ABSTRACT
Background: Invasive pneumococcal disease (IPD) is a major cause of morbidity and mortality globally. However, the literature on the vaccine effectiveness (VE) of 23-valent polysaccharide vaccine (PPV23) and 13-valent conjugated vaccine (PCV13) against IPD in adults is sparse. The aim was to summarize the available evidence on the VE of the PPV23 and the PCV13 in elderly individuals against IPD and to investigate how age and comorbidities influence VE against IPD. Methods: A systematic search was conducted in Medline and Embase in February 2021. We used combinations of terms related to PPV23, PCV13, elderly, high-risk populations, and IPD. Eligible articles published since 2010 were included. Two authors reviewed and extracted data. Results: Eight studies met the inclusion criteria for PPV23. The meta-analysis showed a reduced OR for all-type IPD with the use of PPV23 vaccine compared with unvaccinated controls (OR 0.69; 95%CI 0.54, 0.88) and a reduced OR for vaccine-type IPD compared with non-vaccine type IPD (0.69; 95%CI 0.63, 0.76). VE against vaccine-type IPD ranged from 28% to 54.1% for individuals aged 65–79 and from 7.5% to 34% for those aged ≥80–85 years. Most studies found a lower VE of PPV23 in populations with comorbidities and in immunocompromised populations compared with the VE for individuals without comorbidities.One study met the inclusion criteria for PCV13. The vaccine efficacy of PCV13 against IPD in individuals aged ≥65 was 75.0% (95% CI, 41.4 to 90.8).
Conclusion: The results from this review show a reduction of IPD in elderly and high-risk populations vaccinated with PPV23 and PCV13. The protective effect may be lower in elderly individuals aged >80 and in individuals with comorbidities. However, the literature is sparse; large-scale prospective studies are required to evaluate the VE of PPV23 and PCV13 vaccination in adults against IPD.
Introduction
Streptococcus pneumoniae (S. pneumoniae) are gram-positive bacteria with almost 100 serotypes with different polysaccharide capsules. Pneumococcal infections are a major global cause of morbidity and mortality [Citation1]. Asymptomatic carriers are most prevalent among children since S. pneumoniae are a part of the normal microbiota in the upper respiratory tract [Citation2]. The clinical presentation of pneumococcal infection varies from mild presentations (e.g. otitis media and sinusitis) to severe diseases, such as pneumonia, meningitis, or sepsis [Citation2–4]. Pneumococcal infection is a frequent cause of pneumonia in adults, second only to the increase in Haemophilus influenzae cases in a Scandinavian setting [Citation5–7].
Invasive pneumococcal disease (IPD) is defined as an infection with detection of S. pneumoniae in a normally sterile site of the body (e.g. blood or cerebrospinal fluid). The incidence of IPD is highest among infants younger than two years of age, elderly individuals, and individuals with underlying comorbidities [Citation8]. The incidences of IPD in Europe range from 0.2 to 16.0 per 100,000 citizens per year. The highest incidence is seen in the Nordic countries, the Netherlands, and Ireland, while the lowest incidence occurs in southern and eastern European countries [Citation9].
Pneumococcal vaccines have been developed to protect against serious pneumococcal infections [Citation3,Citation4]. The 23-valent polysaccharide pneumococcal vaccine (PPV23) and the 13-valent conjugated vaccine (PCV13) are the most commonly used vaccines worldwide. PPV23 includes 23 serotypes (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9 V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F) and PCV13includes 13 serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9 V, 14, 18C, 19A, 19F, and 23F). PCV13 stimulates T-cell response and induces formation of memory B-lymphocytes, thus providing long-lasting immunological memory and anamnestic response [Citation10,Citation11]. This can provide increased protection for people with decreased immune response and for infants under two years of age [Citation12,Citation13]. PPV23 induces antibodies, primarily by T-cell-independent mechanisms, which is a short-lasting response that is not anamnestic. The antibody response to PCV23 is poor in infants under two years of age because of an immature immune system [Citation14].
