ABSTRACT
Cough is a condition that can be caused by several different mechanisms. There are numerous guidelines for diagnosing the cause of cough, yet the effect of a well-constructed examination framework has not been investigated. At the Department of Internal Medicine, Lillebaelt Hospital, Vejle, a systematic examination framework for diagnosing cough was introduced. Two hundred consecutive patients referred to the pulmonary outpatient clinic with cough were included. The first 100 patients (Group 1) were included before implementation of the examination framework and diagnosed as usual. The next 100 patients (Group 2) were examined using the systematic framework. The primary endpoint was the number of appointments required to establish a diagnosis. A multivariable Poisson regression was performed, adjusting for age, sex, body mass index, pulmonary function (FEV1/FVC), duration of cough, and smoking status. A diagnosis was established within 1–2 visits in 47% in Group 1 compared to 83% in Group 2. When adjusting for confounders, fewer appointments was required to establish a diagnosis in Group 2 (Incidence rate ratio = 0.713 (95% confidence interval: 0.592–0.859), P = 0.000). Using a systematic examination framework for diagnosing cough may reduce the number of appointments required to establish a diagnosis, seemingly without compromising the diagnostic outcome.
Introduction
Chronic cough in adults is a prevalent health problem that has been estimated to occur in about 10% of the global population [Citation1]. It is generally defined as a cough with a duration of ≥8 weeks [Citation2], and if the underlying cause cannot be established, it is classified as unexplained chronic cough [Citation3].
Chronic cough can be characterized as both a symptom of an underlying condition and as a distinct condition, known as cough hypersensitivity syndrome [Citation2]. Chronic cough is often triggered by mechanical, chemical, or thermal stress [Citation4], but an underlying condition is not established in many cases. It has been estimated that up to 46% of patients with chronic cough have unexplained chronic cough [Citation3]. Known underlying conditions of chronic cough include respiratory diseases such as chronic obstructive pulmonary disease (COPD), asthma, interstitial lung disease, bronchitis (due to smoking), bronchiectasis, and pulmonary cancer [Citation4]. Extrapulmonary conditions include gastro-esophageal reflux disease (GERD) [Citation4], iatrogenic cough caused by angiotensin-converting enzyme (ACE) inhibitor treatment [Citation5], and postnasal drip syndrome, rhinitis, and rhinosinusitis, collectively known as upper airways cough syndrome [Citation6].
The burden of chronic cough is substantial for both society and the individual. Cough as a symptom is associated with a significant socioeconomic impact and increased healthcare consumption [Citation7,Citation8], and chronic cough is associated with a considerable and resource consuming diagnostic examination work-up in a large amount of patients [Citation9]. Furthermore, chronic cough is associated with lower job productivity and time missed from work [Citation10,Citation11]. The increased individual patient burden of chronic cough is also well established with an increased risk of a wide range of negative physical and psychological consequences [Citation12–14], and cough is associated with a decrease in the quality of life [Citation12,Citation15]. Physical complications include stress urinary incontinence, syncope, vomiting, sleep disturbance, hernias, and interference with speech [Citation4,Citation15–17]. Psychological complications include anxiety, depression, social embarrassment, concern about serious underlying illness, and annoyance to family, friends, and coworkers [Citation12,Citation18,Citation19].
Because of the personal and societal burden, it is important to reach a diagnosis and commence effective treatment without unnecessary delays. Several guidelines for the diagnosis and management of cough have been published, and the recommended management is generally diagnostic testing followed by stepwise empirical treatment [Citation2,Citation4,Citation11]. This stepwise approach has been criticized in the management of cough and respiratory diseases, as it may delay relevant diagnosis and treatment due to prolonged diagnostic testing, and a treatable traits paradigm has been proposed instead [Citation2,Citation20–22]. A different way of tackling this issue, is introducing dedicated patient pathways, which has reduced the time from suspicion of disease to a diagnosis in other clinical fields. Denmark introduced standardized cancer patient pathways (CPPs) in 2008 and 2009, which include guidelines and descriptions of selected red flag symptoms [Citation23]. The CPPs in Denmark have resulted in faster diagnosis and treatment initiation in cancer patients [Citation24–26] and most likely an increase in survival [Citation27].
