ABSTRACT
Background
Sedating antihistamines such as promethazine are used as anxiolytics and hypnotic agents for patients with chronic obstructive pulmonary disease (COPD) with and without asthma despite limited knowledge of its effects and side effects. We evaluated if treatment with promethazine had a lower risk of harmful outcome.
Methods
Nationwide retrospective cohort study of Danish specialist diagnosed outpatients with COPD treated with promethazine or an active comparator (melatonin). Patients with collection of promethazine or melatonin were propensity score matched 1:1. The primary outcome was a composite of severe COPD exacerbations and death from all causes analyzed by Cox proportional hazards regression. We performed an interaction analysis for comorbid asthma.
Results
In our registry of 56,523 patients with COPD, 5,661 collected promethazine (n = 3,723) or melatonin (n = 1,938). A cohort of 3,290 promethazine- or melatonin-treated patients matched 1:1 was available for the primary analysis.
Within 1-year patients treated with promethazine were at higher risk of the primary outcome than matched controls with a Hazard Ratio (HR) of 1.42 (CI 1.27–1.58, p < 0.0001). Similarly, the risk of death was higher for promethazine-treated patients (HR 1.53, CI 1.32–1.77, p < 0.0001). An interaction analysis for comorbid asthma showed no interaction between comorbid asthma and the likelihood of a primary outcome when collecting promethazine (p = 0.19). Adjusted Cox analysis on the entire population indicated a further increased risk with more promethazine (HR for primary outcome among patients collecting ≥ 400 promethazine tablets/year=2.15, CI 1.94–2.38, p<0.0001).
Conclusions
Promethazine-treated patients with COPD had a concerning excess risk of a composite outcome of severe exacerbations and death from all causes compared to melatonin.
Introduction
The current use of sedating antihistamine promethazine includes that of an anxiolytic or hypnotic agent for patients with COPD. Promethazine is a first-generation sedative antihistamine belonging to the phenothiazine class, blocking the H1-receptor, and weakly antagonizing dopamine D2, muscarinic acetylcholine, alpha adrenergic and other receptors [Citation1–3]. Originally it was predominantly used as an antiemetic to treat nausea and vomiting and to prevent motion sickness [Citation4].
Promethazine inhibits the H1 receptor in the posterior hypothalamus which affects the regulation of sleep-wakefulness [Citation5]. Studies of people without respiratory illness have pointed to an increased length of sleep with fewer sleep interruptions [Citation6], but also a dose-response dependent inhibition of rapid eye movement sleep [Citation7]. Studies on treatment with promethazine in patients with obstructive pulmonary disease are few and small [Citation8–10], and there is no available knowledge regarding the harmful or beneficial effects of treatment with promethazine beyond one month. The available data point to a positive effect on breathlessness and exercise tolerance [Citation8,Citation9]. In patients with COPD and comorbid asthma, knowledge on treatment with promethazine is even more sparse.
Because of the sparse knowledge on the benefit or harmful effect of promethazine among large groups of patients with COPD treated for prolonged time, we aimed to explore the association between treatment with promethazine and a composite outcome of severe exacerbations of COPD and death from all causes in a large nationwide cohort of patients with COPD. Based on the little available study-based data on promethazine including a possible positive effect on exercise tolerance we hypothesized that use of promethazine would be associated with a reduction in our primary outcome (severe COPD exacerbations or death from all causes).
Methods
Study design
A nationwide retrospective cohort study was conducted by combining information from the following registries:
The Danish Register of Chronic Obstructive Pulmonary Disease (DrCOPD): A nationwide database established in 2008 containing information on the quality of treatment of all patients with COPD treated by a respiratory medicine specialist at a Danish Hospital in an out-patient clinic [Citation11]. Covariates included in this study were smoking status, dyspnea assessed using the Medical Research Council (MRC) Dyspnea Scale, BMI (body mass index) assessed as kilograms per square meter, and lung function assessed as forced expiratory volume in the first second as percent of predicted (FEV1%) [Citation12].
The Danish Civil Registration System: All citizens in Denmark acquire a unique personal identification number at birth or immigration. This unique personal identification number yields data on date of birth and gender, and links individual information for each resident in all Danish registries [Citation13].
The Danish National Health Service Prescription Database holds information on all prescriptions dispensed by Danish pharmacies since 2004 (coded according to ATC classification), including date of dispensation, quantity dispensed, strength, and formulation. All pharmacies are required by Danish legislation to provide information that ensures complete and accurate registration [Citation14].
