ABSTRACT
Background
Lung cancer patients undergoing treatment with immune checkpoint inhibitors (ICIs) are at risk of developing immune-related (ir-)pneumonitis. Since lung cancer patients have competing reasons for respiratory symptoms, this poses a diagnostic challenge. This study aimed to explore diagnosis and management of ir-pneumonitis in this patient group.
Materials and Methods
Retrospective database retrieved analysis of patients with non-small cell lung cancer undergoing treatment with PD-1/PD-L1 inhibitors at Department of Oncology, Copenhagen University Hospital, Herlev. Patients being suspected of ir-pneumonitis during the period 01.07.2016 – 15.06-2020 were selected.
Results
Out of 377 eligible patients, 83 were suspected of ir-pneumonitis. A thoracic computed tomography (CT) was made in 93% of the patients, and 34% had a sputum sample made. A specialist in pulmonology was consulted in seven patient cases. Ir-pneumonitis was radiologically confirmed in 34 patients, however, in 12 patients symptoms could not be confined to ir-pneumonitis and only 22 patients were included in the analysis of the course of treatment. Solely corticosteroids were used as systemic treatment, and the median duration of treatment was 16 weeks (4-34). In seven patients pneumonitis was consistent with recall radiation pneumonitis (RRP).
Conclusion
Suspected ir-pneumonitis was frequent in this group of patients. The cohort was characterized by high heterogeneity and lack of unequivocal diagnostic conclusions. Treatment of ir-pneumonitis was longer than recommended and involvement of pulmonologist was very infrequent. The result of this study reflects the difficulties in a daily clinical setting to diagnose and manage patients with lung cancer presenting with pulmonary symptoms.
Background
In Denmark, approximately 5000 patients are annually diagnosed with non-small cell lung cancer (NSCLC), and half of these patients present with metastatic disease [Citation1]. For a long period, systemic treatment of this patient group has consisted of chemotherapy only, with a median survival of 8–10 months on first-line platinum-based chemotherapy [Citation2] being a modest improvement. Recent development of systemic antineoplastic treatment has introduced options that are more promising. Treatment with immune checkpoint inhibitors (ICI) using PD-1/PD-L1 inhibitors in NSCLC has shown significantly longer survival compared to chemotherapy [Citation3], and is generally better tolerated [Citation4]. However, ICIs are associated with a completely different range of adverse reactions; immune-related adverse events (irAEs). The majority of irAEs are largely manageable but can vary in frequency and severity. However, they can escalate, become life-threatening and in rare cases become fatal [Citation5]. The incidence of ir-pneumonitis in patients undergoing treatment with ICI in is 3–5% [Citation6], however, being a severe type of ir-AE with a high mortality compared with other ir-AEs. In a large meta-analysis, the mortality rate of all type irAE was 0,37% in patients undergoing treatment with monotherapy PD-1/PD-L1 inhibitor, with ir-pneumonitis representing 42% of fatal cases [Citation5].
Lung cancer patients are at higher risk of developing ir-pneumonitis compared to patients with other cancer types [Citation7,Citation8]. The mechanism behind this is largely unknown, but it might include risk factors such as pulmonary comorbidity, prior radiotherapy and location of tumour burden [Citation9]. Patients with ir-pneumonitis presents with respiratory symptoms such as cough and dyspnoea. This poses a diagnostic challenge due to multiple differential diagnosis including infections, progression of cancer, pleural or pericardial effusions, lung embolism, and in lung cancer patients a high prevalence of comorbidities such as chronic obstructive lung disease (COLD), interstitial lung disease (ILD) or cardiac failure.