With this study, we aimed to investigate if introducing a systematic examination framework for diagnosing chronic cough would lead to a reduction in the time required to establish a diagnosis without compromising the diagnostic quality.
Methods
Study inclusion
At the pulmonary outpatient clinic at the Department of Internal Medicine, Lillebaelt Hospital, Vejle, a systematic examination framework for diagnosing chronic cough was introduced. Two hundred consecutive patients referred with the diagnosis ‘DR059 Cough’ were enrolled in this study. The first 100 patients were diagnosed using the usual approach (Group 1), and the next 100 patients were diagnosed using a systematic approach (Group 2).
With the usual approach, the first appointment was always with a doctor. However, with the systematic approach the patients consulted either a nurse or a doctor who performed a systematic screening of symptoms and medical history using a systematic screening tool (). Further examinations, tests and/or medication were ordered based on the screening tool evaluation. The screening tool was developed by experienced respiratory physicians at the pulmonary outpatient clinic.
Table 1. Systematic screening tool used in the study.
Data collection
Data were collected retrospectively via patient records. The number of appointments required to establish a diagnosis was the primary endpoint. The number of days from referral to 1st appointment and from 1st appointment to diagnosis were also collected. A diagnosis was considered established on the date where the patient received information about the diagnosis. This could be based on abnormal test results or by clinical judgment from a senior doctor. A diagnosis of asthma was considered established on the date of a positive bronchodilator reversibility test, significant peak flow variability, asthma provocation test, or explicit clinical judgement from a senior doctor. For discontinuation of angiotensin-converting enzyme inhibitor treatment, the diagnosis was considered established only after verified cough disappearance on follow-up. For gastroesophageal reflux disease, the diagnosis was considered established only after verified cough disappearance following initiation of proton pump inhibitor treatment.
We collected baseline characteristics such as sex, age, body mass index (BMI), pulmonary function, smoking status, and cough duration. Chronic cough was binarily defined as cough lasting longer than 8 weeks. Blood samples and a chest x-ray were routinely obtained prior to the first appointment, and a spirometry is always performed. We also collected data regarding which type of clinician the patients consulted on 1st appointment (nurse, junior doctor, or senior doctor). A senior doctor was defined as a doctor with an obtained specialization in pulmonology.
Secondary endpoints to investigate the quality of the diagnostic framework were the final diagnosis established and the number of patients having performed secondary diagnostic examinations (asthma provocation tests (methacholine or mannitol test as appropriate), bronchoscopies, high-resolution computed tomography (HRCT) scans, referrals to the CPP). These were collected as binary variables (yes/no).
Statistics
Univariable analyses were made with t-test or Wilcoxon Rank Sum test for continuous variables depending on the distribution of the variable. For categorical variables with two groups, the Fischer’s exact test was used. For categorical variables with more than two groups, a Chi-squared test was performed. Wilcoxon Rank Sum test was used to test for differences in time and appointments required to reach a diagnosis (), and median and interquartile ranges (IQR) were calculated.
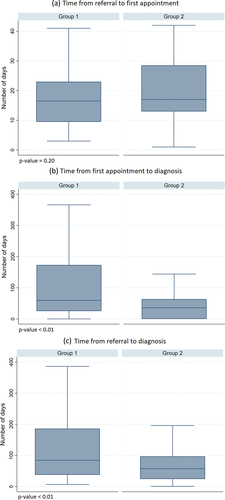
Figure 2. Time in days required to establish a diagnosis. (A) time from referral to 1st appointment, (B) time from 1st appointment to diagnosis, (C) time from referral to diagnosis. Outside values are excluded from graphs.
To assess the relationship between the intervention and the number of appointments required to establish a diagnosis, we performed a multivariable Poisson regression adjusting for pre-determined relevant confounders (sex, age, FEV1/FVC, and BMI). Multiple imputation was used as recommended to account for missing data in the variables BMI, FEV1/FVC, cough duration (Chronic cough >8 weeks), and smoking status [Citation28]. The model was checked for overdispersion, mean misspecification, and overall goodness of fit, and the model did not violate any assumptions. Complete case analysis was made to assess the model’s reliance on multiple imputation, and this showed a high reliance likely due to loss of observations in complete case analysis (Appendix A, Table S1).
