day), sedentary behavior (estimated by the idle time percentage in a day), and physical capacity (estimated by VO2max). Sedentary behavior is defined as the percentage of the day (24 hours) with idle time (sitting, reclining, or lying). PA and sedentary behavior data are collected in the 4 weeks between the two visits, while physical capacity is measured at each of the two visits.
Participants
Adults with asthma were included, most of whom were recruited through respiratory outpatient clinics at Gentofte Hospital, North Zealand Hospital, Roskilde Hospital, and Hvidovre Hospital, Denmark. Patients were also recruited from an out-of-hospital asthma clinic and from advertising on social media.
Asthma diagnosis was confirmed by one of the following methods: 1) historic reversibility, 2) historic PEF variation, 3) historic airway hyperresponsiveness to mannitol, 4) historic airway hyperresponsiveness to methacholine, 5) historic eucapnic voluntary hyperventilation (EVH), or in few cases 6) asthma diagnosis in the opinion of an asthma expert. Only patients with active asthma are included, thus meaning either as-needed treatment or daily maintenance medication is actively used. An equal distribution between severe asthma and mild-moderate asthma was sought through the inclusion, resulting in 33 patients with severe asthma and 27 with mild-moderate asthma – see for details. The GINA 2019 guidelines were used throughout the study [Citation1].
Table 1. Division into mild-moderate asthma vs. severe asthma defined by controller medication and the GINA Steps in the GINA 2019 Guidelines [Citation1].
The exclusion criteria were 1) presence of lung disease other than asthma, 2) pregnancy, 3) musculoskeletal diseases or injuries with physical impairment, 4) on-going infection detected by elevated level of CRP, and 5) exacerbation in asthma disease needing systemic corticosteroids, either in the 4 weeks prior to enrollment or during data collection.
Physical activity and sedentary behavior
At the first visit, participants were instructed in wearing an accelerometer for 24 hours per day for 4 weeks without exceptions. All participants were encouraged to continue an unchanged PA level. The activity monitors used in the present study were SENS Motion®, a 3-axis accelerometer placed on the lower part of either thigh. The sensor holds an internal storage device and the participant’s smartphone automatically transfers the data from the device to the cloud. SENS Motion® has been tested and validated as a reliable monitor for physical activity and sedentary behavior [Citation24].
Only data from participants with at least 7 whole days of activity measurement were used in the analyses. All activity data were examined through a visual day-by-day histogram tool with particular focus on 1) a normal sleeping pattern between 5 and 10 hours per day where the night-time sleep occurred between 10 PM and 10AM for most days of the week, and 2) most days having a structure compatible with an everyday life (i.e. with higher activity intensity in the morning, around midday and late afternoon).
Only completed whole day measurements (from midnight to midnight) were included. Any days (midnight to midnight) with more than 15 minutes of ‘no data’, either because the monitor was dismounted or because of technical errors, were excluded from the analyses.
Sedentary behavior was defined as the total number of minutes spent lying, reclining, or sitting, including sleep, divided by number of minutes over the 4 weeks and expressed as percentage. Sedentary behavior measured via accelerometer is more accurate and reliable compared to self-measured or self-reported of sedentary behavior [Citation25].
Steps per day were estimated and counted via a built-in algorithm in the SENS Motion accelerometer. The total number of steps was divided by the total number of whole days.
Physical capacity
All participants underwent spirometry including reversibility less than 1 h prior to CPET testing, thereby preventing bronchospasm during testing. Cardiopulmonary fitness was evaluated on an ergometer bike (Monark 739E, Varberg, Sweden) using gold standard CPET testing in accordance with the American Thoracic Society’s (ATS) guidelines [Citation26,Citation27]. The test was designed for patients to reach the maximum oxygen uptake between 8 and 12 minutes from start. The test started at an intensity of 70 watt for 2 minutes and thereafter continuously increased in resistance with steps between 10 and 20 watts until exhaustion and R-value >1. Oxygen uptake was simultaneously recorded breath-by-breath with a gas analyzer system (Cosmed Quark CPET, Rome, Italy, in case of breakdown Vyaire Vyntus CPX, Höchberg, Germany). VO2max was calculated as the highest oxygen consumption divided by the body weight.