Severity of ir-pneumonitis is graded based on the symptomatology using Common Terminology Criteria for Adverse Events (CTCAE) [Citation10]. In a Danish setting, management is based on the guidelines from the European Society of Medical Oncology (ESMO) [Citation11]. In case of severe or persistent symptoms, it is recommended to perform a high-resolution computed tomography (HRCT). Ir-pneumonitis can present various radiological patterns such as organizing pneumonia (OP), hypersensitivity pneumonia (HP), nonspecific interstitial pneumonia (NSIP) and bronchiolitis [Citation12]. Bronchoalveolar lavage (BAL) is recommended in case of persistent symptoms, mainly to rule out alternative diagnoses. However, caution should be made towards the possible complications to this procedure. First choice of treatment is corticosteroids, slowly tapered in 4–6 weeks depending on the CTCAE grade; higher grades usually entailing longer taper. In case of no improvement after 48 hours, intensification of treatment is recommended according to ESMO guidelines [Citation11]. In case of CTCAE grade 3–4 this includes an additional immunosuppressive strategy. The preferred drug is the choice of the clinician, and might include infliximab, mycophenolate mofetil, cyclophosphamide or tocilizumab [Citation6,Citation11]. However, very limited evidence supports the effect, nor indicates an optimal strategy.
Cessation of ICI is necessary in CTCAE grade 2–4 ir-pneumonitis, however, in case of CTCAE grade 2 symptoms, re-introduction of ICI can be considered depending on the individual case.
This retrospective study was carried out to explore and describe the use of diagnostic tools in patients with NSCLC undergoing treatment with ICI and being suspected of ir-pneumonitis, and to evaluate the treatment and the course of symptoms in patients with radiologically and clinically confirmed ir-pneumonitis.
Materials and methods
Patients with NSCLC treated with PD-1/PD-L1 inhibitors at the Department of Oncology, University Hospital Herlev were selected based on information from the patient files. Cancer treatment could be either monotherapy with PD-1/PD-L1 inhibitors, a combination of PD-1 inhibitor and chemotherapy, or adjuvant treatment with a PD-L1 inhibitor. Each electronic patient file was screened for the word ‘pneumonitis’, and the patients were included in the study if they had been suspected for ir-pneumonitis during the period of 01.07.2016–15.06.2020. The patients with suspected ir-pneumonitis were all treated at the Department of Oncology, University Hospital Herlev. Both hospitalized patients and patients in the outpatient clinic were included.
Patients with radiological signs of pneumonitis were included in the analysis of the treatment and course of ir-pneumonitis. In case of concurrent progression of cancer, symptoms were not confined to ir-pneumonitis, and the CT findings could not be interpreted with certainty; hence, patients with concurrent signs of progression of cancer were excluded from the analysis, together with patients already undergoing treatment with corticosteroid for other reasons than respiratory symptoms.
Statistical descriptive analyses were performed using IBM SPSS Statistics 25.
Results
A total of 377 patients with NSCLC received treatment with ICI during the study period. Of these, 83 patients (22%) were suspected of ir-pneumonitis and were included in the study. Baseline characteristics are described in . The majority of patients received monotherapy ICI in first line, and the dominant histological type being non-squamous cell carcinoma.
Table 1. Baseline characteristics of patients suspected of ir-pneumonitis.
Initially, a thoracic CT or HCRT was conducted in 77 patients (93%), and 16 patients had an additional HCRT conducted within the following 1–12 days to confirm or refute ir-pneumonitis (). In four patients, an additional HRCT changed the radiological diagnosis from the primary thoracic CT, either from pneumonitis to no pneumonitis, or from non-specific findings to pneumonitis.
Table 2. The use of diagnostic tools and management in patients suspected of ir-pneumonitis.
Infectious workup was only performed in case of hospitalization. A Sputum sample was done in 34% of patients; most samples being inconclusive (). A pulmonologist was consulted in seven cases, and BAL was performed in one case.
Treatment with corticosteroid was initiated in 76% of patients, and 51% of patients were additionally treated with antibiotics, suspected of concomitant infection ().
The majority of patients were hospitalized (63%), and hospitalization was generally associated with a worse outcome, 44% of hospitalized patients were not alive 100 days after initial contact versus 10% of outpatients. Ir-pneumonitis was refuted with certainty in 49 cases, with progression of cancer being the dominant reason for respiratory symptoms (35%) ().