A significant difference in the clinician type at 1st appointment was found between the two groups. This difference was a direct result of the intervention, and we did not include this variable in the primary analysis as recommended by the guidelines on adjustment for baseline covariates by the European Medicines Agency [Citation29]. We included the variable in a secondary analysis as recommended (Appendix A, Table S2) [Citation29]. We also investigated the number of appointments required when only including patients who consulted a senior doctor at 1st appointment (Appendix A, Figure S1). Stata 17 was used for all statistical analyses. A significance level of 0.05 was used.
Results
Population baseline characteristics
displays baseline characteristics of the two groups. Hereafter, all instances of ‘significant’ refer to statistical significance. There were no significant differences in age, BMI, sex, smoking status, or the number of patients with chronic cough. Group 1 had a significantly lower FEV1/FVC, although most patients had a normal FEV1/FVC in both groups. There was no difference between the two groups in FEV1% of predicted. FVC % of predicted was higher in Group 1, but the difference was not significant (p = 0.051).
Table 2. Baseline patient characteristics.
There was a significant difference between the two groups in the type of clinician the patient consulted on 1st appointment. 38 patients in Group 2 consulted a nurse compared to 0 in group 1. 15 patients in Group 2 consulted a junior doctor compared to 73 in Group 1, and 47 patients consulted a senior doctor in Group 2 compared to 27 in Group 1.
Primary outcomes: number of appointments and time required to reach diagnosis
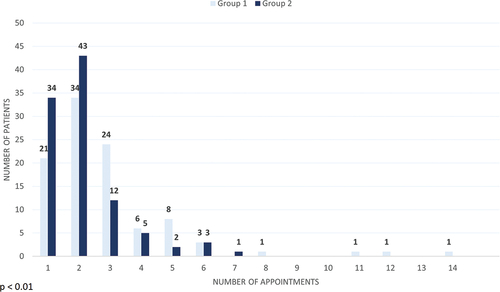
Significantly fewer appointments were required to establish a diagnosis for patients in Group 2 (median = 2, IQR: 1–2) compared to patients in Group 1 (median = 2, IQR: 2–3) (). Correspondingly, significantly less time was required to establish a diagnosis for patients in Group 2 (). shows that significantly fewer days were required from 1st appointment to diagnosis in Group 2 (median = 36, IQR: 0–63.5) compared to Group 1 (median = 59.5, IQR: 25.5–173), and from referral to diagnosis in Group 2 (median = 57.5, IQR:24.5–97) compared to Group 1 (median = 84.5, IQR: 38–186). There was no significant difference between Group 1 (median = 16.5, IQR: 9.5–23) and Group 2 (median = 17. IQR: 13–28.5) in the number of days from referral to first appointment.
Results from the multivariable Poisson regression are shown in . When adjusting for possible confounders, Group 2 (systematic framework) still had significantly fewer appointments required to establish a diagnosis with an incidence rate ratio (IRR) = 0.713 (95% CI: 0.592–0.859) compared to Group 1 (p < 0.001). Sex, age, FEV1/FVC, BMI, smoking status, and chronic cough were not significantly associated with number of appointments required for diagnosis. The results from the secondary analysis are displayed in Appendix A, Table S2 and Figure S1. Table S2 shows that including the variable clinician type at 1st appointment did not alter the regression estimates substantially, and the intervention is still related to fewer appointments required. This can also be seen in Figure S1, where significantly fewer appointments were required in Group 2 when only including patients who consulted a senior doctor at 1st appointment. Table S2 also shows that consulting a nurse at 1st appointment is significantly related to more appointments required compared to a senior doctor (IRR = 1.425 (95% CI: 1.075–1.888)).
Table 3. Multivariable Poisson regression model for the number of appointments required to establish a diagnosis.
