ABSTRACT
Background
Bronchoscopy and EBUS are standard procedures in lung cancer work-up but have low diagnostic yield in lesions outside the central airways and hilar/mediastinal lymph nodes. Growing evidence on introducing the EBUS endoscope into the oesophagus (EUS-B) in the same session as bronchoscopy/EBUS gives access to new anatomical areas that can be safely biopsied.
Objective
To summarize the current evidence of the added value of EUS-B-FNA to bronchoscopy and EBUS-TBNA in lung cancer work-up.
Methods
A narrative review.
Results
Few randomized trials or prospective studies are available. Prospective studies show that add-on EUS-B-FNA increases diagnostic yield when sampling abnormal mediastinal lymph nodes, para-oesophageal lung and left adrenal gland. A large retrospective series on EUS-B-FNA from retroperitoneal lymph nodes suggests high diagnostic yield without safety concerns, as do casuistic reports on EUS-B-FNA from mediastinal pleural thickening, pancreatic lesions, ascites fluid and pericardial effusions. No study has systematically assessed both diagnostic yield, safety, patient reported outcomes, adverse events and costs.
Conclusion
The diagnostic value of add-on EUS-B to standard bronchoscopy and EBUS in lung cancer work-up appears very promising without safety concerns, giving the pulmonologist access to a variety of sites out of reach with other minimally invasive techniques. Little is known on patient-reported outcomes and costs. Future and prospective research should focus on effectiveness aspects to clarify whether overall benefits of add-on EUS-B sufficiently exceed overall downsides.
Background
Worldwide, lung cancer is the most frequent cause of cancer death, and both lung parenchyma and pleura are common sites of metastases from extra-thoracic malignancy of almost any kind. The population is growing in number and life expectancy, and more patients survive their first episode of cancer, thus both the absolute and relative population at risk for primary or secondary lung cancer will continue to increase [Citation1]. Accurate diagnosis and staging is the mainstay of optimal treatment and is obtained across all cancer subtypes by a combination of advanced imaging and tissue sampling for histopathological diagnosis [Citation2–4].
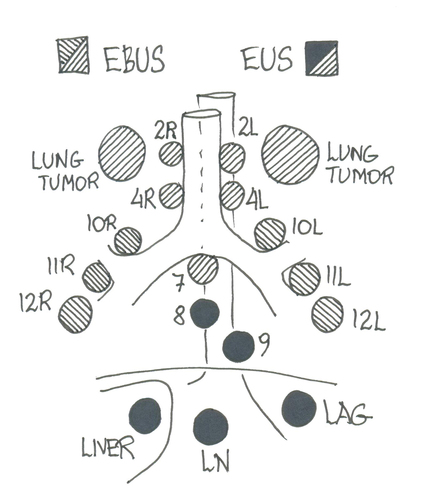
Tumor sampling by flexible bronchoscopy and same-day endobronchial ultrasound with transbronchial needle aspiration (EBUS-TBNA) from mediastinal/hilar lymph nodes is the golden standard in lung cancer work-up [Citation5–7]. Staging by EBUS-TBNA revolutionised lung cancer work-up when shown to improve mediastinal staging and reduce futile thoracotomies compared to staging mediastinoscopy [Citation8]. However, lung lesions or abnormal lymph nodes may be located out of reach of bronchoscopy or EBUS, such as tumors close to the pleura, lymph nodes in lower mediastinum (stations 8 and 9) or lateral to aorta (stations 5 and 6) [Citation5].
Endoscopic ultrasound via the oesophagus (EUS) allows fine-needle aspiration (FNA) from lesions adjacent to the oesophagus, stomach or duodenum with the dedicated gastroenterological ultrasound-endoscope (EUS-FNA), and from oesophagus and stomach with the EBUS endoscope (EUS-B-FNA). Both can be performed as same-day procedures following bronchoscopy and EBUS. EUS-B is attractive to pulmonologists and administrators since it requires little additional training and no additional equipment when running an EBUS service, and the number of scientific reports on EUS-B is growing [Citation9,Citation10]. The aim of this narrative review is to summarize current evidence on utility of EUS-B-FNA in lung cancer work-up, alone or in combination with EBUS, including which structures that can be biopsied.