Figure 1. Flowchart of patients suspected of ir-pneumonitis and course of treatment in patients with radiological signs of ir-pneumonitis.
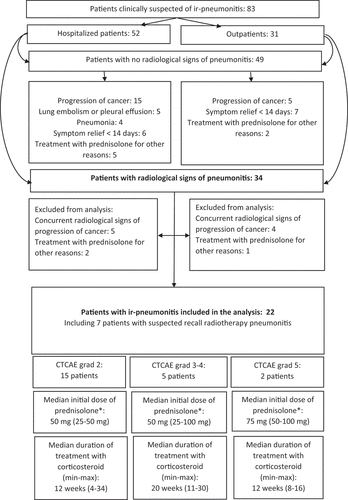
The radiologist specifically described ‘Pneumonitis’ in 34 patients. Of the 34 patients, nine had concurrent evidence of progression of cancer, and three patients were treated with corticosteroid for other reasons than pulmonary symptoms; hence, 22 patients were included in the final analysis of the treatment and course of ir-pneumonitis (). In these 22 patients, pulmonary symptoms presented after a median of 4.5 (1–30) series of ICI, and the median duration of treatment since start of ICI was 20 weeks (1–122). All 22 patients were treated with monotherapy ICI, and three of the patients received ICI as adjuvant treatment. None of these 22 patients were consulted with a pulmonologist.
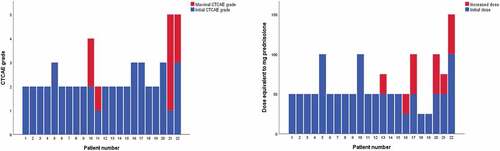
The majority of patients presented with CTCAE grade 2 symptoms and the median starting dose of corticosteroid was 50 mg (25 mg −100 mg) prednisolone daily. Median duration of corticosteroid treatment was 16 weeks (4–34). Patients with CTCAE grade 2 symptoms had a median duration of treatment of 12 weeks (4–34) vs 20 weeks (11–30) in patients with CTCAE grade 3–4 ().
It was necessary to increase the dose of corticosteroid to achieve symptom control in six patients, and four patients developed a higher CTCAE grade during the course of treatment (). No patients were treated with another immunosuppressant treatment than corticosteroid.
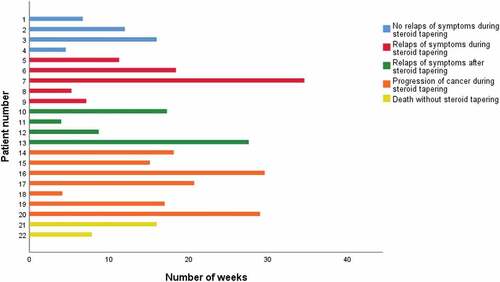
Only four patients underwent steroid tapering without relapse of pulmonary symptoms (). Progression of cancer during steroid tapering was seen in seven patients and in these cases, relapse of pulmonary symptoms could not be considered as solely due to ir-pneumonitis.
Fatal development of pneumonitis was seen in two patients. Both were clinically considered as having progression of cancer, however, there were no radiological signs of progressive disease.
Radiotherapy was given prior to ICI in 10 patients, and in seven of these patients, pneumonitis was located in the same pulmonary lobe as the field of radiotherapy, suspecting recall radiation pneumonitis (RRP). In all seven patients with RRP, radiotherapy was finalized < three months prior to the onset of pulmonary symptoms. In the remaining three patients, radiotherapy was finalized>6 months prior to ir-pneumonitis.
ICI was re-introduced in two patients. Both presented initially with CTCAE grade 2 symptoms during adjuvant treatment following concomitant chemo-radiotherapy, and both with suspected RRP. Symptoms developed at day 92 and 57, respectively, after end of radiotherapy. Both had a relapse of metastatic cancer after day 60 and 458, respectively, and both were re-challenged with ICI with no signs of relapse of ir-pneumonitis.