Secondary outcomes: quality control parameters
shows the difference in quality control variables between the two groups. There were no significant differences in number of HRCT scans, bronchoscopies, or referrals to the CPP. There was no significant difference in the diagnoses established, but the number of patients diagnosed with asthma was numerically lower in Group 2 (19 compared to 28) and the number of infections was numerically higher (12 compared to 2). Significantly less asthma provocation tests were performed in Group 2. To explore the reason for this, we conducted a post-hoc analysis to investigate the relationship between the number of asthma provocation tests performed and the clinician type at 1st appointment (Appendix A, Table S3). This unveiled significant differences, where senior doctors ordered fewer asthma provocation tests compared to junior doctors and nurses.
Table 4. Quality control parameter comparison between the two groups.
Discussion
To our knowledge, this study is the first to report the impact of using a systematic examination framework in the diagnosis of cough. We found that introducing a systematic approach resulted in significantly fewer appointments required and significantly shorter time to diagnosis. These reductions lie solely in the time from the first appointment to diagnosis, as no reduction in the time from referral to the first appointment was seen. Interestingly, most patients in Group 2 (Systematic framework) are diagnosed within 2 appointments or less, and no patients required more than 7 appointments – compared to four patients in Group 1.
The reasons for this reduction in both time and appointments required can only be speculated upon, but a reduction in waiting time for diagnosis and treatment has been seen after the introduction of CPPs in both Denmark [Citation24–26] and other countries [Citation30–32]. Dedicated patient pathways have also been associated with favorable outcomes in hospitalized patients with a decrease in hospitalization time within various medical specialties [Citation33–35], a better patient flow and a decrease in readmission rates in ischemic stroke patients [Citation36], and they have even been associated with quality of care improvements in several settings [Citation37–39].
In our study, it is difficult to conclude whether the use of a systematic framework or the clinician type at first appointment is more important in decreasing the number of appointments required for a diagnosis. In our secondary analysis we have shown that both are significantly associated with fewer appointments required until reaching a diagnosis. This is in accordance with existing evidence: a study by Sreih et al. found that patients with vasculitis were diagnosed faster if they were initially seen by a specialist [Citation40]. Furthermore, limited access to tertiary level expertise has been associated with a less timely diagnosis in interstitial lung disease [Citation41].
Having a lower ratio of FEV1/FVC seemed, although not significantly, associated with less appointments required. It is well known that it requires more time to reach a diagnosis in complex diseases that do not readily fit into our pre-defined diagnostic boxes [Citation42], and more time and investigations are likely required to reach a diagnosis in patients without a clear pulmonary disease. The two patient groups in our study are very similar, and only the pulmonary function (FEV1/FVC) is significantly different between them. The FEV1/FVC of both groups, however, is well above the threshold of 0.70, so this does not appear clinically relevant. Therefore, differences between the two patient groups are not likely to explain the difference in the number of appointments needed to establish a diagnosis.
The only significant difference in quality parameters (secondary diagnostic examinations and diagnoses) between the two groups was the lower number of asthma provocation tests performed in Group 2. Importantly, the number of patients with an unresolved etiology of the cough was similar at about 40% in both groups, which also corresponds to existing literature [Citation43]. This indicates that the patients in Group 2 are, in general, adequately examined and diagnosed when compared to the usual approach in Group 1. The lower number of asthma provocation tests in Group 2 may, at least partly, be explained by the fact that more patients in Group 2 consulted a senior doctor at 1st appointment, and that senior doctors were less likely to order asthma provocation tests. The non-significant reduction in the number of patients diagnosed with asthma in group 2 might be caused by the lower number of asthma provocation tests. To correctly diagnose asthma in chronic cough is difficult, as it is a clinical diagnosis without a single diagnostic tests to either diagnose or exclude asthma, and opinions are divided about the use of asthma provocation tests in cough variant asthma [Citation4]. It may be speculated that senior doctors due to experience are better at not ordering asthma provocation tests for patients, in whom asthma is highly unlikely. These tests can result in false positives [Citation44], which may result in a subsequent unnecessary or potentially harmful treatment regimen. On the other hand, undertesting may result in underdiagnosis of asthma.
