ABSTRACT
Background
The management of pulmonary nodules plays a critical role in early detection of lung cancer. Computed tomography (CT) has led to a stage-shift towards early-stage lung cancer, but regional differences in survival rates have been reported in Denmark. This study aimed to evaluate whether variations in nodule management among Danish health regions contributed to these differences.
Material and Methods
The Danish Health Data Authority and Danish Lung Cancer Registry provided data on CT usage and lung cancer stage distribution, respectively. Auditing of lung cancer stage IA patient referrals and nodule management of stage IV lung cancer patients was conducted in seven Danish lung cancer investigation centers, covering four of the five Danish health regions. CT scans were performed up to 2 years before the patients’ diagnosis from 2019 to 2021.
Results
CT usage has increased steadily in Denmark over the past decade, with a simultaneous increase in the proportion of early-stage lung cancers, particularly stage IA. However, one Danish health region, Region Zealand, exhibited lower rates of early-stage lung cancer and overall survival despite a CT usage roughly similar to that of the other health regions. The audit did not find significant differences in pulmonary nodule management or a higher number of missed nodules by radiologists in this region compared to others.
Conclusion
This study suggests that a high CT scan volume alone is not sufficient for the early detection of lung cancer. Factors beyond hospital management practices, such as patient-related delays in socioeconomically disadvantaged areas, may contribute to regional differences in survival rates. This has implications for future strategies for reducing these differences.
Background
Pulmonary nodules are small, rounded opacities within the lung interstitium with a size between 4 and 30 mm. They are often detected incidentally during imaging examination such as chest X-ray or computed tomography (CT) scans [Citation1,Citation2]. Most pulmonary nodules are benign and are often due to infection, scaring or autoimmune diseases, however, some represent early-stage lung cancer [Citation3]. Early detection and management of pulmonary nodules thus play a crucial role in the early diagnosis and treatment of lung cancer [Citation4].
Screening for lung cancer with low-dose CT is offered for a high-risk population of current and previous smokers in the US [Citation5], while the majority of European countries do not have a full-scale lung cancer screening program [Citation6,Citation7]. The implementation of CT screening in the US has led to an increase in the proportion of stage I lung cancer [Citation8,Citation9]; however, some non-screening countries have also reported an uptick in early-stage lung cancer cases [Citation10–12]. This has been speculated to be linked to the globally increased use of CT scans and subsequent detection and management of incidental pulmonary nodules (IPNs) [Citation13,Citation14]. The management of IPNs is a significant challenge for clinicians, as they need to differentiate between benign and malignant nodules [Citation15], which often requires follow-up CT scans, supplementary imaging with positron-emission-tomography CT (PET-CT) or invasive procedures. Additionally, the failure to detect pulmonary nodules can result in missing a valuable opportunity to diagnose lung cancer at an early, resectable stage [Citation10].
The 5-year survival rate for lung cancer varies by country and is typically in the range of 15–25% [Citation16]. However, the 5-year survival rate in Japan is 32.9%, which can be attributed to a favorable stage distribution, with 36.7% of lung cancer cases being diagnosed at stage I [Citation16]. Moreover, with a significant number of CT scans conducted in Japan (235 per 1,000 inhabitants) [Citation17], there is a high likelihood of a causal relationship. However, in a prior investigation that compared the five regions of Denmark, it was revealed that only three out of the five regions displayed a distinct association between the proportion of stage IA lung cancer and number of chest CT per 1,000 inhabitants [Citation10].
In Denmark, a significant variation in lung cancer stage distribution and survival has been observed among the five Danish health regions [Citation18]. We hypothesized that regional differences in the in-hospital management of nodules might explain the observed variations in proportion of lung cancers found in early stages and in the survival rates.
Material and methods
Auditing
This retrospective study aimed to examine the management of any pulmonary nodules in the preceding 2 years before patients were diagnosed with stage IV lung cancer, as well as the referral pattern for CT scans that lead to diagnosis of stage IA lung cancer in seven respiratory departments across Denmark specialized in workup of suspected cancer as well as the management of pulmonary nodules.