Endo-ultrasonography in upper gastrointestinal tract as part of lung cancer work-up: EUS or EUS-B
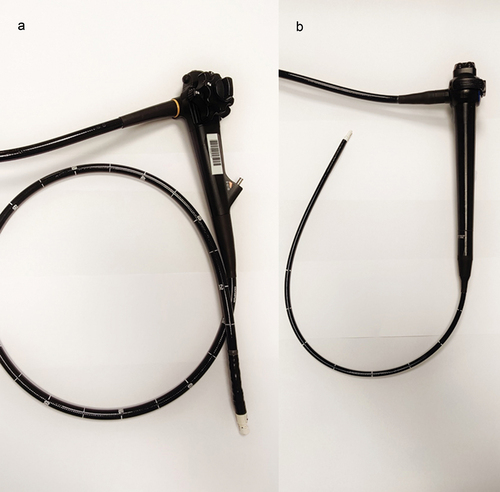
The evidence on conventional EUS – with the dedicated gastroenterological ultrasound-endoscope – in the work-up of suspected lung cancer is very solid [Citation11]. Most reports on EUS-B repeat evidence already established concerning EUS. In the following figure, we will highlight technical differences and similarities between the EUS and the EBUS endoscope ().
EUS endoscope and lung cancer
EUS systems were developed for gastroenterology in early 1980s [Citation12], and the conventional curvilinear gastrointestinal ultrasound-endoscope has been the instrument of choice. Pentax and Olympus are the main producers of radial and linear EUS endoscopes. Linear EUS endoscopes are the most widely used, e.g. the TGF-UC180J (Olympus Optical, Tokyo, Japan): length 125 cm, working channel width 3.7 mm, ultrasonic window angle 180 degrees, high resolution (5–12 MHz), a handle similar to that of a gastroscope with wheels allowing 180°–90° flexibility up-down and 90°–90° right–left, manoeuvrability of the needle with an ‘elevator’ allowing the operator to change the angle of the needle without changing the position of the transducer, and insufflation of water in the oesophagus through the endoscope to lower the risk of perforation. The Pentax EG34-J10U (Pentax Medical, Tokyo, Japan) has approximately same specifications. Usually, a 22 G needle with a maximal length of 80 mm for real-time biopsy is used, but 25 G and 19 G are also available.
In 1996, it was suggested that EUS-FNA could be a useful diagnostic tool for mediastinal lesions, as the gastrointestinal tract traverses through the mediastinum [Citation13]. Due to its outer diameter, conventional EUS scopes are only suitable for use in the gastrointestinal tract. Over the years, many publications described the use of conventional EUS for diagnosing and staging of lung cancer. EUS can be used to diagnose para-oesophageally located lung tumors [Citation14–18]. Besides, EUS has a high specificity and negative predictive value (NPV) for the T4 assessment of a primary lung tumor and offers further value to chest CT scan [Citation19]. Another important indication for EUS-FNA is mediastinal nodal staging in patients with proven or suspected lung cancer [Citation5,Citation20,Citation21]. In a randomized study, Larsen et al. found that addition of routine-EUS-FNA to standard work-up in routine clinical practice improved selection of surgically curable patients with NSCLC [Citation22]. In a non-randomized study, EUS-FNA was superior to mediastinoscopy in the examination of para-tracheal- and subcarinal-regions of patients considered for resection of lung cancer [Citation23]. These results are in accordance with a randomized study showing that EUS-FNA, when added to mediastinoscopy, improves the preoperative staging of lung cancer due to the complementary reach of EUS-FNA in detecting mediastinal lymph node metastases and the ability to assess mediastinal tumor invasion [Citation24]. Also, for assessment of distal metastases e.g. located in the left adrenal gland (LAG) or liver in a lung cancer staging setting, the conventional EUS scope has proven its usefulness [Citation17,Citation18].
The curvilinear endobronchial ultrasound, EBUS, endoscope
The curvilinear EBUS endoscope was developed in the early 2000s. Most EBUS endoscopes are 60 cm long, working channel width 2.2 mm, ultrasonic window angle of 50°–60°, lower resolution than EUS (7.5–12 MHz), with a handle similar to that of a bronchoscope allowing 120°–90° flexibility in one dimension (up-down), an inflatable balloon to improve contact between transducer and bronchial wall, no ‘elevator’ to improve needle manoeuvrability, no insufflation of water. Most often, a 22-gauge needle with a maximal length of 40 mm for real-time biopsy is used, but other sizes for example 25, 21 and 19 G are also available [Citation25–27].
The ultrasound transducer on the tip prevents the operator from looking straight ahead: the EB19-J10U from Pentax (Pentax Medical, Tokyo, Japan has a 45° oblique viewing angle and the BF-UC180F from Olympus (Olympus Optical, Tokyo, Japan) has a 35° oblique viewing angle. Furthermore, the tip angulation is inferior when comparing with a flexible bronchoscope, which reduces manoeuvrability. An EBUS endoscope with a 10° forward oblique view exists (Fujifilm) [Citation28] but a randomized study failed to show any superiority compared to 35° endoscope [Citation29].