Questionnaires
Level of asthma control was assessed with the GINA level of asthma symptom control 4-item yes/no questionnaire [Citation1], the validated 6-item version of the Asthma Control Questionnaire (ACQ6) [Citation28] and the validated 15-item Mini Asthma Quality of Life Questionnaire (MiniAQLQ) [Citation29]. ACQ responses are given on a 7-point scale and the overall score is the mean of the six items ranging from 0 for totally controlled to 6 for severely uncontrolled. The MiniAQLQ responses are also given on a 7-point scale but the questionnaire has a total of 15 questions and the overall score is mean ranging from 1 for the worst to 7 for the best answer. Exacerbations were assessed by interviewers questioning patients and were defined as asthma flare-up needing systemic corticosteroids, either through GP or in hospital. Health‐related quality of life was assessed using the validated Short Form‐12 (SF-12) that generates information in eight domains: physical health, physical role activities, bodily pain, general health perceptions, vitality, social function, emotional role activities, and mental health [Citation30]. Answers are transformed to a scale from 0 (worst score) to 100 (best score) for both physical health and mental health.
Comorbidity with chronic rhinosinusitis (CRS) was assessed with the validated Sino-Nasal Outcome Test 22 (SNOT-22). SNOT-22 responses are given on a 6-point scale and the overall score is the sum of the 22 items ranging from 0 for the best to 110 for the worst [Citation31].
Specific use of medication and treatment compliance was assessed through interviewing participants about both reliever medication and regular medication, cross-checked with the current medication in the national digital Danish medication database. Compliance for regular medication was scored in three categories: 1) medication used more than 80% of time 2) medication used between 50% and 80% of time or 3) medication used less than 50% of time.
Blood testing
Blood samples, including hemoglobin concentration (Hgb), C-reactive protein (CRP), immunoglobulin E (IgE), and eosinophil count (EOS, 109/L), were drawn by venipuncture prior to exercise testing. Blood eosinophil counts were obtained from full blood cell counts. All blood analyses were performed at hospital laboratories in the Capital Region. Blood samples taken at local hospitals up to 3 months prior to enrollment were used as substitute for missing blood samples.
Pulmonary testing
Airway inflammation was assessed by measuring FeNO on NIOX VERO (Circassia, Morrisville, USA). FeNO was measured once at each visit following the European Respiratory Society (ERS)/ATS recommendations [Citation32].
Spirometry was undertaken before and 15 minutes after inhalation of 5 puffs of 200 mg salbutamol on Vyaire Vyntus Spiro (Höchberg, Germany) with forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and bronchodilator reversibility test measured according to the guidelines by ERS and ATS [Citation33]. Predicted normal values based on sex, height, and age were calculated from the National Health and Nutrition Examination Survey reference values [Citation34].
Peak flow (PEF) was used to assess variation in lung function. Diurnal peak flow variability was calculated from two sets of daily peak flow readings for 4 consecutive weeks, three in the morning and three in the evening. The difference between the highest and the lowest values was divided by their mean – done according to GINA guidelines [Citation1].
Statistical analyses
All analyses were performed in IBM SPSS Statistics (Version 25) and SAS (Version 9.4). The continuous data were summarised by the mean and standard deviation (SD), the categorical data by numbers and percentages.
The primary analysis consisted of analysis of variance between patients with mild-moderate disease (GINA1-3) and patients with severe disease (GINA4-5) at both Week 0 and Week 4. The Week 4 confirmatory analysis was done as a sensitivity analysis to strengthen the results, confirm findings, and reduce the risk of type 1 errors.
The major outcomes were sedentary percentage, steps per day, and VO2max. PA data were included in only the Week 0 analysis since the data were collected continuously between Week 0 and 4. Secondary outcomes eosinophil count, immunoglobin E, and diurnal PEF variability were all measured once and, consequently, included in only the Week 0 analysis.