The Danish National Patient Registry holds information on all admissions to Danish hospitals since 1977, and hospital outpatient clinic visits since 1995. Each visit is coded by physicians with one primary diagnosis and one or more secondary diagnoses, according to the International Classification of Diseases, eighth revision (ICD-8) codes until 1994 and ICD-10 thereafter [Citation15].
Population
All Danish residents with a COPD diagnosis by a respiratory medicine specialist in an outpatient clinic from NaN Invalid Date NaN, to NaN Invalid Date NaN were included. We used melatonin as an active comparator, since this drug is roughly given on the same indication (mild anxiety and sleeplessness, but a wish to avoid benzodiazepines [Citation16–19]). We performed an interaction analysis on the risk of severe exacerbations or all-cause mortality for patients collecting promethazine with comorbid asthma.
A patient’s study entry date was defined as the date of their first collection of promethazine (ATC (Anatomical Therapeutic Chemical) code R06AD02) or melatonin (ATC code N05CH01). All patients who collected both drugs within 365 days, were excluded. Only patients collecting either promethazine or melatonin were included in the propensity score matched cohort.
Follow-up
Patients were followed for one year from study entry. This time frame served as the study period, during which patients were eligible to develop an event.
Outcomes
All outcomes were assessed for one year. During follow-up patients were followed for events of the primary outcome; composite outcome of severe exacerbations (admissions diagnosed as DJ44 Chronic obstructive pulmonary disease and all belonging subcodes) and all-cause mortality, and for secondary outcomes; all-cause mortality, all-cause admissions and moderate exacerbations of COPD requiring receptions of prednisolone (ATC-code H02AB06 and H02AB07) but not admission.
Statistics
Patients treated with promethazine and melatonin were propensity score matched on known and likely confounders; age (as a continuous variable), gender, tobacco exposure (divided into the categories ‘never smoking’, ‘passive smoking’, ‘previous smoking’, ‘active smoking’ and ‘unknown tobacco exposure’), MRC (with the options 1, 2, 3, 4 and 5), BMI (as a continuous variable), FEV1% divided into GOLD (Global initiative for Chronic Obstructive Lung Disease) stages (GOLD I Mild COPD FEV1% ≥ 80%, GOLD II Moderate COPD 50% ≤ FEV1% < 80%, GOLD III Severe COPD 30% ≤ FEV1% < 50% and GOLD IV Very severe COPD FEV1% < 30%), collection of inhaled corticosteroids (ICS) and long-acting muscarinic receptor antagonist (LAMA) within one year before study entry and Charlson comorbidity index (CCI). Propensity score matching was performed using the Greedy Match algorithm from the Mayo Clinic [Citation20]. Missing values on scoring variables were imputed before propensity score match.
Baseline characteristics were compared by chi-square test. Cox regression model and cumulative incidence curves with Grays analysis was used to assess the risk of events in the compared groups.
Some patients had more than one event during the follow-up period, and in this case only the first event was counted for all analysis, however total number of severe exacerbations during the one-year follow-up period was also compared.
For sensitivity analysis, we conducted an adjusted Cox proportional hazard regression model of the primary outcome in the unmatched population. This analysis was adjusted for the variables included in the propensity score match (age, gender, tobacco exposure, MRC, BMI and FEV1% GOLD stage, ICS use, LAMA use and CCI). An additional analysis examined the effect of collection of >100 tablets of promethazine 25 mg for the primary outcome.
Model control investigating the proportional hazards assumption was performed to validate the Cox proportional hazards regression, in all cases yielding p values > 0.05.
All statistical analyses were performed using SAS 9.4, Cary, NC, USA, and Microsoft Excel, Windows 365. A two-sided 95% confidence interval was used for all statistical analyses, and p < 0.05 was considered statistically significant. Cumulative Incidence plots were customized by the NewSurv macro [Citation21].
Results were presented as hazard ratios with 95% confidence intervals (CI).
Risks were visualized by cumulative incidence plots with Gray’s analyses. HR profiles of variables were visualized by forest plot.
Ethics
The study was approved by the Capital Region of Denmark by the Knowledge Center for Data reviews (P-2022-952). In Denmark, retrospective use of register data does not require ethical approval or patient consent.