Information regarding the yearly usage of CT scans in Denmark was obtained from the Danish Health Data Authority, while details about the stage distribution and survival rates of lung cancer patients in Denmark were extracted from the Danish Lung Cancer Registry [Citation18].
Clinical auditing was performed in the following seven lung cancer investigation sites: Aalborg University Hospital (North Denmark Region), Aarhus University Hospital and Gødstrup Hospital (Central Denmark Region), Lillebælt Hospital Vejle and Odense University Hospital (Region of Southern Denmark), Zealand University Hospital Roskilde and Næstved (Region Zealand).
During the auditing of patients who were diagnosed with stage IA lung cancer between 2019 and 2021, the cause for referral for CT was documented in order to assess whether the early-stage lung cancer was identified incidentally or because of a suspicion of lung cancer.
As part of the auditing process for patients with stage IV lung cancer diagnosed between 2019 and 2021, CT scans visualizing parts or the entire thorax and conducted up to 2 years before the diagnosis was reviewed. The review aimed to determine if the CT descriptions mentioned any nodules or infiltrates, whether these were localized at the same site and likely had progressed to stage IV lung cancer, and if appropriate follow-up procedures had been conducted. Nodules less than 6 mm were not deemed inappropriately monitored. Furthermore, if nodules or infiltrates were not mentioned in the description, the CT scan was reviewed to see if any nodules or infiltrates had been missed or ignored in the examination. Experienced invasive pulmonologists were responsible for reviewing the CT scans.
Statistics
The clinical auditing data was gathered through REDCap [Citation19], while lung cancer stage and CT usage information was compiled and analyzed using Microsoft Excel version 16.71. Statistical tests for differences in proportions were done in Stata 17.0 BE (StataCorp, 4905 Lakeway Drive, College Station, Texas).
Ethics
The hospital administrations granted permission to conduct an audit of patient charts and CT scans as part of a quality improvement study. The Danish Health Data Authority provides publicly available information on CT scans, while the Danish Lung Cancer Registry also makes its information accessible to the public in yearly reports [Citation18].
Results
Background for the audit
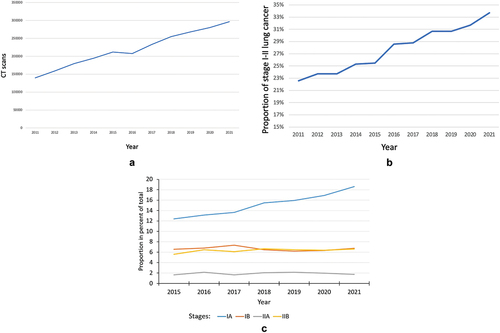
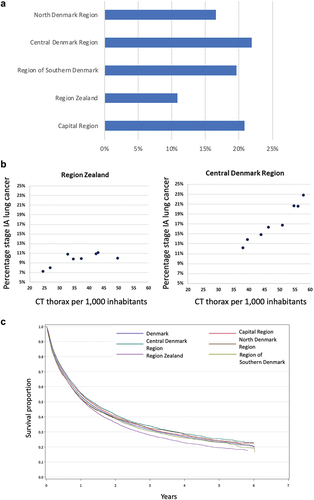
The usage of chest CT increased steadily in Denmark with 112% from 2011 to 2021 (). The slight decrease between 2015 and 2016 was attributed to the introduction of new radiological and clinical IT systems that temporarily reduced CT capacity. The proportion of stage I-II lung cancer increased steadily throughout the period () primarily due to increase in stage IA (). However, the proportion of stage IA lung cancer varied considerably and significantly between the five Danish health regions () with stage IA rate in Region Zealand roughly 50% of that in Central Denmark Region, Capitol Region and Region of Southern Denmark. Chest CT use correlates with stage IA rate in the Central Denmark Region but not in Region Zealand (). The correlations in the other regions have been described previously [Citation10]. The 5-year over-all survival rates after the diagnosis of lung cancer (all stages) were significantly lower in Region Zealand than in other regions, reflecting the less favorable stage distribution ().