Pneumococcal vaccination recommendations are constantly evolving. The PPV23 has been available in Scandinavia since the 1980s and a 7-valent conjugated pneumococcal vaccine (PCV7) was introduced into the Danish vaccination program for children in 2007 [Citation12,Citation15]. A 13-valent vaccine is now offered to all infants under two years of age and as a result the annual incidence of IPD has decreased significantly in infants under two years of age [Citation16]. In addition to the direct protection against IPD in children, the introduction of PCV7 and PCV13 into the children vaccination program has resulted in herd protection against IPD in the adult population. This has led to an overall reduction of IPD across the world [Citation17–20]. The annual incidence of IPD in Denmark for individuals aged ≥65 years decreased from 66 to 36 per 100,000 between 2007 and 2018 [Citation16]. Particularly IPD caused by PCV13 serotypes has decreased markedly in recent years whereas pneumococcal serotypes, which are not covered by the PCV13, are becoming more prevalent causes of IPD. For instance, serotype 9N, which is covered by PPV23 and not PCV13, accounted for 10% of IPD cases in Norway in 2020–2021 [Citation13]. Overall, PPV23 vaccine serotypes are found in approximately 40–60% of all IPD cases in adults [Citation15].
Pneumococcal vaccination programs for adults are widely used in multiple countries [Citation13,Citation21–25]. PPV23 vaccination is recommended for citizens <65 years with increased risk of severe pneumococcal disease due to comorbidities and for all citizens ≥65 years in the Nordic countries [Citation13,Citation23]. PCV13 is generally recommended for individuals in particular high risk of IPD (e.g. asplenic and immunosuppressed individuals) [Citation12,Citation13,Citation26]. However, the evidence for the vaccine effectiveness (VE) of PPV23 and PCV13 against IPD in adults is limited [Citation27]. The present review aims to review and summarize in a meta-analysis the available evidence on the VE of PPV23 and PCV13 against IPD in adults and to investigate how age and comorbidities influence the VE against IPD.
Method
Inclusion and exclusion criteria
Original observational case-control studies, cross-sectional studies, cohort studies and clinical trials investigating the vaccine effectiveness or efficacy of PPV23 and PCV13 against IPD were eligible for inclusion. The primary outcome of IPD was defined as either vaccine-type IPD (VT-IPD: i.e. IPD caused by serotypes covered by PPV23 or PCV13) or all-cause IPD (i.e. IPD without specification of serotypes). The population included adults aged 60 years or above and adults above the age of 18 years with medical conditions predisposing to IPD. Studies published between January 2010 and January 2021 were included. Reviews and case reports were excluded. Articles in languages other than English, Danish, Norwegian, and Swedish were excluded, as were abstracts where the article could not be extracted in full version.
Search strategy
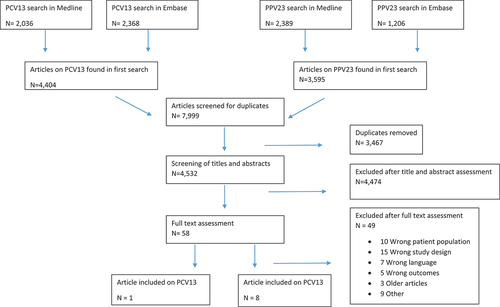
We used the PICO method to build our search string [Citation28]. The population of interest was elderly and high-risk populations. The intervention was PPV23 and PCV13and the outcome was IPD. The final search strategy was developed with the help of a science librarian, and two separate literature searches for PCV13 and PPV23 were conducted in February 2021 in MEDLINE and Embase (Table S1). Titles and abstracts were screened by two authors (MSW and SSS). The articles eligible for full-text review were then reviewed two authors and included based on fulfilment of the inclusion and exclusion criteria ().
Supplementary searches in Google Scholar for literature published outside the two databases (i.e. grey literature) were conducted from March to April 2021. In addition, references of relevant articles were explored, from which further abstracts and articles were reviewed.
Data extraction
Data extraction was performed by MSW and SSS with input from MGS, AAP, and AL and charted in predefined tables. VE or vaccine efficacy were reported either as the percent reduction in IPD in cases compared with controls or as the odds ratio (OR) for IPD in cases compared with controls [Citation29]. The VE was reported as either vaccine-type IPD (i.e. IPD caused by serotypes covered by PPV23) or all-cause IPD (i.e. IPD without specification of serotypes). The quality of the studies was assessed using the Newcastle – Ottawa Scale for cases-control and cohort studies [Citation30]. The grading criteria are listed in Table S2.
