ABSTRACT
Pulmonary lophomoniasis is a rare and life-threatening disease, most commonly reported across Asian and Latin American countries. Here, we have reported two cases of pulmonary lophomoniasis presenting with atypical manifestations. Case #1 represents a 19-year-old male patient with clinical characteristics suggestive of tuberculosis, presenting with hemoptysis and receiving antituberculosis treatment. Case #2 represents a 69-year-old man with post-tuberculosis pulmonary disease with cystic bronchiectasis presenting with polymicrobial co-infection. Based on our case experience, lophomoniasis should be considered in patients with pneumonia who do not respond to antibiotic treatment, and the corresponding epidemiological factors should be carefully considered in addition to bronchoscopy for precise diagnosis.
Introduction
Lophomonas sp. is a parasitic, multiflagellate endocomensal protozoan detected in the intestine of certain arthropods, such as cockroaches, but rarely causing bronchopulmonary infection [Citation1].
Pulmonary lophomoniasis majorly affects Latin American and Asian countries, such as Panama and Iran, with a prevalence of 35% and 22.7%, respectively, specifically among people aged >60 years [Citation2,Citation3]. The transmission of Lophomonas to humans remains unclear, albeit certain environmental situations such as rain, temperature, humidity, and geographic latitude have been considered to facilitate the development of the transmitter (L. blattarum) [Citation3,Citation4]. Second, the lifestyle of the population at risk, such as a work environment with dirty and muggy environments, allowing continuous inhalation or ingestion of Lophomonas cysts [Citation5,Citation6].
For the development of this disease, immunosuppression or underlying diseases such as tuberculosis (TB) or COPD were considered contributing factors [Citation7–9]. However, up to 68% of these patients have been reported to be immunocompetents [Citation2,Citation10], possibly due to the temperature and humidity of the bronchial tree, which allows favorable conditions that allow the release of the cyst and adhering firmly to the respiratory mucosa to cause cell damage via cytoadherence, protease, rupture of epithelial barriers, and induction to apoptosis [Citation11].
The clinical manifestations of this disease are non-specific, as such the presence of productive cough and fever, in > 60% of all cases. Similarly, chest pain and dyspnea may occur in a lower percentage (<40%) of individuals, while the finding of significant hemoptysis is rare [Citation12,Citation13]. Some reports have described the presence of eosinophilia in up to 35% of cases, with computed tomography showing a consolidation pattern and linear reticular opacities [Citation1]. Definitive diagnosis is made via direct visualization of the parasite, and the sample is collected via bronchofibroscopy as it improves the diagnostic performance [Citation12,Citation14].
Pulmonary lophomoniasis can present as a differential diagnosis of other respiratory diseases [Citation2,Citation15]. Therefore, we have presented two cases with a description of the epidemiological characteristics, clinical manifestations, and tomographic lesions that can allow the reader to consider the diagnosis of this disease.
Case report
Case 1
A 19-year-old male patient, from the Andean region of Peru, who was working at a poultry market for the past 7 months. Five months ago, he experienced an episode of cough and mild hemoptysis that self-limited after 2 days. He did not utilize any biosafety equipment while working and had no history of previous illnesses or hospitalizations.
As his sporadic dry cough continued for 3 weeks along with headache and a feverish sensation, he visited the health center. He was administered as a ‘symptomatic’ patient, presenting slight improvement. Ten days before his admission, the patient experienced stabbing chest pain and diaphoresis. Nine days prior to admission, during some sports activities, he experienced hemoptysis (125 mL), and a day before, two episodes of hemoptysis (250 mL), for which he then visited our hospital.
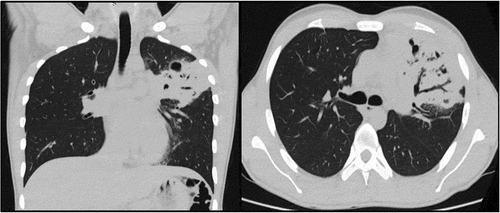
On admission to the emergency room, the patient’s blood pressure was stable (108/63 mmHg) with mild tachycardia (109 beats/min) and tachypnea (26 breaths/min); SatO2 was 96%. During his physical examination, the patient exhibited decreased vesicular murmur in the anterior hemithorax vertex of the left lung with wet crackles. Laboratory tests revealed a hemoglobin level of 11 (normal:12.5–16 g/dL), leukocyte count of 11,277 (normal: 4,000–10,000 mm3), and eosinophil count of 162 (normal: 0–500 mm3). The CRP levels were elevated at 244 (normal: 0–10 mg/dL) and the D-dimer levels were elevated at 2.72 (normal: ≤0.5 ug/mL). Serological testing was negative for HIV, HTLV, hepatitis B, and hepatitis C. Computed tomography revealed a lobar consolidation with a cavitated area in S3 of the left lung, as well as a reduction of the rib cage on the same side ().