Our findings have several potential implications. A reduction in number of required appointments and time to diagnosis has the potential to decrease healthcare expenditures, and it may also increase the quality of life of the individual patient. A decrease in time to relevant diagnosis and treatment may reduce the duration of symptoms, and also reduce the duration of concern of a serious underlying illness, which is known to cause increased levels of anxiety in patients with chronic cough [Citation12]. For these reasons, we consider it important to investigate further upon our results, and we stress the relevance of randomized controlled trials to replicate our results in a more controlled setting. Furthermore, we have documented that visiting a nurse at 1st appointment is associated with more appointments required compared to visiting a senior doctor, and this also warrants further exploration.
This study has various limitations. First, this was a relatively small study, which decreases generalizability and replicability. However, the demographic characteristics of our study populations appear to be consistent with existing literature [Citation2,Citation4,Citation43], which increases the probability that our study population is a representative sample. Second, this was conducted as a before-after cohort study instead of a randomized controlled trial. Residual confounding is likely present, and we cannot for certain conclude that a causal relationship exists between the systematic framework and the reduction in time to diagnosis, although this appears highly likely. For example, it is possible that some clinicians were involved in the diagnosis in patients from both Group 1 and Group 2. The more inexperienced clinicians (junior doctors primarily) might have adopted a more focused approach during the two stages of the study, which may account for some of the observed reduction. Third, as a direct result of the intervention, we saw a difference in which type of clinician the patients consulted at the first appointment, and it is difficult to completely distinguish the effect of the systematic framework from the effect of the clinician type. This issue was handled by performing a secondary analysis adjusting for clinician type, where we saw a significant effect of both the systematic framework and the clinician type. Fourth, the retrospective data collection resulted in missing data, but this was not to an extent that made multiple imputation unwarranted [Citation28].
Conclusions
Using a systematic examination framework for diagnosing chronic cough seems to reduce the number of appointments and time required to establish a diagnosis without compromising the diagnostic outcome. This should be investigated further, as it holds the potential to reduce health expenses and improve patients’ overall quality of life by commencing relevant treatment earlier and reducing uncertainty of serious underlying illness among patients.
CRediT authorship contribution statement
Allan Klitgaard: Methodology, Formal analysis, Investigation, Data Curation, Writing – Original Draft, Writing – Review and Editing, Visualization. Anders Løkke: Supervision, Writing – Review and Editing. Jannie Frølund: Formal Analysis, Investigation, Writing – Review and Editing, Project Administration, Funding Acquisition. Steffen Kristensen: Conceptualization, Methodology, Writing – Review and Editing. Ole Hilberg: Supervision, Writing – Review and Editing, Project Administration.
Data-sharing statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved as a quality assurance project (project number: 18/44304) and included in the Region of Southern Denmark’s register of processing of personal data in connection with research. All analyses and data handling comply with the General Data Protection Regulation (GDPR) and the Danish Data Protection Agency guidelines for research on sensitive data. In accordance with Danish law and National Research Ethics Committee guidelines, no additional ethical approval is needed for regionally approved quality assurance projects.
Supplemental Material
Download PDF (176.3 KB)Acknowledgments
We would like to thank pulmonologist Kristian Rasmussen from the Department of Internal Medicine, Lillebaelt Hospital, for valuable help with data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20018525.2023.2273026
Additional information
Funding
References
- Song WJ, Chang YS, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J. 2015;45(5):1479–10. doi: 10.1183/09031936.00218714
- Morice A, Dicpinigaitis P, McGarvey L, et al. Chronic cough: new insights and future prospects. Eur Respir Rev. 2021;30(162):210127. doi: 10.1183/16000617.0127-2021
- Gibson P, Wang G, McGarvey L, et al. Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest. 2016;149(1):27–44. doi: 10.1378/chest.15-1496
- Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. 2020;55(5): 1901136. Eur Respir J 56 (2020).