Results
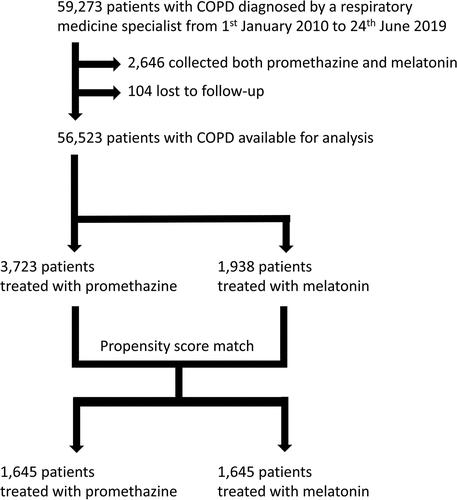
Of the 59,169 patients in DrCOPD, collection of promethazine was found among 3,723 patients, and 2,324 patients collected >100 tablets of promethazine 25 mg. Similarly, 1,938 patients collected melatonin, and 50,862 patients collected neither promethazine nor melatonin. We excluded the 2,646 patients, who collected both. Of the patients with collection of promethazine, 1,645 patients could be matched 1:1 to a control patient with collection of melatonin, .
Baseline data
There were few differences in the baseline characteristics, ; collection of bronchodilators (long and short acting) was higher, and the profile of collection of benzodiazepines and its derivates differed among patients treated with promethazine and melatonin.
Table 1. Baseline characteristics of the propensity score matched cohorts.
Primary outcome
We found an increased risk of our primary outcome; acomposite outcome of severe exacerbations of COPD and all-cause mortality among patients treated with promethazine in comparison to propensity score matched patients treated with melatonin, . The HR for the primary outcome was 1.42 (CI 1.27–1.58, p < 0.0001) in patients who collected promethazine. Similarly, Cumulative incidence curve () and Grays analysis showed an increased risk of the primary outcome (HR 1.41, CI 1.27–1.57, P < 0.0001).
Figure 2. Cumulated incidence plots of the primary outcome; composite outcome of severe exacerbations of COPD and all-cause mortality of propensity score matched patients.
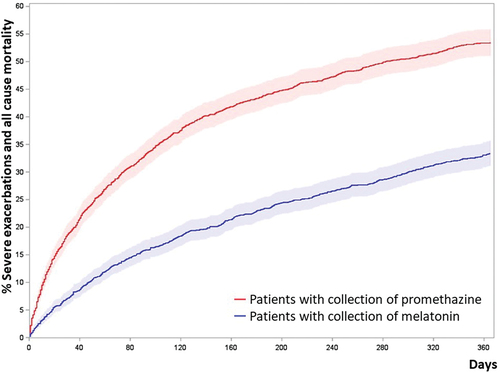
Table 2. Primary and secondary outcome analyses.
Secondary outcomes
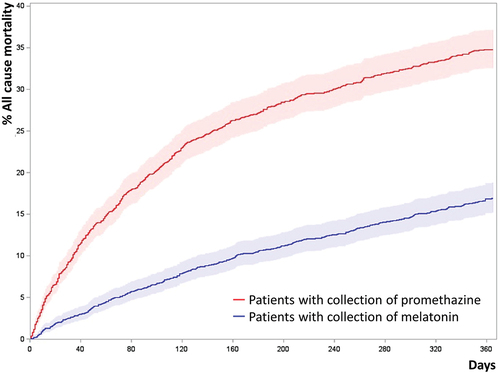
The risk of death from all causes was higher for promethazine-treated patients (HR 1.53, CI 1.32–1.77, p < 0.0001, Cox proportional hazards model), .
Figure 3. Cumulated incidence plots of all-cause mortality of propensity score matched patients.
The risk of experiencing a severe exacerbation …
During the one-year follow-up, patients who collected promethazine had an average of XX severe exacerbations, in contrast to YY severe exacerbations among patients collecting melatonin.
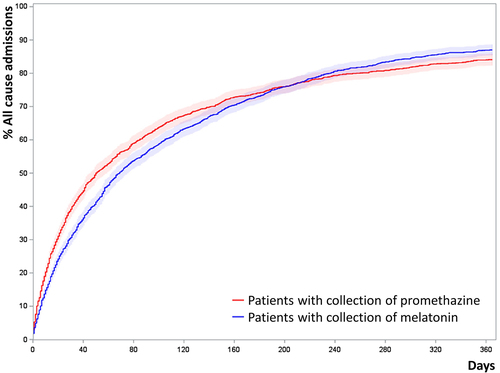
There was also an increased risk of all-cause admissions (HR 1.21, CI 1.12–1.30 p < 0.0001), Appendix I, and moderate exacerbations of COPD (requiring prednisolone but not admission) with HR 1.22 among promethazine-treated patients (CI 1.08–1.39, p = 0.0019).