Meta-analysis
A meta-analysis was conducted on the included studies on PPV23 stratified by their study design using the random effects model. Information on the number of cases, controls, and their vaccination status were extracted from the studies for all patients older than 60 years. Adjusted numbers were used when available. Studies were grouped according to study design to mitigate potential heterogeneity resulting from differences in potential selection bias, recall bias, and confounder adjustments. Case-control studies were grouped into studies evaluating all-type IPD and studies using the Broome methods (i.e. indirect cohort studies). The Broome methods compares VT-IPD with non-vaccine type IPD (NVT-IPD) [Citation31].
Results
Characteristics of PPV23 studies
Eight studies met the inclusion criteria for PPV23. Seven studies were case-control studies [Citation3,Citation8,Citation32–36], and one was a cohort study [Citation37]. No randomized clinical trials were identified. All eight studies were evaluated as being of high quality on the Newcastle – Ottawa Scale. An overview of study characteristics can be seen in .
Table 1. Characteristics of PPV23 and PCV13 studies.
Six studies reported the VE of PPV23 against IPD as the percent reduction of IPD in cases compared with controls and two studies reported the OR of IPD in cases compared with controls [Citation33,Citation37]. Three of the eight studies used stratified analysis to assess VE by time since vaccination [Citation3,Citation8,Citation35], while others included patients who were vaccinated <1 year [Citation37] or <5 years [Citation32,Citation33,Citation36] before IPD diagnosis. One study defined the patients as vaccinated if they had received a vaccination at any time before the IPD diagnosis [Citation34].
The diagnostic criteria for IPD varied between studies. The majority confirmed IPD by presence of S. pneumoniae in a normally sterile site [Citation3,Citation32,Citation34–36]. One study used ICD-9 diagnosis codes from hospital discharge or out-patient follow-up [Citation33], one study used ‘cause of death’ registry and the ‘IPD notification’ database maintained by the Ministry of Health and Welfare in Taiwan [Citation37], and one study reported that the diagnosis was laboratory confirmed without further specification [Citation8].
VE of PPV23 against all-type IPD
Three studies reported the VE against all-type IPD [Citation32,Citation33,Citation37]. Gutierrez Rodriguez et al. reported VE results for both VT-IPD and all-type IPD. Thus, the results on all-type IPD were omitted from the meta-analysis to ensure that no duplicates of cases or controls were included in the meta-analysis [Citation34].
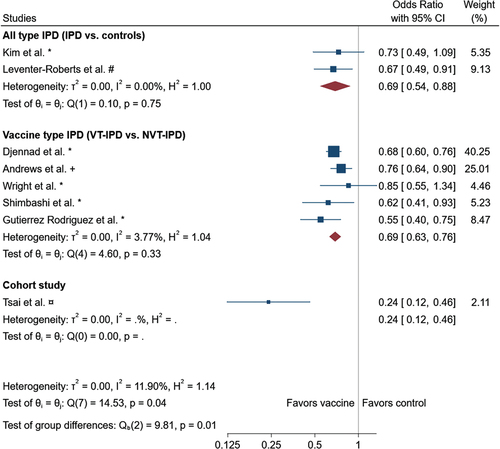
Kim et al. investigated the overall VE of PPV23 against IPD in adults ≥65 years of age who were vaccinated <5 years before being diagnosed with IPD, and found a VE of PPV23 against all-type IPD of 28.5% (95% CI −5.8 to 51.6) [Citation32]. Leventer-Roberts et al. found an OR for all-type IPD of 0.58 (0.41–0.8, p < 0.01) in adults ≥65 years of age [Citation33]. In the meta-analysis, we found a reduced OR for all-type IPD with the use of PPV23 vaccine: OR 0.69 (95%CI 0.54, 0.88) compared with unvaccinated controls ().
Figure 2. Forest plot of the meta analysis. Adjusted data was used when available. * : unadjusted/crude, #: age, sex and risk matched controls, +: age matched. The meta-analysis performed using the random-effects-model with subgroups according to study design. Combined or for the subgroups are indicated by the diamond.
VE of PPV23 against vaccine-type IPD
Five studies investigated the VE against VT-IPD compared with NVT-IPD which ranged from 24–78% [Citation3,Citation8,Citation34–36]. We found a reduced OR for VT-IPD compared with NVT-IPD in the meta-analysis of 0.69 (0.63, 0.76) (Figure 3). Notably, the total I2 of 11.90% combined for all studies suggests low heterogeneity.