The oesophageal route versus the tracheal route
Recent guidelines suggest that optimal mediastinal staging is performed as lymph node sampling both from the endobronchial route and via the oesophagus [Citation5,Citation30]. Many central structures can be reached by both routes, but EBUS provides unique access to hili and structures anterior to the large airways, whereas the oesophageal route (EUS or EUS-B) is excellent for the left and lower para-oesophageal structures plus structures under the diaphragm [Citation31]. A combination of the procedures can give a full staging of the patient with lung cancer. Thus, in the majority of cases, choosing one method over the other is not the issue. However, when a choice is possible, EBUS is associated with more coughing and longer procedure duration – perhaps due to interposing cartilage rings between needle and target – and more frequent oxygen desaturation episodes [Citation32].
EUS-FNA versus EUS-B-FNA
The EUS endoscope is overall technically superior – e.g. length, US window, flexibility, manoeuvrability, needle length – to the EBUS endoscope (). So theoretically, it should be superior concerning diagnostic yield. However, there are no randomized studies showing a better diagnostic outcome or a reduced rate of complications when performing EUS-FNA compared with EUS-B-FNA [Citation18].
Therefore, there are obvious practical and logistical advantages in performing both tracheal and oesophageal ultrasound with the use of one single endoscope, compared to training and costs associated with purchasing a dedicated EUS endoscope. The control handle of the EUS endoscope do not resemble and is more complicated (reflecting the technical advantages) than EBUS handles, thus even experienced pulmonologists are novices with the EUS endoscope but already familiar with the EBUS endoscope, and just have to learn new landmarks to perform EUS-B-FNA [Citation33,Citation34]. Same-day EBUS-TBNA and EUS-B-FNA therefore offers several advantages. In the following, the utility of EUS-B-FNA from various anatomical sites are discussed.
EUS-B and mediastinal lesions
Mediastinal staging of the lower paratracheal (stations 4 R and 4 L) and subcarinal (station 7) lymph nodes in a potentially resectable lung cancer is usually performed using EBUS [Citation4,Citation5]. Mediastinal staging can also be done via EUS-B (); however, station 4 R can be hidden behind the trachea [Citation35].
Following EBUS-TBNA in the mediastinal staging, the accessibility to mediastinal nodal stations increased by adding EUS-B-FNA [Citation36]. A systematic endobronchial ultrasound combined with an oesophageal investigation (EUS-B) using the same EBUS bronchoscope increases the sensitivity for mediastinal nodal staging by 5% compared with a systematic EBUS procedure and by 9% compared with a PET-CT targeted EBUS procedure [Citation37]. The ASTER study showed that complete endosonography (ie. EBUS-TBNA and EUS-FNA) followed by surgical staging (ie. mediastinoscopy) in absence of metastases at endosonography resulted in greater sensitivity for mediastinal nodal metastases compared with surgical staging alone (78% vs 85%) [Citation8]. Guidelines recommend EUS or EUS-B-FNA in combination with EBUS-TBNA in the work-up of lung cancer when an indication for tissue verification of mediastinal nodal disease based on imaging is present due to a 20% risk of missed N2/N3 disease with imaging alone [Citation5]. Lower mediastinal lymph node stations [Citation8] and [Citation9] are positioned adjacent to the oesophagus and out of reach by EBUS, but can easily be targeted via EUS-B ().
EUS-B-FNA and lung lesions
In a retrospective study, adding EUS-B-FNA to bronchoscopy and EBUS-TBNA increased the diagnostic yield for diagnosing lung cancer in patients with para-oesophageal lung tumors from 51% to 91% [Citation14]. An observational prospective study reported that the diagnostic accuracy of EUS-B-FNA from para-oesophageal pulmonary lesions was 95.3% [Citation38]. According to the European guidelines, it is possible to biopsy lung tumors ‘immediately adjacent to the oesophagus’ with EUS-B-FNA () [Citation5]. Christiansen et al. defined this term more precisely in a prospective study of 70 patients with lung tumors and showed that ‘immediately adjacent’ most often means less than 31 mm when measured at CT [Citation39]. In this study, she also showed that the probability of achieving a biopsy depended on both the distance and the size of the tumor and was possible to predict the probability of success with a biopsy index formula [Citation39]. The study also indicated that the operator could move the oesophagus closer to the target with the endoscope. The biopsy index formula still needs to be validated in larger multi centre studies. Moreover, a prospective systematic registration of side effects as pneumothorax, bleeding and other side effects when performing EUS-B-FNA is needed. An alternative procedure for biopsying intrapulmonary lesions is percutaneous lung biopsy, and in contrast to EUS-B-FNA, systematic data from percutaneous lung biopsy guided by computed tomography (CT) has been presented and the risk of pneumothorax may be up to 30% and increases with the distance to the lesion, lesion size <20 mm and presence of emphysema [Citation40]. No randomized studies comparing EUS-B-FNA and CT guided lung biopsy exists. Furthermore, it would be interesting to compare the costs when comparing the two techniques.