Group differences for both primary and secondary outcomes were analysed using general linear models with a factor GINA group (two levels). The analyses were done for week 0 and week 4 separately to confirm any statistically significant findings. From these models, unpaired point estimates (least squares means) for each GINA group were extracted together with two-sided 95% confidence intervals and p-values at both Week 0 and Week 4. To account for possible confounding from age and body mass index (BMI), the analyses were repeated with adjustment for age and BMI.
The secondary analysis was done using bivariate correlation analyses (Pearson’s r) to assess the association between all major and secondary outcomes. Analyses were done on Week 0 data, and any statistically significant correlations were repeated using the Week 4 data set, thus highlighting correlations at both Week 0 and Week 4 and strengthening the secondary analysis.
Statistical significance was considered for p-values below 0.05. All p values and 95% confidence intervals are double-sided.
Results
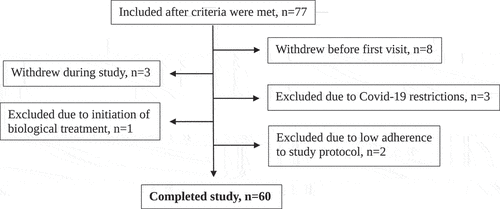
A total of 77 patients were initially included, of whom 3 were excluded due to the COVID-19 restrictions, one was excluded due to initiating biologic treatment and 13 failed to complete the study program for various other reasons ().
Thus, 60 participants with asthma: 27 with mild-moderate disease (GINA step 1 through 3) and 33 with severe disease (GINA step 4 and 5) had complete data and were included in the analyses. Demographic and clinical characteristics at both visits are presented in .
Table 2. Demographic characteristics of all participants.
There was no difference in activity data collecting time between patients with mild-moderate asthma and patients with severe asthma (24.3 days vs. 24.5 days, respectively, p = 0.005).
Association between asthma severity and physical capacity, physical activity, or sedentary behavior
No statistically significant difference was found between patients with mild-moderate asthma and those with severe asthma for level of cardiopulmonary fitnees measured as Vo2Max, sedentary behavior and steps per day ().
Table 3. Comparison of outcomes between GINA1-3 and GINA4-5 at Week 0 and Week 4 adjusted for age and BMI. There is only one comparison of sedentary percentage, steps per day and diurnal PEF variability as this is an average of 4 weeks (from Week 0 to Week 4).
Association between asthma severity and supportive outcomes
A tendency toward a lower level of lung function (FEV1% predicted) was found amongst those with severe asthma when compared with those with mild-moderate asthma at the first visit at Week 0 (99% vs. 107%, respectively, p = 0.17), as well as at the second visit at Week 4 (101% vs. 110%, respectively, p = 0.1) ().
All other associations are presented in .
Cross-related correlations between all outcomes
Allcorrelations between outcomes at the first visit at Week 0 and, if present, a confirmatory correlation at the second visit at Week 4, are shown in .
Table 4. Correlation matrix showing Pearson’s correlation coefficients between outcomes. Lower left half represents correlations between outcomes at week 0. Upper right half (grey shade) represents correlations between outcomes at week 4 that are statistically significant at both week 0 and 4.
A statistically significantly negative correlation was found between VO2max and FeNO (r = −0.30, p < 0.05) along with ACQ6 score (r = −0.35, p < 0.05), and a positive correlation was found between VO2max and miniAQLQ score (r = 0.36, p < 0.05) along with SF-12 Mental Health score (r = 0.37, p < 0.05) (). A negative association was found between percentage of sedentary time and the level of FeNO (r = −0.26, p < 0.05, ), while SF-12 Physical Health score was positively correlated with level of FeNO (r = 0.32, p < 0.05) ().
All other cross-related correlations are presented in .