Differences in VE by age
A VE of the PPV23 vaccine against IPD or VT-IPD was found in all studies with age-stratified analysis of healthy adults aged 60 to 79 years [Citation3,Citation8,Citation32–35]. Increasing age at the onset of the IPD was associated with a decrease in vaccine protection in most studies [Citation3,Citation32–35]. Kim et al. reported a significant protection against all-type IPD in adults 65–75 years of age with an adjusted VE of 57.4% (95% CI 19.4–77.5). Adults with ages ≥75 years had an adjusted VE of 6.7% (95% CI −73.9 to 49.9) and thus showed no significant protection [Citation32]. Leventer et al. [Citation33] found a protective VE of PPV23 against all-type IPD (OR = 0.54; 95% CI 0.32–0.90; P = 0.02) in younger cases (aged 65–74). However, the protectiveness was not significant among individuals aged 75 years or older (OR 0.80; 95% CI 0.53–1.22).
VE against VT-IPD ranged from 28 to 54.1% for ages 65–79 and from 7.5 to 34% in those aged ≥80–85 years [Citation3,Citation8,Citation34,Citation35].
Differences in VE by levels of comorbidities
Stratified analyses on the VE of PPV23 in subgroups with risk factors are presented in [Citation3,Citation8,Citation33–36]. The study participants were defined in groups as ‘no known risk factors’, ‘high-risk immunocompetent’, and ‘immunocompromised’, depending on their comorbidity status. In all five studies evaluating VT-IPD the VE was generally lower for the ‘high-risk immunocompetent’ and ‘immunocompromised’ groups than the ‘no known risk factors’ groups, and statistical significance was not reached in most of the estimates for the groups with risk factors and for the immunocompromised groups [Citation3,Citation8,Citation34–36].
Table 2. Vaccine effectiveness of PPV23 on IPD.
Two studies evaluated VE against all-type IPD by risk group. Djennad et al. found a VE of 45% (95% CI, 27–59) among those without any risk factors followed by a VE among the high-risk immunocompetent of 25% (95% CI, 11–37) and 13% (95% CI, −9 to 30) among the immunocompromised [Citation8]. Leventer-Roberts et al. [Citation33] evaluated subgroups of low-, moderate-, and high-risk groups. The moderate- and high-risk groups had a marginal protective VE of PPV23 against all-type IPD with an OR of 0.70 (95% CI 0.49–0.99; P = 0.05) while the low-risk group did not show a significant VE with an OR of 0.63 (95% CI 0.30–1.33; P = 0.23).
Vaccine efficacy of PCV13 against IPD
We identified one study reporting on the vaccine efficacy of PCV13 vaccination against IPD. The study was a randomized double-blinded placebo controlled study from The Netherlands (CAPITA) [Citation38]. Vaccine efficacy for IPD after PCV13 vaccine was investigated in approximately 85,000 individuals ≥65 years. Cases were randomly assigned to a PCV13 vaccination group or a placebo group. The mean follow-up period was 3.98 years. The prevalence of vaccine-type IPD was seven cases in the PCV13 vaccinated group and 28 cases in the placebo group. The vaccine efficacy was 75.0% (95% CI, 41.4 to 90.8) for PCV13-specific serotypes IPD [Citation38].
Discussion
We identified eight studies that investigated the VE of PPV23 and one study that investigated the clinical effect of PCV13 against IPD. Overall, the studies included in the review found that PPV23 and PCV13 reduce the risk of IPD in elderly vaccinated individuals but that VE against IPD decreased as age increased and was decreased in individuals with underlying diseases and immune deficiencies.
Ppv23
In our present review, we found a VE of PPV23 against IPD in adults ≥60 years of age but estimates of VE varied vastly between studies. All eight studies were observational studies, heterogeneous in their methodologies, and varied widely in their definitions and diagnostic criteria of IPD as quantified by the meta-analysis heterogeneity analysis.
Most studies evaluated hospitalized patients diagnosed with IPD. Thus, a lack of research was identified on the VE of PPV23 in cases where death or IPD occurred out of hospital. The time from vaccination to incidence of IPD also varied between studies. Most studies used time limitations of <5 years or <1 year since vaccination. Despite the association of waning protection 5 years after the vaccination [Citation3,Citation4,Citation8], one study counted the patients as vaccinated in the overall analysis if they had received a vaccination at any time before the IPD infection [Citation34].