- Morice AH, Lowry R, Brown MJ, et al. Angiotensin-converting enzyme and the cough reflex. Lancet. 1987;2(8568):1116–1118. doi: 10.1016/S0140-6736(87)91547-9
- Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):1s–23s. doi: 10.1378/chest.129.1_suppl.1S
- Morice AH. Epidemiology of cough. Pulm Pharmacol Ther. 2002;15(3):253–259. doi: 10.1006/pupt.2002.0352
- Axelsson M, Lindberg A, Kainu A, et al. Respiratory symptoms increase health care consumption and affect everyday life – a cross-sectional population-based study from Finland, Estonia, and Sweden. Eur Clin Respir J. 2016;3(1):31024. doi: 10.3402/ecrj.v3.31024
- Backer V, Porsborg A, Hansen V, et al. A register-based study: cough - a frequent phenomenon in the adult population. BMC Pulm Med. 2022;22(1):426. doi: 10.1186/s12890-022-02228-z
- Koskela HO, Lätti AM, Pekkanen J. The impacts of cough: a cross-sectional study in a Finnish adult employee population. ERJ Open Res. 2018;4(4):00113–2018. doi: 10.1183/23120541.00113-2018
- Irwin RS, French CT, Lewis SZ, et al. Overview of the management of cough: CHEST guideline and expert panel report. Chest. 2014;146(4):885–889. doi: 10.1378/chest.14-1485
- French CL, Irwin RS, Curley FJ, et al. Impact of chronic cough on quality of life. Arch Intern Med. 1998;158(15):1657–1661. doi: 10.1001/archinte.158.15.1657
- Raj AA, Birring SS. Clinical assessment of chronic cough severity. Pulm Pharmacol Ther. 2007;20(4):334–337. doi: 10.1016/j.pupt.2006.10.002
- Song WJ, Morice AH, Kim MH, et al. Cough in the elderly population: relationships with multiple comorbidity. PLoS One. 2013;8(10):e78081. doi: 10.1371/journal.pone.0078081
- Young EC, Smith JA. Quality of life in patients with chronic cough. Ther Adv Respir Dis. 2010;4(1):49–55. doi: 10.1177/1753465809358249
- Everett CF, Kastelik JA, Thompson RH, et al. Chronic persistent cough in the community: a questionnaire survey. Cough. 2007;3(1):5. doi: 10.1186/1745-9974-3-5
- Dicpinigaitis PV, Lim L, Farmakidis C. Cough syncope. Respir med. 2014;108(2):244–251. doi: 10.1016/j.rmed.2013.10.020
- Kuzniar TJ, Morgenthaler TI, Afessa B, et al. Chronic cough from the patient’s perspective. Mayo Clin Proc. 2007;82(1):56–60. doi: 10.1016/S0025-6196(11)60967-1
- Hulme K, Deary V, Dogan S, et al. Psychological profile of individuals presenting with chronic cough. ERJ Open Res. 2017;3(1):00099–2016. doi: 10.1183/23120541.00099-2016
- Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47(2):410–419. doi: 10.1183/13993003.01359-2015
- Mazzone SB, Chung KF, McGarvey L. The heterogeneity of chronic cough: a case for endotypes of cough hypersensitivity. Lancet Respir Med. 2018;6(8):636–646. doi: 10.1016/S2213-2600(18)30150-4
- Shrimanker R, Choo XN, Pavord ID. A new approach to the classification and management of airways diseases: identification of treatable traits. Clin Sci (Lond). 2017;131(10):1027–1043. doi: 10.1042/CS20160028
- Probst HB, Hussain ZB, Andersen O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians–a national Danish project. Health Policy. 2012;105(1):65–70. doi: 10.1016/j.healthpol.2011.11.001
- Lyhne NM, Christensen A, Alanin MC, et al. Waiting times for diagnosis and treatment of head and neck cancer in Denmark in 2010 compared to 1992 and 2002. Eur J Cancer. 2013;49(7):1627–1633. doi: 10.1016/j.ejca.2012.11.034
- Dyrop HB, Safwat A, Vedsted P, et al. Cancer patient pathways shortens waiting times and accelerates the diagnostic process of suspected sarcoma patients in Denmark. Health Policy. 2013;113(1–2):110–117. doi: 10.1016/j.healthpol.2013.09.012
- Jensen H, Tørring ML, Olesen F, et al. Diagnostic intervals before and after implementation of cancer patient pathways – a GP survey and registry based comparison of three cohorts of cancer patients. BMC Cancer. 2015;15(1):308. doi: 10.1186/s12885-015-1317-7