Discussion
We found that 22% of the lung cancer patients undergoing treatment with ICI, presented with respiratory symptoms that lead to suspicion of ir-pneumonitis.
The main diagnostic tool for identifying ir-pneumonitis was thoracic CT, however, often performed in the acute setting in order to identify several possible diagnoses and not chosen specifically to identify ir-pneumonitis. In addition, the scan was seldomly described by a thoracic radiologist; hence, the diagnostic value could in several cases be questioned. In many patient, other evident reasons for respiratory symptoms could be clearly identified, and an additional investigation was not indicated. In case of an additional HRCT, to confirm or refute ir-pneumonitis, it led to a change of diagnosis in 4 of 16 patients. Although numbers are small, they support the need to address the radiological assessment. We suggest ensuring re-evaluation of the CT scan by a thoracic radiologist, in order to optimize the diagnostic value in patients with suspected ir-pneumonitis where no other evident reason for symptoms can be clearly identified.
Infectious workup was limited and often inconclusive; hence, very little could be said about possible microbiological agents in these patients. Since pneumonia is an important differential diagnosis, we suggest to improve follow-up on microbiological tests, and to repeat infectious workup in case of relapse of respiratory symptoms during steroid tapering.
Few patients were consulted with a pulmonologist, and in only one patient, BAL was performed, however, not prompted by ir-pneumonitis. In most cases, ir-pneumonitis were clearly refuted; hence, involvement of a pulmonologist was often not indicated. In the group of patients we included in the final analysis of the course and treatment of ir-pneumonitis, none were consulted with a pulmonologist, which raises concern.
Generally, we found several areas in need of improvement when diagnosing ir-pneumonitis, however, one-third of all patients were re-introduced to ICI, and in most cases without recurrence of pulmonary symptoms, suggesting that ir-pneumonitis was confirmed or refuted correctly in far most cases.
We included 6% of patients in the analysis of the course and treatment of ir-pneumonitis. This study did not set out to determine the incidence of ir-pneumonitis; hence, this number should be interpreted with caution. In phase III studies, the incidence of ir-pneumonitis in patients with NSCLC being treated with ICI was 1–6% [Citation7], however, in retrospective real-world studies the incidence was as high as 9.5–19% [Citation9,Citation13,Citation14]. The reasons for this discrepancy is unclear, but might reflect the diagnostic challenges in the real-world setting where lung cancer patients have a higher prevalence of comorbidity, and more frequently presents with respiratory symptoms, compared with patients included in clinical studies.
The majority of patients with ir-pneumonitis presented with CTCAE grade 2 symptoms, and 45% of patients needed hospitalization. In one retrospective study more than half of patients presented with CTCAE grade 3–4 symptoms and 60% of patients were in need of hospitalization [Citation13]. Considering the high number of suspected cases (22%) in our cohort, we speculate that many cases were identified at an early stage and had started treatment before symptoms developed more severely.
Main treatment of ir-pneumonitis was corticosteroid. The median duration of corticosteroid treatment was longer in the group of patients presenting with CTCAE grade 3–4 symptoms compared to those presenting with CTCAE grade 2, however, in both groups, the treatment course was much longer than recommended. In no cases other immunosuppressant than corticosteroid were given. In one study of 71 cases of ir-pneumonitis in all type cancers, mean duration of treatment with corticosteroid was 27 days (4–251) [Citation15], and no patients were treated with other immunosuppressant. One study of 30 cases of ir-pneumonitis in patients with lung cancer found a similar median duration of treatment with corticosteroid of 30 days (11–78 days) [Citation13], also with no patients being treated with other immunosuppressant. Since the mortality in case of ir-pneumonitis is high compared with other irAEs [Citation5], it seems conspicuous that several retrospective studies, including ours, could not report use of a more intensified immunosuppressant strategy.