On the day of his admission, the patient underwent bronchofibroscopy and bronchoalveolar lavage. The report indicated inflammation and erythema in the left upper bronchus along with active bleeding. Accordingly, samples were collected for acid-fast bacilli (AFB) and culturing.
Due to the patient’s massive hemoptysis and clinical and radiological suspicion of TB, treatment with a sensitive scheme (isoniazid 300 mg, rifampicin 600 mg, ethambutol 1200 mg, and pyrazinamide 1500 mg) was initiated in addition to intramuscular codeine administration (60 mg every 8 h). Although the AFB results were negative, it was decided to continue the antituberculosis treatment and to conduct a GeneXpert assay as soon as the hemoptysis was under control.
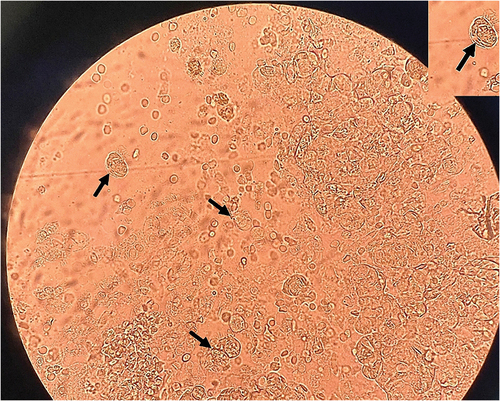
Four days after the bronchofibroscopy, the results of bronchoalveolar lavage indicated the presence of trophozoites of Lophomonas sp. with long and irregular flagella under direct microscopy, without additional staining (). The diagnosis of pulmonary lophomoniasis was considered after the GeneXpert and microbiological assays yielded negative outcomes. As a result, antituberculosis treatment was discontinued on day 7 and intravenous metronidazole was initiated at a dosage of 500 mg every 8 h. The patient was discharged from the hospital after 10 days. Upon follow-up, it was confirmed that the patient’s sputum culture for tuberculosis was negative. Thus, the patient successfully completed a 3-week treatment for pulmonary lophomoniasis without any complications.
Case 2
A 69-year-old male patient from the coast of Peru presented with a productive cough, dyspnea, and fever for the past 15 days. The patient worked as a recycler until 2 months ago, and his only medical history was pulmonary tuberculosis, which was treated for 6 months.
At the time of this admission to the emergency room, his findings were blood pressure: 110/70 mmHg, heart rate: 78 lat./min, respiratory rate: 27 breaths/min., and SatO2: 94% with oxygen support via binasal cannula (FiO2: 40%). Preferential physical examination revealed chest and lungs: intercostal indrawing, decreased vesicular murmur with left hemithorax crepites, and bilateral snoring. Neurological tests showed a Glasgow scale: of 14/15 and a decrease in visual scale. Laboratory tests showed Hb 9,6 g/dL, leukocytes: 19360 mm3, neutrophils: 96.5%, lymphocytes: 21%, eosinophils: 5%, albumin 2,30 g/dL. Serological (HIV, HBV, and HTLV) test results were negative. The analysis of arterial gases showed P02: 66.8 mmHg, PCO2: 44,5 mmHg, HCO3: 28.3 mEq/L, and PaFi02: 134 mmHg.
Non-contrast chest tomography showed sequelae bronchiectasis due to tuberculosis, with acute inflammatory signs and the presence of intracavitary mycetoma in the apical segments of the left hemithorax (). Sputum culture showed ESBL E. coli, hyphae (+), and the AFB results were negative. Direct microscopy identified Lophomonas sp. with multiple flagella at one pole with characteristic to-and-fro movements (). Given the patient’s occupational history, microscopic findings, and sputum culture results, a diagnosis of infected bronchiectasis of polymicrobial origin was made. Consequently, coverage with broad-spectrum antibiotics and intravenous metronidazole was started. However, the patient developed further respiratory insufficiency and was transferred to the intensive care unit (ICU) on a mechanical ventilator.
Figure 3. Chest CT scan, evidence of a cavity with content compatible with mycetoma, and multiple bronchiectasis in S1 and 2.