The various settings in which the studies were conducted are likely to affect VE estimates. The studies were conducted in countries with diverse vaccination programs, and with differences regarding the prevalence of pneumococcal disease and healthcare systems. Child vaccination with PCV13 has been proven to have a positive indirect effect on elderly individuals because of herd protection [Citation39]. Thus, differences in vaccine programs and stances on vaccination in the respective countries may affect the level of VE observed in the studies.
Pcv13
The CAPITA study [Citation38] showed significant vaccine efficacy in preventing vaccine-type IPD for adults ≥65 years. A post-hoc analysis of the CAPITA study found that PCV13 vaccination remained protective during the whole five year study period and that no waning of vaccine efficacy was observed [Citation40]. The CAPITA study was a robust study with a large population group and long follow-up period. However, the study population was a homogenous group, given that the study was designed as a single-country study. Moreover, incident IPD was low because of the low incidence of pneumococcal disease in The Netherlands and a long-standing tradition of providing infant pneumococcal vaccination thus facilitating the indirect protective effect. This limits the generalizability of the study to other populations with different demographics, vaccination programs, and IPD prevalence. Given the study was a single-nation clinical trial, more research is required to support a VE of PCV13 in elderly individuals [Citation38].
Clinical implications
This review provides an updated summary of the evidence for the use of PPV23 and PCV13 in the elderly and high-risk populations. Pneumococcal vaccination recommendations for elderly and high-risk groups to prevent IPD have existed in several countries for decades, but the evidence for the use of these vaccines is not comprehensive. Berild et al. reviewed the literature published from 2016 to 2019 on the VE of PPV23 and PCV13 against IPD in elderly individuals. They included two studies on the VE of PPV23 against all-type IPD [Citation8,Citation32]. We included eight studies that investigated the VE of PPV23 against VT-IPD and/or all-cause IPD for meta-analysis. Of these eight studies only one study was published after 2019. Our review revealed that no new evidence of VE of PCV13 against IPD in adults has been published since the CAPITA study in 2015 except the previously mentioned post-hoc analysis from the CAPITA study.
No studies aimed at evaluating the VE of IPD in the very old age group. However, most studies evaluated in this review found that the VE of PPV23 against IPD decreases with age and the lowest VE against IPD was seen in the oldest age-strata (). Moreover, the VE of PPV23 against IPD decreased in individuals with comorbidity as well as in immunocompromised individuals. Moreover, research has found that antibody concentration after a booster dose of PPV23 is lower than after the first PPV23 administration (i.e. hyporesponsiveness), thus limiting the VE of PPV23 against IPD for repeated vaccinations [Citation41]. We found no studies investigating the VE against IPD of dual vaccination with PPV23 and PCV13. Studies on immunogenicity have found that elderly and high-risk groups previously vaccinated with PPV23 have a more robust immunogenic response when they received a booster of PCV13 compared to a booster of PPV23 [Citation42,Citation43]. Also, the immune response after PCV13 vaccination in elderly individuals >70 years has been found to be higher than the immune response after PPV23 vaccination [Citation42]. Since August 2022 a 20-valent conjugated vaccine (PCV20) has been recommended in Denmark for some high-risk groups in addition to PPV23, but revaccination with PCV20 is not recommended [Citation12].
It is recommended to administrate PPV13 one year before PPV23 in individuals with no prior PPV23 vaccination when both vaccines are indicated [Citation44]. This is in concordance with Rezai et al [Citation43]. who showed a better immune response in immunocompromised cases receiving PCV13 before PPV23 compared with cases receiving PPV23 before PCV13. This finding might be caused by the hyporesponsiveness of the immune system which seems to persist years after PPV23 vaccination [Citation41]. However, the correlation between the immunological response measured in these studies and VE against IPD is unknown. Thus, the clinical benefits of dual vaccination with PPV23 and PCV13 in adults remain unclear. Future studies should focus on investigating the VE of the combined vaccines in elderly and high-risk individuals.
Strengths and limitations
A general limitation in this review is the lack of randomized controlled studies of PPV23. Comparison of VE across studies was challenging because of variations in the age ranges used in the age subgroups and variant distribution of risk groups. Moreover, only one study investigated the vaccine efficacy of PCV13 in elderly individuals. Thus, more research is required to support the findings of our current study.