Audit of stage IA lung cancers: incidental finding
In total, 1464 patient files and CT scans were reviewed, and a mean of 87.4% (between-site range 76.0–94.3%) of stage I lung cancers were discovered incidentally on CT for other reasons than suspicion of lung cancer, such as suspicion of pulmonary embolism, staging or follow-up of other solid cancers, cardiac CT, or abdominal CT for gastrointestinal symptoms.
Audit of stage IV lung cancer: missed nodules
Medical files and CT scans from a total of 4066 patients diagnosed with stage IV lung cancer were reviewed (). A total of 14% (552 patients) had undergone a partial or complete chest CT within 2 years prior to diagnosis. In Region Zealand, this prevalence was significantly lower compared to the other regions (9.3% vs. 15.5%, p < 0.001). Of the 552 patients, 245 had an incidental nodule described in their CT scan. Among these cases, 64% of patients received adequate follow-up CT scans for the incidental nodules, but still developed stage IV lung cancer, while in 36% (between-site range 11.1–70.4%) of cases, the incidental nodule was inappropriately monitored with only insignificant difference between Region Zealand and other regions (38.6% vs. 34.9%, p = 0.58).
Table 1. Audit of stage IV lung cancer: follow-up of nodules.
In 307 patients with a CT scan within 2 years prior to their lung cancer diagnosis, no pulmonary nodules were mentioned in the radiologist’s report (). However, upon reviewing these CT scans, for 42 patients (mean 14%, between-site range 4–24%) we found nodules at the same location where stage IV lung cancer later developed. Again, the prevalence in Region Zealand differed only insignificantly from other regions (6.8% vs. 7.8%, p = 0.70).
Table 2. Audit of stage IV lung cancer: missed nodules.
Discussion
CT usage and lung cancer stage distribution
According to the study, CT usage has steadily increased in Denmark during the past decade, and there has been a corresponding increase in stage I-II lung cancer which is largely attributable to an increase in the number of stage IA lung cancer. While there is generally a close correlation between CT usage and the proportion of early-stage lung cancer, this is not always true. In Region Zealand, this correlation was not observed, despite CT usage being largely comparable to the other regions.
As previously found, the majority of early-stage lung cancer is diagnosed as the result of the incidental discovery of pulmonary nodules [Citation20]. Hence, given that Region Zealand has lower rates of early-stage lung cancer and lower overall survival compared to other Danish regions, the absence of a correlation between CT usage and the proportion of early-stage lung cancers could lead to the hypothesis that lack of identification and/or monitoring of IPNs (representing small, early-stage lung cancers) led to a less favorable stage distribution with poorer prognosis and survival rates.
Auditing results
The hypothesis could not be substantiated in the current study, making it more likely that the reason for the less favorable stage distribution and lower survival rates of lung cancer patients in Region Zealand is not related to different hospital management practices across regions, but rather to regional differences in pre-hospital delays corresponding to the significantly lower proportion of stage IV patients with a prior CT scan in Region Zealand compared with the other hospitals. Previous studies have found substantial patient-related delays with a mean of 188 days [Citation21,Citation22]. As much as 38.8% of patients felt they had delayed lung cancer investigation, and the major reasons for delay were denial, anxiety, and non-recognition of symptoms. This delay might be more pronounced among residents in Region Zealand when compared with the national average, given the region’s general association with a lower socioeconomic status compared to other regions [Citation23]. Improved awareness campaigns have showed short-term benefits [Citation24,Citation25], indicating that addressing these factors could be achieved through campaigns. The significance of such initiatives is underscored by the fact that the prognosis of patients receiving curative treatment worsens with delay [Citation26,Citation27]. Apart from the aforementioned direct patient related delays, a sparse coverage with general practitioners in areas of Region Zealand may also contribute to a delay from the first symptoms are felt and until a doctor is consulted.