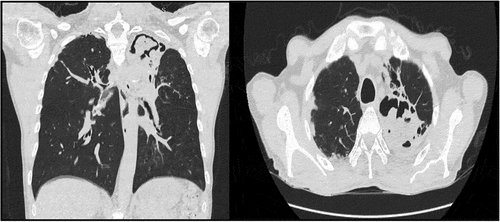
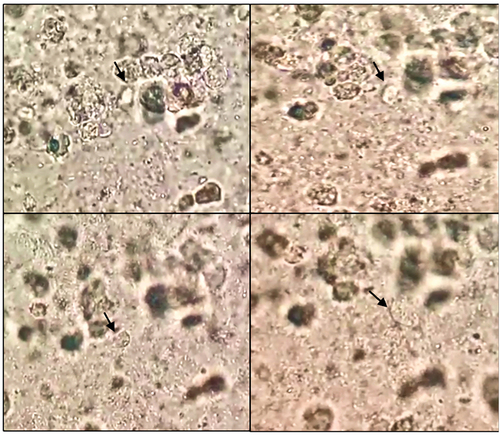
Figure 4. Sequential microscopic examination of Lophomonas (black arrow) in the direct smear of bronchial aspirate.
During the patient’s ICU stay, his clinical condition worsened. No AFB was detected in the secretion sample. A bronchial secretion sample was taken, revealing extremely drug-resistant Acinetobacter baumannii and confirming the diagnosis of ventilator-associated pneumonia. Although treatment with colistin, vancomycin, metronidazole, and vasopressors was provided, the patient passed away after 10 days of hospitalization.
Discussion
Pulmonary lophomoniasis is an emerging disease, and multiple mechanisms that facilitate infection in humans have been identified [Citation3]. Occupation should be considered as one of the main contributing factors as it increases direct contact with the transmitter, as in our patients. In support, a review by Nakhaei et al. dismisses the idea of classifying this disease as an opportunistic one, as no significant differences were noted between healthy patients and those with an underlying condition [Citation2].
Latin America and Asia are the regions with the highest number of lophomoniasis cases [Citation1,Citation2], which can be explained by certain environmental and occupational conditions that promote the presence of cockroaches [Citation5,Citation16]. Our patients worked in unhygienic environments that favored continuous exposure to Lophomonas cysts [Citation5]. It is therefore important that appropriate preventive measures should be applied in such workshops to avoid the development and spread of infectious diseases, such as hand washing, safe disposal of sanitary waste, and the use of personal protection [Citation17].
Underlying lung diseases, such as COPD, asthma, or tuberculosis sequelae, can cause structural alterations that promote the growth of Lophomonas [Citation18–20]. This may trigger acute exacerbation and may even complicate patients’ prognosis with other respiratory diseases during hospitalization. It is therefore essential to consider such factors to improve the treatment outcomes in the affected patients [Citation2,Citation8].
It is challenging to diagnose this disease due to the non-specific and potentially chronic nature of the pulmonary clinical expression reported in cases, which can also result in the misdiagnosis of COPD, bacterial pneumonia, or tuberculosis [Citation21–23]. In a study published by H. Kalani et al., a relatively high incidence of Lophomonas sp. Was detected among patients presenting with symptoms similar to those of tuberculosis [Citation24]. In general, the authors recommend the suspicion of pulmonary lophomoniasis in (a) patients with or without comorbidities who have an occupational history that allows contact with cockroaches or certain arthropods [Citation3,Citation5]. Also, patients who come from endemic areas with environmental factors that favor the presence of this vector [Citation3]. (b) Woking areas or rooms with few biosecurity measures, especially without the use of face masks [Citation6]. (c) Patients with persistent signs or symptoms of cough, fever, chest pain, dyspnea, and hemoptysis [Citation2,Citation25]. In these patients, clinical improvement is rare despite the use of antibiotics. In our context, it is reasonable to start antituberculosis therapy in Peru as it has one of the highest tuberculosis prevalence rates across Latin America [Citation26].
Different presentations have been observed in imaging studies, with the most common being pneumonic infiltrations resembling the present first case, such as pulmonary abscesses, pleural effusion, and bronchiectasis [Citation27]. A recent study by Amirmasoud T. et al. reported the first case of cavitary pulmonary infiltration as a form of presentation of pulmonary lophomoniasis [Citation19]. In the second case of this report, a cavity with mycetoma was detected, suggesting the possibility of a co-infection between fungi and parasites (Lophomonas sp).
In laboratory studies, most patients show an elevated peripheral white blood cell count and only one-third of the patients exhibit eosinophilia [Citation28]. Both of our case patients had leukocytosis, but neither had peripheral blood eosinophilia, which implies that eosinophilia is not a reliable predictor for the diagnosis of lophomoniasis [Citation27].