Discussion
This study demonstrated no difference in sedentary behavior, steps per day or level of cardiopulmonary fitness between patients with severe asthma and those with mild-moderate asthma. Further, we demonstrated that those with the highest VO2max had the best quality of life (scoring better on MiniAQLQ, SF-12 Mental Health, and ACQ) and the lowest level of airway inflammation (FeNO).
We found that the patients with severe asthma reported slightly more asthma symptoms, even with an acceptable level of lung function and level of quality of life. On inclusion, participants were instructed to sustain their level of day-to-day exercise and to continue unchanged with any type of activity during the study period. On this background and considering the present study showed stable VO2max between the two visits, our findings indicate that this level of cardiopulmonary fitness and sedentary behavior could be generalizable to a larger population of patients with asthma. Surprisingly, neither steps per day or sedentary behavior correlated with VO2max, which may be because the accelerometer algorithm did not capture vigorous activity such as physical exercise training or biking.
The difference in daily steps between those with mild-moderate asthma and those with severe asthma has previously been described by researchers only once [Citation7], as has sedentary behavior among patients with asthma [Citation6]. The previous study on sedentary behavior excluded sleep from sedentary behavior and is therefore not comparable to our study.
To the best of our knowledge, this is the first study to examine the difference in sedentary behavior between patients with severe asthma and mild-moderate asthma.
Our findings are contrary to the findings of a similar study in 2017 by Bahmer et al., which concluded that patients with severe asthma took 21% fewer steps per day [Citation7]. The devices used for measuring steps differ and therefore a different algorithm was used; accordingly, although the exact numbers are not necessarily comparable, the difference between groups can be compared. It is worth noting that the characteristics of the participants differ between the two studies. For example, the listed lung function (FEV1) and level of obesity (BMI) in the group with severe asthma in the study by Bahmer et al. were 73.3% predicted and 28.2 kg/m2, respectively [Citation7], compared with FEV1 predicted at 99.3% and a BMI of 25.9 kg/m2, respectively, in the current study. Mild-moderate disease also varies with a FEV1 at 88.7% in Bahmer et al.’s study compared with a FEV1 at 107.6% in the present study. Bahmer et al. has a sample with a surprisingly high percentage of smokers: 22% and 24% for patients with mild-moderate asthma and severe asthma, respectively. Taken together, this indicates the data are extracted for two different groups of asthma patients, making direct comparison difficult and calling for further research on the matter.
A meta-analysis by Cordora-Rivera et al. in 2018 calculated a standardized mean on steps per day in patients with asthma from seven different studies – with an overall average of 8390 (SD 1029) steps per. day [Citation3]. Our findings for, respectively, mild-moderate and severe asthma were higher at 9888 (SD 641) and 9287 (SD 572). The averages in the different studies mentioned range from 5983 to 11,125 steps per day, indicating that our Danish asthma population performs a number of steps within this range, albeit in the upper end. Overall, the number of steps found in this study is comparable to that in international studies.
Apart from the correlations between our major outcomes, we also examined other measurements. The cardiopulmonary fitness level is correlated with the ACQ6 score, indicating that those with uncontrolled asthma had the lowest level of fitness score and probably also the lowest level of activity. These findings confirm that exercise training is associated with better asthma control, which is supported by former research in the field [Citation4,Citation5,Citation15,Citation17,Citation35,Citation36].
The cardiopulmonary fitness level also correlates with airway inflammation (FeNO), which is highly interesting and could be a ‘chicken or the egg’ dilemma. Studies from Brazil on the effect of exercise training on airway inflammation showed that exercise training inhibited the house-dust-mite-induced asthma phenotype [Citation37]. In contrast, a recent meta-analysis concluded that exercise training had no effect on airway inflammation among patients with asthma [Citation4]. In our study, airway inflammation, as indicated by level of FeNO, was negatively correlated with the level of fitness and sedentary behavior (although not correlated with steps per day), and positively correlated with blood eosinophil count and the SF-12 Physical Health score, thus supporting a possible association between low levels of airway inflammation and a high level of physical activity. The association between eosinophilic count and airway inflammation is well understood, but the findings presented here suggest that increased airway inflammation is also associated with a lower level of physical health in general and a higher level of sedentary behavior, thus leading to another ‘chicken or egg’ dilemma: what comes first, inactivity or inflammation? It is known that elite athletes, especially those performing endurance sport [Citation35,Citation38,Citation39], develop asthma during their active elite period, indicating the exercise training might paradoxically induce asthma if it is undertaken too vigorously.