- Jensen H, Vedsted P. Exploration of the possible effect on survival of lead-time associated with implementation of cancer patient pathways among symptomatic first-time cancer patients in Denmark. Cancer Epidemiol. 2017;49:195–201. doi: 10.1016/j.canep.2017.06.006
- Jakobsen JC, Gluud C, Wetterslev J, et al. When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts. BMC Med Res Methodol. 2017;17(1):162. doi: 10.1186/s12874-017-0442-1
- Guideline on adjustment for baseline covariates in clinical trials, 2015. [cited 2022 Dec 12]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-adjustment-baseline-covariates-clinical-trials_en.pdf
- Valentín-López B, Ferrándiz-Santos J, Blasco-Amaro JA, et al. Assessment of a rapid referral pathway for suspected colorectal cancer in Madrid. Fam Pract. 2012;29(2):182–188. doi: 10.1093/fampra/cmr080
- Guzmán Laura KP, Bolíbar Ribas I, Alepuz MT, et al. Impact on patient care time and tumor stage of a program for fast diagnostic and treatment of colorectal cancer. Rev Esp Enferm Dig. 2011;103(1):13–19. doi: 10.4321/S1130-01082011000100003
- Vallverdú-Cartié H, Comajuncosas-Camp J, Orbeal-Sáenz RA, et al. Results of implementation of a fast track pathway for diagnosis of colorectal cancer. Rev Esp Enferm Dig. 2011;103(8):402–407. doi: 10.4321/S1130-01082011000800003
- Mummadi SR, Hahn PY. Outcomes of a clinical pathway for pleural disease management: “pleural pathway. Pulm Med. 2018;2018:2035248. doi: 10.1155/2018/2035248
- Ng JWG, Smith C, Ilo K, et al. Dedicated peri-operative pathway improved day case discharge rate for anterior cruciate ligament reconstructions. Eur J Orthop Surg Traumatol. 2019;29(3):639–644. doi: 10.1007/s00590-018-2326-4
- Zehr KJ, Dawson PB, Yang SC, et al. Standardized clinical care pathways for major thoracic cases reduce hospital costs. Ann Thorac Surg. 1998;66(3):914–919. doi: 10.1016/S0003-4975(98)00662-6
- de Belvis AG, Lohmeyer FM, Barbara A, et al. Ischemic stroke: clinical pathway impact. Int J Health Care Qual Assur. 2019;32(3):588–598. doi: 10.1108/IJHCQA-05-2018-0111
- Isoardi K, Learmont B, Horan B, et al. Dedicated nursing care pathway improved management of opioid-poisoned patients in the emergency department: a before–after observational study. Emerg Med Australas. 2022;35(1):69–73. doi: 10.1111/1742-6723.14056
- Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;3:Cd006632. doi: 10.1002/14651858.CD006632.pub2
- Maxey C. A case map reduces time to administration of thrombolytic therapy in patients experiencing an acute myocardial infarction. Nurs Case Manag. 1997;2(5):229–237.
- Sreih AG, Cronin K, Shaw DG, et al. Diagnostic delays in vasculitis and factors associated with time to diagnosis. Orphanet J Rare Dis. 2021;16(1):184. doi: 10.1186/s13023-021-01794-5
- Cosgrove GP, Bianchi P, Danese S, et al. Barriers to timely diagnosis of interstitial lung disease in the real world: the INTENSITY survey. BMC Pulm Med. 2018;18(1):9. doi: 10.1186/s12890-017-0560-x
- Benito-Lozano J, Arias-Merino G, Gómez-Martínez M, et al. Diagnostic process in rare diseases: determinants associated with diagnostic delay. Int J Environ Res Public Health. 2022;19(11):6456. doi: 10.3390/ijerph19116456
- Haque RA, Usmani OS, Barnes PJ. Chronic idiopathic cough: a discrete clinical entity? Chest. 2005;127(5):1710–1713. doi: 10.1378/chest.127.5.1710
- Sverrild A, Porsbjerg C, Thomsen SF, et al. Airway hyperresponsiveness to mannitol and methacholine and exhaled nitric oxide: a random-sample population study. J Allergy Clin Immunol. 2010;126(5):952–958. doi: 10.1016/j.jaci.2010.08.028