Interaction analysis for comorbid asthma
An interaction analysis for comorbid asthma showed no interaction between comorbid asthma and the likelihood of a primary outcome (severe exacerbations or all-cause mortality) when collecting promethazine. (p = 0.19)
Sensitivity analysis: multivariate cox regression and forest plot on the entire population
A multivariable model analyzing the primary outcome (severe exacerbations or all-cause mortality) and adjusted for promethazine, melatonin, comorbid asthma, and the variables used in the propensity score match was carried out. This analysis examined 56,523 patients and yielded the results visualized in the forest plot shown in and , including a HR for promethazine of 1.88 (CI 1.75–2.03, p < 0.0001). The risk of a primary outcome (severe exacerbations of COPD or all-cause mortality) in melatonin-treated patients was also increased (HR 1.30, CI 1.18–1.43, p < 0.0001). Finally, the risk of a primary outcome (severe exacerbations of COPD or all-cause mortality) was also increased among patients with comorbid asthma, patients collecting ICS, patients with a higher MRC score, patients with low BMI, and patients with a more severe FEV1% GOLD stage.
Figure 4. Forest plot of hazard ratios for primary outcome in the entire population: Severe exacerbations of COPD and all-cause death.
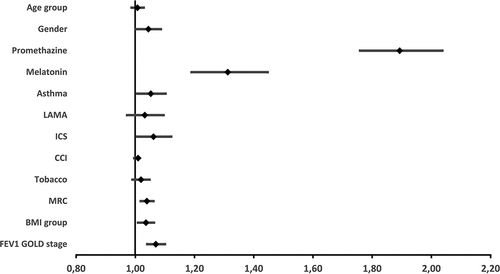
Table 3. Multivariable Cox analysis of the entire population.
Sensitivity analysis: risk with increased doses of promethazine in the entire population
In an adjusted Cox analysis, an additional increase in risk of a primary outcome (severe exacerbations of COPD or all-cause mortality) was observed among patients treated with a higher dose of promethazine plateauing at a cumulated dose equivalent to daily use (collection of ≥ 400 tablets within one year). Patients who collected 100 tablets of promethazine had HR of 1.74 (CI 1.60–1.89, p < 0.0001), increasing to HR of 2.03 (CI 1.80–2.30, p < 0.0001) among patients who collected 200 tablets, and further to HR 2.31 (CI 1.98–2.69) for patients collecting 300 tablets of promethazine within one year, . There was no further increase in risk of our primary outcome (severe exacerbations of COPD or all-cause mortality) among patients collecting ≥400 tablets within one year (HR 2.15, CI 1.94–2.38, p < 0.0001).
Table 4. Analysis of increased doses of promethazine in the entire population.
Loss to follow-up
Loss to follow-up due to emigration from Denmark was seldom in all investigated groups: In total N = 104 (0.2%) were lost to follow-up and no meaningful differences were observed between groups at baseline.
Discussion
In this active-comparator designed study, which is internationally the largest study of patients with COPD receiving promethazine, we found that treatment with promethazine was associated to a substantially higher risk of a composite outcome of severe exacerbations of COPD and death from all causes than with melatonin. Patients seemed closely matched in the active comparator population, and these two drugs are largely given on the same indication.
Secondary outcome analyses all revealed a signal in the same direction and a in separate analysis of death from all causes, we also observed an increase in risk among the promethazine users. The increased risk of admission by all causes could be caused mostly by severe exacerbations, as this is the leading cause of hospitalization among patients with severe COPD and as the risk of a primary outcome was higher than the risk of admission by all causes. An interaction analysis did not point to an altered risk among patients with concomitant COPD and asthma.
In another analysis assessing admissions from all causes, promethazine-users were also at higher risk of hospitalization, and the signal was in the same direction in an exploratory analysis of the risk of moderate exacerbations of COPD. However, this signal was weaker, and thus, we suspect, mainly driven by the severe COPD admissions.
A Cox proportional hazards multivariable analysis of the entire population of patients with COPD, treating promethazine as a covariate, confirmed the results. Further, patients using presumably higher doses (those collecting higher accumulated doses within the preceding year) had higher risk than those using lower doses, thus confirming a dose-response association.