Lastly, the MiniAQLQ and the SF-12 Mental Health score was associated with a reduced fitness score, leading us to conclude that good cardiopulmonary fitness not only improves the level of asthma control and airway inflammation but also the quality of life and mental health status. The association with mental health and health-related quality of life and the association with exercise training is well known [Citation40]. This helps to validate our methods and adds support to what is already known on the subject. Nonetheless, the association with airway inflammation is a relatively under-research subject and studies, including ours, call for more investigation into the matter [Citation41,Citation42]. A recent meta-analysis concluded that, based on the current research, there is no apparent relation between the two [Citation4].
When compared with other similar studies, we believe that some of the strengths of this study are the following: 1) long wearing time of the PA measuring device, 2) a low tolerance for non-adherence of the device with 15 minutes of no-data or non-wearing time per 24 hours allowed, giving an only 1% error margin, and 3) the confirmatory analysis with both major outcomes and the cross-correlated matrix enhance validity and accuracy.
Especially, the amount of activity data as a result of the long wearing time really elevates the validity compared to similar studies, as in our study the mean collecting time for activity data was 24.4 days. Each individual week for one person is different and activity levels can vary greatly, and therefore long period of collecting data was essential for us. Of the six comparable studies, five studies measured activity for 7 days straight and the last one 14 days (but excluded sleeping and non-wear time) [Citation3].
We also experienced some limitations. As this study was done in the fall of 2019 and the spring of 2020, it was cut short due to the global Covid-19 pandemic. This resulted in the inclusion of fewer participants in both groups. Although we originally aimed for considerably more participants, after the analyses, this seemingly does not affect the results. Also, the many statistical tests may increase the risk of type 1 errors. However, as we repeat the analyses at both week 0 and 4, the risk is somewhat reduced as we replicate the findings immediately. Nevertheless, our results should be interpreted as exploratory and need replication in independent cohorts.
Finally, the participants were very adherent to their asthma medication, which is both a strength and a limitation. It limits the study results as the participants are not completely representative of the general asthma population. On the other hand, the results are strengthened as the study participants were adherent when entering the study and thus less likely to optimize adherence during the observation-period, which might have biased the results.
Conclusions
Our results found no significant differences in sedentary behavior, steps per day or level of fitness between patients with severe asthma and those with mild-moderate asthma. Those with the highest VO2max were found to have the lowest level of airway inflammation and the best quality of life.
Airway inflammation (FeNO) was positively correlated with sedentary time but, interestingly, not with steps per day. Considering this and that this study is the first to compare sedentary behavior between asthma disease severity groups, these results underline the need for more research on the association between sedentary behavior and airway inflammation independent of physical activity.
Authors’ contributions
MH and VB were major contributors to the protocol and the statistical analysis plan. LF was a major contributor in collecting data, the rest of the data collecting and all of the manuscript is written by NBH. All authors read, made useful comments and approved the final manuscript.
Consent for publication
This manuscript does not contain any individual person’s data in any form.
Ethics approval and consent to participate
All potential participants are informed, both orally and in writing, about the purpose of this trial, its process and potential risks, as well as costs and benefits of participation. All participants are informed of their rights to withdraw from the study at any time without this impacting on any future investigations. After the information is delivered, read and understood, voluntary informed consent is given by the participant by signing a consent form.
The study was approved by the regional Health Research Ethics Committee of Copenhagen, Denmark (H-19018443).
Acknowledgments
The authors will like to acknowledge professor Bente Klarlund for her great help by providing office space, physical exercise rooms and much more when help was much needed.