This review aimed to evaluate the VE on IPD however, pneumococcal pneumonia is also an important cause of death in the elderly population. Therefore, the VE of PPV23 and PCV13 on pneumococcal pneumonia should also be taken into account when evaluating the over-all effect of vaccination in the elderly population.
One strength of this review is the wider time frame in our search criteria than a previously published review [Citation27], resulting in a higher share of included studies. Further, to reduce the risk of missing relevant studies, the systematic search and review of the studies were done by two authors. The meta-analysis provides an overview of the PPV23 studies and highlights the heterogeneity as well as an estimation of the overall reduction in OR for IPD.
Conclusion
The results from this review show a reduction of IPD in elderly individuals and high-risk populations vaccinated with PPV23 and PCV13. The meta-analysis revealed a significant protective effect of PPV23 vaccination for the population >65 years of age, not accounting for waning protection with increasing age. However, most studies investigating the VE of PPV23 against IPD are retrospective observational studies, and the literature on particularly PCV13 is sparse. The results from this review demonstrate the reduced effect of the vaccines in elderly individuals and high-risk populations, which unfortunately represent high-risk groups for IPD. Therefore, there is a need for large-scale prospective studies to evaluate the clinical effect of PPV23.
No studies exist that evaluate the VE against IPD of combined vaccination with PPV23 and PCV13 in elderly individuals and high-risk populations. This should therefore be a key area of interest in future research. An evidence-based vaccination strategy for PPV23 and PCV13 in these groups might further reduce morbidity and mortality caused by pneumococcal diseases.
Supplemental Material
Download MS Word (145.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20018525.2023.2168354
Additional information
Funding
References
- GBD. Lower respiratory infections collaborators, “Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016 (in eng). Lancet Infect Dis. 2016 Nov;18(11):1191–10. DOI:10.1016/s1473-3099(18)30310-4
- Donkor ES. Understanding the pneumococcus: transmission and evolution (in eng). Front Cell Infect Microbiol. 2013;3:7.
- Andrews EANJ. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. 2012;30(48):6802–6808.
- Niederman MS, Folaranmi T, Buchwald UK, et al. Efficacy and effectiveness of a 23-valent polysaccharide vaccine against invasive and noninvasive pneumococcal disease and related outcomes: a review of available evidence. Expert Rev Vaccines. 2021;20(3):1–13.
- Harboe ZB, Thomsen RW, Riis A, et al. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLOS Med. 2009 [May 26, 2009];6(5):e1000081. DOI:10.1371/journal.pmed.1000081.
- Statens Serum Institut. “Fakta om pneumokoksygdom og vaccination.” ssi.dk. [cited 2020 November 2]. Available from: https://www.ssi.dk/-/media/arkiv/dk/vaccination/risikogrupper/infografik-fakta-om-pneumokoksygdom-og-vaccination.pdf?la=da
- Fally M, Israelsen S, Anhøj J, et al. The increasing importance of Haemophilus influenzae in community-acquired pneumonia: results from a Danish cohort study (in eng). Infect Dis (Lond). 2021 Feb;53(2):122–130. 10.1080/23744235.2020.1846776.
- Djennad A, et al. Effectiveness of 23-valent polysaccharide pneumococcal vaccine and changes in invasive pneumococcal disease incidence from 2000 to 2017 in those aged 65 and over in England and Wales. EClinicalMedicine. 2018;6(101733727):42–50.