EUS-B-FNA and liver lesions
Liver metastases at the time of lung cancer diagnosis affects 5% of patients with NSCLC, and 25% of patients with SCLC [Citation41,Citation42]. The first EUS-B-FNA from a liver metastasis was described in 2015 [Citation43] and was followed by larger series [Citation44]. EUS-B gives access to lesions in the left liver lobe (). However, in analogy with the biopsy index formula for EUS-B-FNA from lung tumors, one should ask how big the influence of the distance from the stomach and the size of the suspected liver metastasis is. In addition, data concerning adverse events and costs should be systematically collected. Adverse events with the larger EUS endoscope are <1% [Citation45,Citation46]. Feasibility, diagnostic yield or adverse event rate associated with EUS-B-FNA from suspected metastatic liver lesions have never been systematically assessed. A single-institution retrospective study reported that EUS-B-FNA of liver lesions was diagnostic in 96% of the 24 cases [Citation47].
EUS-B-FNA and left adrenal gland
The adrenal glands are common sites of lung cancer metastases but adrenal masses are also commonly found in patients with potentially resectable lung cancer with a prevalence of up to 4–7% [Citation48]. Approximately, two-thirds of these masses are benign adenomas [Citation49]. Therefore, pathological verification of a suspicious adrenal gland is mandatory for correct TNM classification and for correct treatment of the patient. The use of EUS-B to visualize the left adrenal gland () was first described by Meena et al. in 2015 [Citation50]. EUS-B-FNA of the LAG is a safe and feasible procedure when a trans-gastric approach is used [Citation51]. In a retrospective study, EUS-B-FNA of 47 left adrenal glands was diagnostic in 89% of the cases [Citation47]. A systematic review also concluded that both EUS and EUS-B guided FNA have good performance and safety when sampling the left adrenal gland in patients with lung cancer [Citation52]. In a randomized study, the success rate was the same for EUS-B-FNA and EUS-FNA of the left adrenal gland [Citation18]. The length of the relatively short EBUS-scope, compared with the EUS-scope, was not a limiting factor, yet inability to visualize LAG was observed with EUS-B in 2 out of 44 cases (0 out of 44 in EUS). In clinical practice, EUS should be considered when EUS-B do not allow for LAG visualization.
There are a few reports saying that it is possible to reach the right adrenal gland with the transduodenal approach using the EUS endoscope [Citation5,Citation53], but there are no data showing that we can reach the right adrenal gland with EUS-B-FNA.
EUS-B-FNA from other structures
EUS-B-FNA also makes it possible to biopsy retroperitoneal lymph nodes () [Citation45], pleura [Citation54] and difficult‑to‑assess posterior mediastinal structures [Citation55], to aspirate ascites [Citation56] and pericardial fluid () [Citation57] and pancreas [Citation58,Citation59].
EUS-B-FNA and respiratory impairment
A complication to EBUS-TBNA may be respiratory dysfunction, since the endoscope partly obstructs the lumen in the airways [Citation60]. However, endoscopy using the oesophageal route may diminish this risk. This applies to both EUS-B-FNA [Citation61–64] and EUS-FNA [Citation32]. In their randomized study, Oki et al. demonstrated that EUS-B-FNA was associated with shorter duration of the procedure, lower doses of Midazolam, less frequent oxygen desaturations and lower scores of cough than EBUS-TBNA [Citation32]. These findings make it likely that the operator should prefer the oesophageal route over the tracheal route when the task is to biopsy a lesion that is located close to both the central airways and to the oesophagus in a patient with respiratory impairment. Although this assumption seems obvious and logical, we have limited data to support it in the literature.
What do the patients say?