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Nikolaj Brix Hansen
Nikolaj Brix Hansen is MD based in Aalborg, Denmark. He currently is in residency training to become a pulmonologist. The other authors are based in and around Copenhagen, and have all being conducting research in physiology and asthma for a long time - for most of the authors decades.
References
- Reddel HK, FitzGerald JM, Bateman ED, et al. GINA 2019: a fundamental change in asthma management. Eur Respir J. [Internet]. 2019 Jun 1;53(6):1901046. http://erj.ersjournals.com/content/53/6/1901046.abstract
- Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. C Can Med Assoc J = J l’Association medicale Can. 2006 Mar;174(6): 801–12.
- Cordova-Rivera L, Gibson PG, Gardiner PA, et al. A systematic review of associations of physical activity and sedentary time with asthma outcomes. J Allergy Clin Immunol Pract. 2018;6(6):1968–1981.e2.
- Hansen ESH, Pitzner-Fabricius A, Toennesen LL, et al. Effect of aerobic exercise training on asthma in adults: a systematic review and meta-analysis. Eur Respir J. [Internet]. 2020 Jul 1;56(1):2000146. http://erj.ersjournals.com/content/56/1/2000146.abstract
- Disabella V, Sherman C. Exercise for asthma patients: little risk, big rewards. Phys Sportsmed. 1998 Jun;26(6):75–84.
- Cordova-Rivera L, Gibson PG, Gardiner PA, et al. Physical activity and exercise capacity in severe asthma: key clinical associations. J Allergy Clin Immunol Pract. 2018;6(3):814–822.
- Bahmer T, Waschki B, Schatz F, et al. Physical activity, airway resistance and small airway dysfunction in severe asthma. Eur Respir J Engl. 2017;49.
- Mendes FAR, Gonçalves RC, Nunes MPT, et al. Effects of aerobic training on psychosocial morbidity and symptoms in patients with asthma: a randomized clinical trial. Chest. 2010 Aug;138(2):331–337.
- Westergren T, Berntsen S, Ludvigsen MS, et al. Relationship between physical activity level and psychosocial and socioeconomic factors and issues in children and adolescents with asthma: a scoping review. JBI Database Syst Rev Implement Rep. 2017 Aug;15(8):2182–2222.
- França-Pinto A, Mendes FAR, de Carvalho-Pinto RM, et al. Aerobic training decreases bronchial hyperresponsiveness and systemic inflammation in patients with moderate or severe asthma: a randomised controlled trial. Thorax. 2015 Aug;70(8):732–739.
- Eichenberger PA, Diener SN, Kofmehl R, et al. Effects of exercise training on airway hyperreactivity in asthma: a systematic review and meta-analysis. Sports Med. 2013 Nov;43(11):1157–1170.
- Rebar AL, Duncan MJ, Short C, et al. Differences in health-related quality of life between three clusters of physical activity, sitting time, depression, anxiety, and stress. BMC Public Health. 2014 Oct;14:1088.
- Rhodes RE, Mark RS, Temmel CP. Adult sedentary behavior: a systematic review. Am J Prev Med. 2012 Mar;42(3):e3–28.
- Park JH, Moon JH, Kim HJ, et al. Sedentary lifestyle: overview of updated evidence of potential health risks. Korean J Fam Med. 2020 Nov;41(6):365–373.
- Carson KV, Chandratilleke MG, Picot J, et al. Physical training for asthma. Cochrane Database Syst Rev. 2013. Sep;(9):CD001116. DOI:10.1002/14651858.CD001116.pub4.
- Aggarwal B, Mulgirigama A, Berend N. Exercise-induced bronchoconstriction: prevalence, pathophysiology, patient impact, diagnosis and management. NPJ Prim Care Respir Med. 2018 Aug;28(1):31.
- Jaakkola MS, Aalto SAM, Hyrkäs-Palmu H, et al. Association between regular exercise and asthma control among adults: the population-based northern Finnish asthma study. PLoS One. 2020;15(1):e0227983.