- European Centre for Disease Prevention and Control. Invasive pneumococcal disease - annual epidemiological report for 2018. https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2018_IPD.pdf
- Statens Serum Institut. Fakta om pneumokoksygdom og vaccination. [cited 2021 april 9]. Available from: https://www.ssi.dk/-/media/arkiv/dk/vaccination/risikogrupper/infografik-fakta-om-pneumokoksygdom-og-vaccination.pdf?la=da
- Sings HL. Pneumococcal conjugate vaccine use in adults – Addressing an unmet medical need for non-bacteremic pneumococcal pneumonia. Vaccine. 2017;35:5406–5417.June. [Online]. Availablehttps://reader.elsevier.com/reader/sd/pii/S0264410X1730734X?token=9E2F3C10AF2207F95A30E226731ECAA7BC0692EA3EEBC2FE695B28852B0D497C5C20EC9A1E1D1DAA5038C07B8A633394&originRegion=eu-west-1&originCreation=20210915112149
- Statens Serum Institut. Personer med særlig risiko for invasiv pneumokoksygdom. cited 2021 April 10]. Available from: https://www.ssi.dk/vaccinationer/risikogrupper/invasiv-pneumokoksygdom
- Folkehelseinstituttet. Pneumokokkvaksine - veileder for helsepersonell. [cited 2021 April 9]. Available from: https://www.fhi.no/nettpub/vaksinasjonsveilederen-for-helsepersonell/vaksiner-mot-de-enkelte-sykdommene/pneumokokkvaksinasjon—veileder-fo/
- Pilishvili T, Bennett NM. Pneumococcal disease prevention among adults. Am J Preventive Med. 2015December;49(6, 4):383–390. [Online]. Available. https://reader.elsevier.com/reader/sd/pii/S0749379715005474?token=88ECC1A7B6E6965D5B7329206C96C27821C8654771E8DD193C28C2BF702ADC524BC6C57E7E41138D81008055673D00EB&originRegion=eu-west-1&originCreation=20210915112448
- Norwegian Institute of Public Health. Pneumokokkvaksine - veileder for helsepersonell. [cited 2022 October 10]. Available from: https://www.fhi.no/nettpub/vaksinasjonsveilederen-for-helsepersonell/vaksiner-mot-de-enkelte-sykdommene/pneumokokkvaksinasjon—veileder-fo/?term=ppv23&h=1
- Statens Serum Institute. Invasive pneumococcal disease 2018-2019. https://en.ssi.dk/surveillance-and-preparedness/surveillance-in-denmark/annual-reports-on-disease-incidence/invasive-pneumococcal-disease-2018-2019
- Danish Health Authority. Tilbud om pneumokokvaccination til særlige risikogrupper. [cited 2022 October 10]. Available from: https://www.sst.dk/da/udgivelser/2020/rationel-farmakoterapi-6-2020/tilbud-om-pneumokokvaccination--til-saerlige-risikogrupper
- Løvlie A, Vestrheim DF, Aaberge IS, et al. Changes in pneumococcal carriage prevalence and factors associated with carriage in Norwegian children, four years after introduction of PCV13 (in eng). BMC Infect Dis. Jan 10 2020;20(1):29. DOI:10.1186/s12879-019-4754-0.
- Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance,” (in eng). Lancet Infect Dis. 2015 Mar;15(3):301–309. DOI: 10.1016/s1473-3099(14)71081-3.
- Izurieta P, Bahety P, Adegbola R, et al. Public health impact of pneumococcal conjugate vaccine infant immunization programs: assessment of invasive pneumococcal disease burden and serotype distribution,” (in eng). Expert Rev Vaccines. 2018 Jun;17(6):479–493. DOI:10.1080/14760584.2018.1413354.
- Gov.Canada. Pneumococcal vaccine: Canadian immunization guide. [citrd 2022 October 10]. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-16-pneumococcal-vaccine.html#a9
- Yamada M, Li M, Iino T. Pneumococcal vaccine coverage in Japan among patients with a history of splenectomy: results of a retrospective administrative database study,” (in eng). Vaccine. May 6 2021;39(19):2692–2697. DOI:10.1016/j.vaccine.2021.03.045
- Statens Serum Institut. Pneumokokvaccine 23-valent. [cited 2021 april 9]. Available from: https://www.ssi.dk/vaccinationer/vaccineleksikon/p/pneumokokvaccine-23-valent
- Gov. UK. Pneumococcal polysaccharide vaccine: patient group direction template. [cited 2022 October 10]. Available from: https://www.gov.uk/government/publications/pneumococcal-polysaccharide-vaccine-ppv-patient-group-direction-pgd-template
- Folkhalsomyndigheten. “Vaccin mot pneumokockinfektion.” [cited 2022 Octomber 12]. https://www.folkhalsomyndigheten.se/smittskydd-beredskap/vaccinationer/vacciner-a-o/pneumokocker/
- Center for Disease Control and Prevention. Evidence to Recommendations for PCV13 use among adults ≥65 years old. [cited 2022 October 12]. Available from: https://www.cdc.gov/vaccines/acip/recs/grade/PCV13-etr.html
- Berild JD, Winje BA, Frimann Vestrheim D, et al. A systematic review of studies published between 2016 and 2019 on the effectiveness and efficacy of pneumococcal vaccination on pneumonia and invasive pneumococcal disease in an elderly population. 2020. [April 3, 2020]. Pathogens. 9(4):259. DOI: 10.3390/pathogens9040259.