One study reported 6 of 44 (14%) patients with ir-pneumonitis developing chronic pneumonitis, defined as necessity of immunosuppressive treatment≥12 weeks [Citation16], suggesting a unique subset of ir-pneumonitis requiring longer duration of treatment or a different strategy. In our study, seven of 13 (54%) patients received treatment with corticosteroid≥12 weeks, excluding patients who died or had progression of cancer during steroid tapering, suggesting that a large group of patients are in need of more attention.
In the group of 22 patients with ir-pneumonitis, 7 patients had radiological findings corresponding to the field of prior radiotherapy. All seven patients with RRP presented with symptoms within three months after radiotherapy, suggesting a possible relation to acute radiation pneumonitis (RP). Noticeably, in two patients with RRP during adjuvant treatment, ICI were re-introduced after relapse of metastatic cancer with no recurrence of ir-pneumonitis. Though evidence is limited, data suggest the risk of RRP is increased in patients undergoing treatment with ICI [Citation17], and it is not possible to determine whether RRP is related to ICI or not, nor whether the mechanism resulting in ir-pneumonitis is different. The patients with RRP were, therefore, included in our analysis. When comparing patients with suspected RRP after ICI and patients with no relation to prior radiation therapy, we found no difference in management, nor any significant difference in duration of the steroid treatment. In one study of 136 patients being suspected of ir-pneumonitis, 29% of cases were classified as RRP [Citation18], which is similar to our finding. In future studies on ir-pneumonitis, it is relevant to include data on RRP and to explore if re-introducing ICI might be safe for this patient group.
The limitation of this study is the retrospective design and the challenges of identifying cases with ir-pneumonitis. The extent and quality of the collected data were dependent on the data reported by the clinicians. The number of patients suspected of ir-pneumonitis was high; hence, unidentified cases with ir-pneumonitis by the clinician being unlikely. Much depended on the radiological description, and it is very likely that identification of cases with ir-pneumonitis was compromised by the lack of systematic radiological evaluation by a thoracic radiologist. Noticeably, our analysis cannot report findings on patients with CTCAE grade 1 pneumonitis alone. Former studies have found the incidence of cases with CTCAE grade 1 being 7–33% [Citation7]. A possible explanation could be that the clinicians are less likely to mention ir-pneumonitisuntil it entails a clinical consequence; hence, patients with CTCAE grade 1 ir-pneumonitis might be underreported.
The information on the course and treatment of ir-pneumonitis was limited. We could identify the duration of corticosteroid treatment and an increase of dose in case of relapse of symptoms, but not follow the steps of steroid tapering with certainty, and it was, therefore, not possible to measure the cumulative dose of prednisolone for each patient.
The long duration of steroid treatment and frequent relapse of symptoms seems to be the most urgent challenges among patients with ir-pneumonitis. Generally, guidelines were followed with regards to initial dose of corticosteroid according to CTCAE grade, however, it could be questioned, if monitoring of symptoms was sufficient to identify cases, were an intensified treatment would be indicated. According to ESMO guidelines, pulmonary function tests (PFTs) are recommended in CTCAE grade≥2. This tool was not used in any patients in our study, and introduction of repeated PFTs as an objective measurement tool might improve follow-up and early identification of patients with a more severe development. In addition, involvement of a pulmonologist seems urgent in optimizing the treatment and course of the patients, and to identify those who might benefit from other immunosuppressant treatments than corticosteroid only.
In conclusion, suspected ir-pneumonitis was frequent in patients with metastatic or previous NSCLC treated with PD-1/PD-L1 inhibitors. Our cohort was characterized by high heterogeneity and lack of unequivocal diagnostic conclusions. Duration of treatment with corticosteroids was often longer than recommended in the ESMO guidelines and involvement of pulmonologist throughout the course of treatment was lacking. Our results reflect the challenges in a daily clinical setting to diagnose and manage patients with lung cancer presenting with respiratory symptoms. Optimizing the diagnostic certainty and follow-up on treatment of ir-pneumonitis would require dedicated thoracic radiologists to evaluate the radiological findings, and a close corporation with pulmonologists, for a better follow-up on the course of treatment.