Upon reviewing , it is evident that each of the audited hospitals has the potential to enhance its ability to detect early-stage lung cancer. All hospitals have a number of stage IV lung cancer cases that could possibly have been identified at an earlier stage. A comprehensive analysis of the current data indicates that certain nodules are noted by the radiologists in the description of the CT scan, but no direct recommendations are made for follow-up or further investigation for lung cancer, potentially because the radiologists expect the clinicians requesting the CT scan to react alone on the noting of a pulmonary nodule, or they do not deem the nodule suspicious of lung cancer. In instances where radiologists do suggest follow-up or investigation for lung cancer, clinicians may not carry out the recommendations. Therefore, it is crucial to establish clear management protocols for each hospital. In a previous study, a multifaceted communication system was implemented to ensure nodule follow-up, resulting in a significant increase in follow-up rates from 26.5% to 59.7% [Citation28]. In another study, the hospital implemented a system using standardized language (tracker phrases) recommending time-based follow-up in chest CT reports, coupled with a computerized registry. This resulted in an increase in timely follow-up from 46% to 55% [Citation29].
Despite being enrolled in a follow-up program, a small proportion of patients still develop stage IV lung cancer. Upon scrutinizing these cases, it becomes evident that most of these patients exhibit several infiltrates, with the majority of them being infectious in nature. A subsequent CT scan conducted 6–12 weeks later may then reveal that the patients indeed have stage IV lung cancer. Therefore, it is highly probable that the patient had already progressed to this stage at the time of the primary CT scan, and it was therefore not feasible to detect the cancer at an earlier stage.
It is a well-established fact that radiologists occasionally overlook pulmonary nodules even in the setting of screening for lung cancer. However, the rate heavily relies on size of the nodule, use of second reader and access to maximum intensity projection (MIP) at the radiological department [Citation30–32]. Research has shown that detecting nodules adjacent to branching vessels and the posterior mediastinum can be particularly challenging [Citation33]. Nonetheless, providing nodule-specific training for radiologists can enhance their detection rates, as demonstrated in previous studies [Citation33]. Computer-aided assistance may also very well prove to be part of the solution, possibly as a first or second reader of CT scans. In a recent study, the artificial intelligence software was able to outperform four out of five radiologists and drastically diminish the radiologists’ workload, although the positive misclassification rate was higher in the artificial intelligence assessment [Citation34].
Strengths and weaknesses
While medical chart audits can provide valuable insights into clinical practice, it is essential to recognize their limitations and potential biases in interpreting the findings. The accuracy of data recorded in charts is dependent on the quality of the information provided by the clinicians, which may affect the evaluation of the referral patterns for CT scans detecting stage IA lung cancer. In some instances, the referring clinician may have suspected lung cancer but failed to document it in the referral, leading to potential inaccuracies in the data.
The audit involving stage IV patients aimed to evaluate whether previously identified pulmonary nodules or infiltrates were probable origins of subsequently diagnosed stage IV lung cancer. This involved a subjective retrospective assessment relying on the matching location of the pulmonary nodule or infiltrate with the primary tumor of the later diagnosed stage IV lung cancer. Additionally, the interpretation of CT referrals and scans by various reviewers at participating sites may result in inter-observer variability. Nevertheless, the study’s major strength is its coverage of seven sites in four out of five Danish health regions. Thus, making it possible to investigate the factors contributing to Region Zealand’s notable distinction in lung cancer mortality and proportion of early-stage lung cancer by making comparisons with the other sites examined in this study.