Patients who collected 300–400 tablets in one year had a substantially higher risk of severe exacerbations of COPD or death, than those who collected 100–200 tablets. This strengthens the notion of causality, although this cannot be finally established without data from randomized controlled trials. The increased risk of all-cause mortality was not scrutinized, hence the causes for the increased risk of mortality remains speculative.
Previous evidence on promethazine showed i) a single dose of 25 mg promethazine in combination with morphine was associated with a higher exercise tolerance in patients with COPD (n = 7) [Citation8], ii) a daily dose of 25 mg promethazine for two weeks was associated with a lower degree of breathlessness and an improved exercise tolerance, but lung function measures seemed unchanged in patients with ‘pink puffer phenotype’ (n = 15) and likely COPD [Citation9], and iii) 25 mg promethazine daily was not associated to any changes in spirometry, arterial blood gases, 12-minute walk distance or subjective dyspnea rating in patients with chronic airflow obstruction (n = 11) during a one-month study [Citation10].
Our study has some strengths. First, all patients in the study have a respiratory medicine specialist and spirometry verified diagnosis of COPD. Second, the nationwide coverage of our registries assured that only 0.2% patients were lost to follow-up in the observation time. Third, in our COPD registry, we have annual data entry and very high follow-up on crucial confounders such as smoking status, lung function, body mass index and important co-morbidities. Our results seemed robust to different sensitivity analyses and through different analyses of non-desirable outcomes like COPD admissions, moderate COPD exacerbations, death from all causes and admissions from all causes (although the latter seemed to be driven by COPD exacerbations). Further, we identified a dose-response link.
Despite the above-mentioned strengths, our study has important limitations. due to the retrospective nature of the study, the presence of possible systematic bias influencing the results cannot be totally excluded [Citation22]. Although we accounted for confounders in the analysis, our study is observational, with inherent limitations regarding inferring causation. In this context, it is worth mentioning a possible difference in prescription patterns for promethazine and melatonin, such as clinicians prescribing promethazine to patients with poorer prognosis. If such a difference evaded our propensity score match it could confound our results. In our study the risk of severe exacerbations or death was also increased in patients treated with melatonin, which would cause us to underestimate the harmful effect of promethazine, or could be attributed to an underlying risk associated with insomnia and anxiety associated to treatment with both drugs. Also, some data were only available as semi-quantified data, and access to more complete data might have improved our propensity score matching.
Conclusions
Promethazine use in patients with severe COPD was associated with an excess risk of a composite outcome of severe COPD exacerbations and death from all cause, severe COPD exacerbations, death from all causes, as well as moderate COPD exacerbations and admittance to hospital from all causes. Since the indication for using this drug in patients with COPD seems weak, based on the lack of strong evidence for effect, our data should lead to caution when prescribing this drug to patients with COPD, and they do not support prescription of such drugs for mild insomnia or mild anxiety, which are the most common uses currently. Non-harmful alternatives to promethazine and melatonin could be instruction on sleep hygiene and cognitive behavioral therapy.
List of abbreviations
| ATC code | = | Anatomical Therapeutic Chemical code |
| BMI | = | body mass index |
| CCI | = | Charlson comorbidity index |
| COPD | = | Chronic obstructive pulmonary disease |
| DrCOPD | = | The Danish Register of Chronic Obstructive Pulmonary Disease |
| FEV1% | = | forced expiratory volume in the first second as percent of predicted |
| HR | = | Hazard Ratio |
| ICD-8 | = | The International Classification of Diseases, eighth revision |
| ICS | = | Inhaled corticosteroids |
| LAMA | = | Long-acting muscarinic receptor antagonist |
| MRC | = | Dyspnea Scale: Medical Research Council Dyspnea Scale |
| GOLD | = | initiative for Chronic Obstructive Lung Disease |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Golembiewski JA, O’Brien D. A systematic approach to the management of postoperative nausea and vomiting. J Perianesth Nurs. 2002;17(6):364–11. doi: 10.1053/jpan.2002.36596
- Chen X, Ji ZL, Chen YZ. TTD: Therapeutic Target Database. Nucleic Acids Res. 2002;30(1):412–415. doi: 10.1093/nar/30.1.412
- Southard BT, Al Khalili Y. Promethazine. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022 Jul 21 [cited2023 Jan]. https://www.ncbi.nlm.nih.gov/books/NBK544361/
- Promethazine, in LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012: Bethesda (MD).