Acknowledgments
Thanks to Jesper Andreas Palshof, Jon Lykkegaard Andersen, Lene Collatz Laustrup and Jens Ulrik Stæhr Jensen for input and clinical advice.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Dansk lunge cancer register, national årsrapport 2019-2020. 2020.
- Cufer T, Ovcaricek T, O’brien MER. Systemic therapy of advanced non-small cell lung cancer: major-developments of the last 5-years. Eur J Cancer. 2013 Apr;49(6):1216–8. DOI:10.1016/j.ejca.2012.11.021
- W V Ferrara R, Imbimbo M, Malouf R, et al. Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer (Review). Cochrane Database Syst Rev. 2021;2021(4). DOI:10.1002/14651858.CD013257.pub3
- Luo W, Wang Z, Tian P, et al. Safety and tolerability of PD-1/PD-L1 inhibitors in the treatment of non-small cell lung cancer: a meta-analysis of randomized controlled trials. J Cancer Res Clin Oncol. 2018;144(10):1851–1859.
- Wang DY, Salem J-E, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. DOI:10.1001/jamaoncol.2018.3923
- Reuss JE, Suresh K, Naidoo J. Checkpoint inhibitor pneumonitis: mechanisms, characteristics, management strategies, and beyond. Curr Oncol Rep. 2020 Jun 1;22(6). Springer. 10.1007/s11912-020-00920-z.
- Cadranel J, Canellas A, Matton L, et al. Pulmonary complications of immune checkpoint inhibitors in patients with nonsmall cell lung cancer. Eur Respir Rev. 2019;28(153):190058. DOI:10.1183/16000617.0058-2019
- Khoja L, Day D, Wei-Wu Chen T, et al. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28(10):2377–2385.
- Cho JY, Kim J, Lee JS, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer. 2018 Jul;125:150–156.
- NCI. CTCAE. Available from: https://ctep.cancer.gov/protocolDevelopment/adverse_effects.htm.
- Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: eSMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Supplement 4):iv119–142. DOI:10.1093/annonc/mdx225
- Kalisz KR, Ramaiya NH, Laukamp KR, et al. Immune checkpoint inhibitor therapy–related pneumonitis: patterns and management. Radiographics. 2019 Nov;39(7):1923–1937.
- Atchley WT, Alvarez C, Saxena-Beem S, et al. Immune checkpoint inhibitor-related pneumonitis in lung cancer: real-world incidence, risk factors, and management practices across six health care centers in North Carolina. Chest. 2021;160(2):731–742. DOI:10.1016/j.chest.2021.02.032
- Suresh K, Voong KR, Shankar B, et al. Pneumonitis in non–small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13(12):1930–1939. DOI:10.1016/j.jtho.2018.08.2035
- Delaunay M, Cadranel J, Lusque A, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J. 2017;50(2):1700050. DOI:10.1183/13993003.00050-2017
- Naidoo J, Cottrell TR, Lipson EJ, et al. Chronic immune checkpoint inhibitor pneumonitis. J Immunother Cancer. 2020;8(1):1–7. DOI:10.1136/jitc-2020-000840
- Cousin F, Desir C, Ben Mustapha S, et al. Incidence, risk factors, and CT characteristics of radiation recall pneumonitis induced by immune checkpoint inhibitor in lung cancer. Radiother Oncol. 2021 Apr;157:47–55. DOI:10.1016/j.radonc.2021.01.001.
- Smith DA, Radzinsky E, Tirumani SH, et al. Findings on chest CT performed in the emergency department in patients receiving immune checkpoint inhibitor therapy: single-institution 8-year experience in 136 patients. Am J Roentgenol. 2021 Sep;217(3):613–622.