The definitive diagnosis of pulmonary lophomoniasis requires direct observation of the parasite in various samples, including sputum, tissue, and secretions obtained via bronchofibroscopy [Citation5]. The initial case was diagnosed through bronchofibroscopy. However, due to logistical issues at our hospital, the sample processing took 4 days (which generally happens within the day), which resulted in a delay of the treatment. In the second case, the diagnosis was made with the sputum sample. Although both patients were analyzed through direct microscopy, we suggest utilizing alternative staining methods such as trichrome, Papanicolau, and Giemsa [Citation29] as these enable improved visualization of cellular details and better identification of the parasite [Citation30]. The accuracy of the diagnosis also depends on the skill and experience of the microbiologists, as misdiagnosis of hair cells is possible [Citation31].
In this disease, treatment is oral or intravenously administered metronidazole at a dose of 500 mg every 8 h for 20–30 days, depending on the severity of the infection [Citation28]. Metronidazole treatment was initiated in both the present cases, which presented clinical improvement and cessation of hemoptysis in the first case. However, the second case presented a poor clinical evolution due to a polymicrobial infection.
Based on our case experiences, we suggest that pulmonary lophomoniasis should be considered in the differential diagnosis of tuberculosis, especially in those who do not respond to antibiotic treatment. In addition, patients with high-risk epidemiological factors should be evaluated for this disease. Microbiological co-infection should be suspected in cases of hemoptysis and cavitated lesions, as observed in our patients. We thus suggest that bronchoscopy should be the preferred method for sampling. In the absence of any diagnostic laboratory test, the empirical use of metronidazole may be beneficial in preventing complications.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Informed consent
Written informed consent for publication of this case report was obtained from the patient.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Martinez-Girón R, Cornelis van Woerden H. Lophomonas blattarum and bronchopulmonary disease. J Med Microbiol. 2013;62(11):1641–6. doi: 10.1099/jmm.0.059311-0
- Nakhaei M, Fakhar M, Sharifpour A, et al. Global status of emerging lophomonas infection: a systematic review of reported cases (1993-2020). Interdiscip Perspect Infect Dis. 2022;2022:3155845. doi: 10.1155/2022/3155845
- Ghatee MA, Nakhaei M, Sharifpour A, et al. Geospatial analysis and molecular epidemiologic study of emerging pulmonary lophomoniasis in Iran: a national registry-based study. J Parasitol Res. 2023;2023:1039186. doi: 10.1155/2023/1039186
- Camargo-Assis F, Máttar S, González M. Lophomonas blattarum cockroach parasite that causes uncommon pneumonia in humans. Revista MVZ Córdoba. 2020;25(1):1948. doi: 10.21897/rmvz.1948
- Ding Q, Shen K. Pulmonary infection with Lophomonas blattarum. Indian J Pediatr. 2021;88(1):23–27. doi: 10.1007/s12098-020-03311-1
- Mirzazadeh F, Berenji F, Amini M, et al. Lophomonas blattarum in asthmatic patients and control group. JRMDS. 2017;5(5):1–5.
- Ribas A, Martínez-Girón R, Sánchez-Del-Río J, et al. Protozoal forms in the sputum of immunocompromised patients. Scand J Infect Dis. 2005;37(3):205–210. doi: 10.1080/00365540410025177
- Saldaña NG, Mendoza FJO, Larrauri FR, et al. Bronchopulmonary infection by Lophomonas blattarum in a pediatric patient after hematopoietic progenitor cell transplantation: first report in Mexico. J Thoracic Dis. 2017;9(10):E899.
- Zerpa R, Ore E, Patiño L, et al. [Lophomonas sp. in respiratory tract secretions in hospitalized children with severe lung disease]. Hallazgo de Lophomonas sp. en secreciones del tracto respiratorio de niños hospitalizados con enfermedad pulmonar grave. Rev Peru Med Exp Salud Publica. 2010;27(4):575–577. doi: 10.1590/s1726-46342010000400013
- Tyagi R, Anand KB, Teple K, et al. Lophomonas blattarum infection in immunocompetent patient. Lung India. 2016;33(6):667–668. doi: 10.4103/0970-2113.192867
- Martínez-Girón R, van Woerden HC. Bronchopulmonary lophomoniasis: emerging disease or unsubstantiated legend? Parasites Vectors. 2014;7(1):284. doi: 10.1186/1756-3305-7-284
- Yao G, Zhou B, Zeng L. Imaging characteristics of bronchopulmonary lophomonas blattarum infection: case report and literature review. J Thorac Imaging. 2009;24(1):49–51. doi: 10.1097/RTI.0b013e31818c6b72
- Wang Y, Tang Z, Ji S, et al. Pulmonary lophomonas blattarum infection in patients with kidney allograft transplantation. Transplant Int. 2006;19(12):1006–1013. doi: 10.1111/j.1432-2277.2006.00380.x
- Zhang X, Xu L, Wang LL, et al. Bronchopulmonary infection with Lophomonas blattarum: a case report and literature review. J Int Med Res. 2011;39(3):944–949. doi: 10.1177/147323001103900329
- Lee M, Hwang SM, Park JS, et al. Lophomonas blattarum-like organism in bronchoalveolar lavage from a pneumonia patient: current diagnostic scheme and polymerase chain reaction can lead to false-positive results. Parasites Hosts Dis. 2023;61(2):202–209. doi: 10.3347/PHD.22107
- Talebian M, Berenji F, Amini M, et al. A study about clinical symptoms and laboratory signs of adult and pediatric patients with lophomonas blattarum. JRMDS. 2018;6(1):312–317.