- Lewthwaite H, Effing TW, Olds T, et al. Physical activity, sedentary behaviour and sleep in COPD guidelines: a systematic review. Chron Respir Dis. 2017 Aug;14(3):231–244.
- Spruit MA, Pitta F, McAuley E, et al. Pulmonary rehabilitation and physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015 Oct;192(8):924–933.
- Watz H, Waschki B, Meyer T, et al. Physical activity in patients with COPD. Eur Respir J. 2009 Feb;33(2):262–272.
- Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015 Dec;25(Suppl 3):1–72.
- Phillips SM, Cadmus-Bertram L, Rosenberg D, et al. Wearable technology and physical activity in chronic disease: opportunities and challenges. Am J Prev Med. 2018 Jan;54(1):144–150.
- Dobkin BH, Martinez C. Wearable sensors to monitor, enable feedback, and measure outcomes of activity and practice. Curr Neurol Neurosci Rep. 2018 Oct;18(12):87.
- Bartholdy C, Gudbergsen H, and Bliddal H, et al. Reliability and construct validity of the sens motion® activity measurement system as a tool to detect sedentary behaviour in patients with knee osteoarthritis. In: Malemud CJ, editor. Arthritis Mar 2018 (Bethesda, Maryland: PMC) 2018. DOI:.
- Healy GN, Clark BK, Winkler EAH, et al. Measurement of adults’ sedentary time in population-based studies. Am J Prev Med. 2011 Aug;41(2):216–227.
- Ross RM. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003 Jan;167(2):211–277.
- Palange P, Ward SA, Carlsen K-H, et al. Recommendations on the use of exercise testing in clinical practice. Eur Respir J. 2007 Jan;29(1):185–209.
- Juniper EF, O’Byrne PM, Guyatt GH, et al. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999 Oct;14(4):902–907.
- Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the mini asthma quality of life questionnaire. Eur Respir J. 1999 Jul;14(1):32–38.
- Ware JJ, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996 Mar;34(3):220–233.
- Fokkens WJ, Lund VJ, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012 Mar;23:3. preceding table of contents, 1–298
- Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011 Sep;184(5):602–615.
- Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019Oct; 200(8): e70–88.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999 Jan;159(1):179–187.
- Côté A, Turmel J, Boulet L-P. Exercise and asthma. Semin Respir Crit Care Med. 2018 Feb;39(1):19–28.
- Wu X, Gao S, Lian Y. Effects of continuous aerobic exercise on lung function and quality of life with asthma: a systematic review and meta-analysis. J Thorac Dis. 2020 Sep;12(9):4781–4795.
- Almeida-Oliveira AR, Aquino-Junior J, Abbasi A, et al. Effects of aerobic exercise on molecular aspects of asthma: involvement of SOCS-JAK-STAT. Exerc Immunol Rev. 2019;25:50–62.
- Langdeau JB, Turcotte H, Bowie DM, et al. Airway hyperresponsiveness in elite athletes. Am J Respir Crit Care Med. 2000 May;161(5):1479–1484.
- Vergès S, Devouassoux G, Flore P, et al. Bronchial hyperresponsiveness, airway inflammation, and airflow limitation in endurance athletes. Chest. 2005 Jun;127(6):1935–1941.
- Bize R, Johnson JA, Plotnikoff RC. Physical activity level and health-related quality of life in the general adult population: a systematic review. Prev Med. 2007 Dec;45(6):401–415.
- Silva RA, Almeida FM, Olivo CR, et al. Exercise reverses OVA-induced inhibition of glucocorticoid receptor and increases anti-inflammatory cytokines in asthma. Scand J Med Sci Sports. 2016 Jan;26(1):82–92.
- Scott HA, Latham JR, Callister R, et al. Acute exercise is associated with reduced exhaled nitric oxide in physically inactive adults with asthma. Ann allergy, asthma Immunol Off Publ Am Coll Allergy, Asthma, Immunol. 2015 Jun;114(6):470–479.