- Thakkar MM. Histamine in the regulation of wakefulness. Sleep Med Rev. 2011;15(1):65–74. doi: 10.1016/j.smrv.2010.06.004
- Adam K, Oswald I. The hypnotic effects of an antihistamine: promethazine. Br J Clin Pharmacol. 1986;22(6):715–717. doi: 10.1111/j.1365-2125.1986.tb02962.x
- Risberg AM, Risberg J, Ingvar DH. Effects of promethazine on nocturnal sleep in normal man. Psychopharmacologia. 1975;43(3):279–284. doi: 10.1007/BF00429264
- Light RW, Stansbury DW, Webster JS. Effect of 30 mg of morphine alone or with promethazine or prochlorperazine on the exercise capacity of patients with COPD. Chest. 1996;109(4):975–981. doi: 10.1378/chest.109.4.975
- Woodcock AA, Gross ER, Geddes DM. Drug treatment of breathlessness: contrasting effects of diazepam and promethazine in pink puffers. Br Med J (Clin Res Ed). 1981;283(6287):343–346. doi: 10.1136/bmj.283.6287.343
- Rice KL, Kronenberg RS, Hedemark LL, et al. Effects of chronic administration of codeine and promethazine on breathlessness and exercise tolerance in patients with chronic airflow obstruction. Br J Dis Chest. 1987;81(3):287–292. doi: 10.1016/0007-0971(87)90163-X
- Lange P, Tøttenborg SS, Sorknæs AD, et al. Danish register of chronic obstructive pulmonary disease. Clin Epidemiol. 2016;8:673–678. doi: 10.2147/CLEP.S99489
- Miller MR, Hankinson JA, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805
- Schmidt M, Pedersen L, Sorensen HT. The Danish civil registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi: 10.1007/s10654-014-9930-3
- Ehrenstein V, Antonsen S, Pedersen L. Existing data sources for clinical epidemiology: aarhus university prescription database. Clin Epidemiol. 2010;2:273–279. doi: 10.2147/CLEP.S13458
- Schmidt M, Schmidt SA, Sandegaard JL, et al. The Danish National patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125
- Puustinen J, Lähteenmäki R, Nurminen J, et al. Long-term persistence of withdrawal of temazepam, zopiclone, and zolpidem in older adults: a 3-year follow-up study. BMC Geriatr. 2018;18(1):142. doi: 10.1186/s12877-018-0829-9
- Rondanelli M, Opizzi A, Monteferrario F, et al. The effect of melatonin, magnesium, and zinc on primary insomnia in long-term care facility residents in Italy: a double-blind, placebo-controlled clinical trial. J Am Geriatr Soc. 2011;59(1):82–90. doi: 10.1111/j.1532-5415.2010.03232.x
- Nunes DM, Mota RMS, Machado MO, et al. Effect of melatonin administration on subjective sleep quality in chronic obstructive pulmonary disease. Braz J Med Biol Res. 2008;41(10):926–931. doi: 10.1590/S0100-879X2008001000016
- Campos FL, da Silva-Júnior FP, de Bruin VMS, et al. Melatonin improves sleep in asthma: a randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med. 2004;170(9):947–951. doi: 10.1164/rccm.200404-488OC
- Research, D.o.Q.H.S.M.C. [cited 2022 Jan 1]. http://bioinformaticstools.mayo.edu/research/gmatch/. 2022
- Meyers J, NewSurv Macro. https://communities.sas.com/t5/SAS-Communities-Library/Kaplan-Meier-Survival-Plotting-Macro-NEWSURV/ta-p/479747, 2021.
- Suissa S, Dell’aniello S. Time-related biases in pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2020;29(9):1101–1110. doi: 10.1002/pds.5083
Appendix I:
Cumulated incidence plots of all-cause admission of propensity score matched patients
Patients were propensity score matched 1:1 by age, gender, tobacco exposure, MRC, BMI, FEV1% GOLD stages, collection of inhaled corticosteroids, collection of inhaled long-acting muscarinic receptor antagonist and Charlson comorbidity index. (N = 3290)
Figure A1. Cumulated incidence plots of all-cause admission of propensity score matched patients. Patients were propensity score matched 1:1 by age, gender, tobacco exposure, MRC, BMI, FEV1% GOLD stages, collection of inhaled corticosteroids, collection of inhaled long-acting muscarinic receptor antagonist and Charlson comorbidity index. (N=3,290).