Conclusions
In conclusion, this audit falsified our hypothesis that poorer monitoring of nodules explained the significant regional variations in both proportion of lung cancers found in early stages and in survival rates. The study supports the presence of important non-hospital factors (beyond CT capacity and nodule management) responsible for early lung cancer detection, such as patient-related delays or poor access to primary health care in socioeconomically disadvantaged areas, which again has important implications with regard to strategies for alleviating the unfavorable stage distribution in Region Zealand and thus underlining the importance of conducting audits like the current. Ensuring the timely follow-up of IPNs is important for early detection of lung cancer. However, to achieve this, it is imperative to allocate the required resources for the interpretation of CT’s and management of follow-up programs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data on CT examinations and lung cancer stage distribution are publicly available. Anonymized data from the clinical auditing will be made available at reasonable request and after acceptance of the hospital directors.
Additional information
Funding
References
- Schmid-Bindert G, Vogel-Claussen J, Gütz S, et al. Incidental pulmonary nodules – what do we know in 2022. Respiration. 2022;101(11):1024–8. doi: 10.1159/000526818
- Loverdos K, Fotiadis A, Kontogianni C, et al. Lung nodules: a comprehensive review on current approach and management. Ann Thorac Med. 2019;14(4):226. doi: 10.4103/atm.ATM_110_19
- Xu CH, Zhan P, Yu LK. Solitary pulmonary nodule-a case of peripheral adenocarcinoma with rapid metastasis. J Thorac Dis. 2013;5(6):847–850. doi: 10.3978/j.issn.2072-1439.2013.12.21
- Mazzone PJ, Lam L. Evaluating the patient with a pulmonary nodule: a review. JAMA. 2022;327(3):264–273. doi: 10.1001/jama.2021.24287
- Nitz JA, Erkmen CP. New 2021 USPSTF lung cancer screening criteria-an opportunity to mitigate racial disparity. JAMA Oncol. 2022;8(3):383–384. doi: 10.1001/jamaoncol.2021.6708
- van Meerbeeck JP, Franck C. Lung cancer screening in Europe: where are we in 2021? Transl Lung Cancer Res. 2021;10(5):2407–2417. doi: 10.21037/tlcr-20-890
- Van Meerbeeck JP, O’Dowd E, Ward B, et al. Lung cancer screening: new perspective and challenges in Europe. Cancers (Basel). 2022;14(9):2343. doi: 10.3390/cancers14092343
- Singareddy A, Flanagan ME, Samson PP, et al. Trends in stage I lung cancer. Clinical Lung Cancer. 2023;24(2):114–119. doi: 10.1016/j.cllc.2022.11.005
- Potter AL, Rosenstein AL, Kiang MV, et al. Association of computed tomography screening with lung cancer stage shift and survival in the United States: quasi-experimental study. BMJ. 2022:e069008. doi: 10.1136/bmj-2021-069008
- Borg M, Hilberg O, Andersen MB, et al. Increased use of computed tomography in Denmark: stage shift toward early stage lung cancer through incidental findings. Acta Oncologica. 2022;61(10):1256–1262. doi: 10.1080/0284186X.2022.2135134
- Brustugun OT, Grønberg BH, Fjellbirkeland L, et al. Substantial nation-wide improvement in lung cancer relative survival in Norway from 2000 to 2016. Lung Cancer (Amst Neth). 2018;122:138–145. doi: 10.1016/j.lungcan.2018.06.003
- Yang CY, Lin YT, Lin LJ, et al. Stage shift improves lung cancer survival: real-world evidence. J Thorac Oncol. 2023;18(1):47–56. doi: 10.1016/j.jtho.2022.09.005
- Gould MK, Tang T, Liu ILA, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192(10):1208–1214. doi: 10.1164/rccm.201505-0990OC
- OECD. Computed tomography exams [internet]. [cited 2023 Sep 5]. Available from: https://www.oecd-ilibrary.org/social-issues-migration-health/computed-tomography-ct-exams/indicator/english_3c994537-en