- Miller SA, Forrest JL. Enhancing your practice through evidence-based decision making: pICO, learning how to ask good questions. 2001. [2001 10 01]. J Evid Based Dent Pract. 1(2):136–141. DOI: 10.1016/S1532-3382(01)70024-3.
- Centers for Disease Control and Prevention (CDC). 2012. Principles of Epidemiology in Public Health Practice. https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section6.html.
- Wells GA, Shea B, Higgins JP, et al. Checklists of methodological issues for review authors to consider when including non-randomized studies in systematic reviews (in eng). Res Synth Methods. 2013 Mar;4(1):63–77. DOI:10.1002/jrsm.1077.
- Broome CV, Facklam RR, Fraser DW. Pneumococcal disease after pneumococcal vaccination: an alternative method to estimate the efficacy of pneumococcal vaccine,” (in eng). N Engl J Med. Sep 4 1980;303(10):549–552. DOI:10.1056/nejm198009043031003.
- Kim JH, et al. Direct effectiveness of pneumococcal polysaccharide vaccine against invasive pneumococcal disease and non-bacteremic pneumococcal pneumonia in elderly population in the era of pneumococcal conjugate vaccine: a case-control study. Vaccine. 2019;37(21):2797–2804.
- Leventer-Roberts, Leventer-Roberts M, Feldman BS, et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive disease and hospital-treated pneumonia among people aged ≥65 years: a retrospective case-control study. Clin Infect Dis. 2015;60(10):1472–1480. DOI: 10.1093/cid/civ096.
- Gutierrez Rodriguez MA, Ordobás Gavín MA, García-Comas L, et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccine in adults aged 60 years and over in the Region of Madrid, Spain, 2008–2011. Euro Surveill. 2014;19(40):20922. DOI:10.2807/1560-7917.ES2014.19.40.20922
- Wright LB, Hughes GJ, Chapman KE, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in people aged 65 years and over in the North East of England, April 2006-July 2012. Trials Vaccinol. 2013;2(1):45–48.
- Shimbashi R, Suzuki M, Chang B, et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in adults, Japan, 2013–2017. Emerg Infect Dis. 2020;26(10):2378–2386.
- Tsai Y-H, Hsieh M-J, Chang C-J, et al. The 23-valent pneumococcal polysaccharide vaccine is effective in elderly adults over 75 years old—taiwan’s PPV vaccination program. Vaccine. 2015;33(25):2897–2902. DOI:10.1016/j.vaccine.2015.04.068.
- Bonten MJM, Huijts SM, Bolkenbaas M. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–1125.
- Moore MR, et al. Impact of 13-valent pneumococcal conjugate vaccine used in children on invasive pneumococcal disease in children and adults in the United States: analysis of multisite, population- based surveillance. Lancet Infect Dis. 2015 Mars;15(3):301–309. DOI:10.1016/S1473-3099(14)71081-3.
- Patterson S, Webber C, Patton M, et al. A post hoc assessment of duration of protection in CAPiTA (Community Acquired Pneumonia immunization Trial in Adults). Vaccinology 2016;5:92–96 [May 4, 2016].
- Clutterbuck EA, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells (in eng). J Infect Dis. May 1 2012;205(9):1408–1416. Doi:10.1093/infdis/jis212.
- Jackson LA, et al., “Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine,” Vaccine, vol. 31, no. 35, pp. 3585–3593, 2 August 2013. https://www.sciencedirect.com/science/article/pii/S0264410X13005884
- Rezai MS, Ghaffari J, Mahdavi M, et al. Conjugate and 23-valent pneumococcal polysaccharide booster vaccination in asplenic patients with thalassemia major: a randomized clinical trial study. Caspian J Intern Med. 2017;8(1):16–22. [Online]. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5412244/pdf/cjim-8-016.pdf
- Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the advisory committee on immunization practices (ACIP) (in eng). MMWR Morb Mortal Wkly Rep. Oct 12 2012;61(40):816–819.