- Aw TC, Blair I, Babcock HM. Occupational infections. Infect Dis (Auckl). 2017;647–655.e1. doi: 10.1016/b978-0-7020-6285-8.00072-1
- Singh S, Allwood BW, Chiyaka TL, et al. Immunologic and imaging signatures in post tuberculosis lung disease. Tuberculosis. 2022;136:102244. doi: 10.1016/j.tube.2022.102244
- Taheri A, Fakhar M, Sharifpour A, et al. Cavitary pulmonary lesions following emerging lophomoniasis: a novel perspective. Respirol Case Rep. 2022;10(3):e0908. doi: 10.1002/rcr2.908
- Failoc-Rojas VE, Iglesias-Osores S, Silva-Díaz H. Lophomonassp. In the upper and lower respiratory tract of patients from a hospital in Lambayeque, Peru: clinical case studies. Respir Med Case Rep. 2020;31:101142. doi: 10.1016/j.rmcr.2020.101142
- Zeng H, Kong X, Chen X, et al. Lophomonas blattarum infection presented as acute exacerbation of chronic obstructive pulmonary disease. J Thoracic Dis. 2014;6(6):E73–6. doi: 10.3978/j.issn.2072-1439.2014.03.40
- Wahid W, Ahmad Fahmi NA, Mohd Salleh AF, et al. Bronchopulmonary lophomoniasis: a rare cause of pneumonia in an immunosuppressed host. Respir Med Case Rep. 2019;28:100939. doi: 10.1016/j.rmcr.2019.100939
- Taheri A, Fakhar M, Sharifpour A, et al. Lophomonas and mycobacterium co-infection: the first molecular evidence to overcome potential diagnostic pitfalls. Oxf Med Case Reports. 2022;2022(7):omac064. doi: 10.1093/omcr/omac064
- Kalani H, Pangh A, Nakhaei M, et al. High occurrence of emerged lophomonas infection among patients suspected of having pulmonary tuberculosis: in-house PCR-based evidence. Interdiscip Perspect Infect Dis. 2022;2022:2742164. doi: 10.1155/2022/2742164
- Sharifpour A, Zarrinfar H, Fakhar M, et al. First report of emerged pulmonary lophomoniasis in two Afghanian medical tourists. Clin Case Rep. 2022;10(3):e05607. doi: 10.1002/ccr3.5607
- Ranzani OT, Pescarini JM, Martinez L, et al. Increasing tuberculosis burden in Latin America: an alarming trend for global control efforts. BMJ Global Health. 2021;6(3):e005639. doi: 10.1136/bmjgh-2021-005639
- Rao X, Liao Q, Pan T, et al. Retrospect and prospect of lophomonas blattarum infections and lophomoniasis reported in China. Open Access Lib J. 2014;1(9):1–6. doi: 10.4236/oalib.1101121
- Pinos Vélez N, Ordoñez Vintimilla R, Agreda Orellana SS. Lung infection caused by Lophomonas blattarum. Arch Bronconeumol. 2021;57(9):594–596. doi: 10.1016/j.arbr.2021.06.005
- Berenji F, Razieh B, Hosseini Farash BR, et al. Different staining methods in diagnosing lophomonas blattarum in bronchoalveolar lavage samples. Patient Saf Surg. 2021:9. doi: 10.22038/PSJ.2021.60597.1340
- Alam-Eldin YH, Abdulaziz AM. Identification criteria of the rare multi-flagellate lophomonas blattarum: comparison of different staining techniques. Parasitol Res. 2015;114(9):3309–3314. doi: 10.1007/s00436-015-4554-4
- Meng SS, Dai ZF, Wang HC, et al. Authenticity of pulmonary lophomonas blattarum infection: a case report. WJCC. 2019;7(1):95–101.