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Chest. 2013;143(5):e93–e120. doi: 10.1378/chest.12-2351
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet Lond Engl. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3
- Tsushima Y, Taketomi-Takahashi A, Takei H, et al. Radiation exposure from CT examinations in Japan. BMC Med Imaging. 2010;10(1):24. doi: 10.1186/1471-2342-10-24
- Danish Lung Cancer Registry. Danish lung cancer registry annual report. 2021.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010
- Bredtoft EN, Madsen HH, Rasmussen TR. Stage I lung cancer patients with or without symptoms - are the patients different and should we treat them differently? Acta Oncol Stockh Swed. 2021;60(9):1169–1174. doi: 10.1080/0284186X.2021.1931959
- Kotecha J, Clark A, Burton M, et al. Evaluating the delay prior to primary care presentation in patients with lung cancer: a cohort study. BJGP Open. 2021;5(2): BJGPO.2020.0130. doi: 10.3399/BJGPO.2020.0130
- Koyi H, Hillerdal G, Brandén E. Patient’s and doctors’ delays in the diagnosis of chest tumors. Lung Cancer (Amst Neth). 2002;35(1):53–57. doi: 10.1016/S0169-5002(01)00293-8
- Statistics Denmark. Regionalt nationalregnskab [Internet]. [cited 2023 Jan 8]. Available from: https://www.dst.dk/da/Statistik/emner/oekonomi/nationalregnskab/regionalfordelt-nationalregnskab
- Ironmonger L, Ohuma E, Ormiston-Smith N, et al. An evaluation of the impact of large-scale interventions to raise public awareness of a lung cancer symptom. Br J Cancer. 2015;112(1):207–216. doi: 10.1038/bjc.2014.596
- Athey VL, Suckling RJ, Tod AM, et al. Early diagnosis of lung cancer: evaluation of a community-based social marketing intervention. Thorax. 2012;67(5):412–417. doi: 10.1136/thoraxjnl-2011-200714
- Christensen ED, Harvald T, Jendresen M, et al. The impact of delayed diagnosis of lung cancer on the stage at the time of operation. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 1997;12(6):880–884. doi: 10.1016/S1010-7940(97)00275-3
- O’Rourke N, Edwards R. Lung Cancer Treatment Waiting Times and tumour growth. Clinical Oncology. 2000;12(3):141–144. doi: 10.1053/clon.2000.9139
- Lim PS, Schneider D, Sternlieb J, et al. Process improvement for follow-up radiology report recommendations of lung nodules. BMJ Open Qual. 2019;8(2):e000370. doi: 10.1136/bmjoq-2018-000370
- Dyer DS, Zelarney PT, Carr LL, et al. Improvement in follow-up imaging with a patient tracking system and computerized registry for lung nodule management. J Am Coll Radiol. 2021;18(7):937–946. doi: 10.1016/j.jacr.2021.01.018
- White CS, Romney BM, Mason AC, et al. Primary carcinoma of the lung overlooked at CT: analysis of findings in 14 patients. Radiology. 1996;199(1):109–115. doi: 10.1148/radiology.199.1.8633131
- Gurney JW. Missed lung cancer at CT: imaging findings in nine patients. Radiology. 1996;199(1):117–122. doi: 10.1148/radiology.199.1.8633132
- Kakinuma R, Ohmatsu H, Kaneko M, et al. Detection failures in spiral CT screening for lung cancer: analysis of CT findings. Radiology. 1999;212(1):61–66. doi: 10.1148/radiology.212.1.r99jn1461
- Digumarthy S, Gullo R, Levesque MH, et al. Cause determination of missed lung nodules and impact of reader training and education: simulation study with nodule insertion software. J Cancer Res Ther. 2020;16(4):780. doi: 10.4103/jcrt.JCRT_312_17
- Lancaster HL, Zheng S, Aleshina OO, et al. Outstanding negative prediction performance of solid pulmonary nodule volume AI for ultra-LDCT baseline lung cancer screening risk stratification. Lung Cancer. 2022;165:133–140. doi: 10.1016/j.lungcan.2022.01.002