We have limited knowledge with respect to the patient’s experiences and opinions in connection with EUS-B-FNA. A small study suggests that the patients are satisfied with the procedure, when conscious sedation is used [Citation65]. In a larger prospective study on transnasal EUS-B, 99% were willing to repeat the procedure if necessary [Citation66]. Thus, at least on the same level as for bronchoscopy or EBUS [Citation67,Citation68].
Training and Education
In contrast to what is the case for bronchoscopy and EBUS-TBNA, we still have to practice EUS-B-FNA on patients, since no simulation-based educational program exists despite a huge and increasing need. Both a simulator and a training program for EBUS-TBNA is available [Citation69], recommended in the international guidelines [Citation5], and offered by the European Respiratory Society [Citation70]. A validated assessment tool for competences in EUS-FNA exists [Citation71,Citation72] but not for EUS-B-FNA. Since no EUS-B simulator has been developed it is still not possible to develop an evidence-based EUS-B training program in which simulator training is integrated in the training and competency assessment. Development of an EUS-B simulator is thus warranted if EUS-B training should reach same standards as is the case for bronchoscopy and EBUS.
Directions for future research
Future studies should focus on effectiveness aiming at all aspects of lung cancer work-up: diagnostic yield, patients’ perspectives, adverse events, costs, number of visits to finalize diagnostic work-up, time to final diagnosis and time to treatment start. This will ensure that benefits and downsides of EUS-B are elucidated to ensure a robust evidence base on which the use of EUS-B can be further integrated into future international guidelines on lung cancer work-up.
When we one day have an EBUS endoscope with a 0° viewing angle and optimal manoeuvrability, the operator will hopefully be able to perform bronchoscopy, EBUS-TBNA and EUS-B-FNA with one single endoscope in one session.
Until then, we may soon change the name of the EBUS endoscope to EBOUS, endobronchial & oesophageal ultrasound endoscope, as the endoscope has utility beyond EBUS only.
Conclusion
There are many arguments for using the EBUS endoscope both in the trachea and in the oesophagus in the diagnosis and staging of lung cancer patients. Addition of the oesophageal approach increases the sensitivity for mediastinal nodal staging, it gives access to biopsy distal metastases for example retroperitoneal lymph nodes, the left adrenal gland and the left liver lobe, and it makes it possible to biopsy para-oesophageal lung tumors. In patients with respiratory impairment, the oesophageal approach may spare the patient from aggravation.
Acknowledgments
Aras Kamal Mohammad Ali, MD: for clinical photos.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209–12. doi: 10.3322/caac.21660
- Stein MK, Oluoha O, Patel K, et al. Precision medicine in oncology: a review of multi-tumor actionable molecular targets with an emphasis on non-small cell lung cancer. J Pers Med. [2021 Jun 5];11(6):518. doi: 10.3390/jpm11060518
- Finazzi T, Schneiders FL, Senan S. Developments in radiation techniques for thoracic malignancies. Eur Respir Rev Off J Eur Respir Soc. [2021 Jun 30];30(160):200224. doi: 10.1183/16000617.0224-2020
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American college of chest Physicians evidence-based clinical practice guidelines. Chest. 2013 May;143(5 Suppl):e142–e165. doi: 10.1378/chest.12-2353
- Vilmann P, Clementsen P, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European society of gastrointestinal endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy. [2015 Jun 1];47(6):545–559. doi: 10.1055/s-0034-1392040
- Silvestri GA, Bevill BT, Huang J, et al. An evaluation of diagnostic yield from bronchoscopy: The Impact of Clinical/Radiographic Factors, Procedure Type, and Degree of Suspicion for Cancer. Chest. 2020 Jun;157(6):1656–1664. doi: 10.1016/j.chest.2019.12.024
- Gaga M, Powell CA, Schraufnagel DE, et al. An official American Thoracic Society/European Respiratory Society statement: the role of the pulmonologist in the diagnosis and management of lung cancer. Am J Respir Crit Care Med. [2013 Aug 15];188(4):503–507. doi: 10.1164/rccm.201307-1269ST
- Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA. [2010 Nov 24];304(20):2245–2252. doi: 10.1001/jama.2010.1705
- Hong G, Oki M. Transesophageal endoscopic ultrasound with bronchoscope-guided fine-needle aspiration for diagnostic and staging purposes: a narrative review. J Thorac Dis. [2023 Sep 28];15(9):5088–5098. doi: 10.21037/jtd-23-681
- Biondini D, Tinè M, Semenzato U, et al. Clinical applications of endobronchial Ultrasound (EBUS) Scope: Challenges and opportunities. Diagn Basel Switz. [2023 Aug 1];13(15):2565. doi: 10.3390/diagnostics13152565
- Bhatia S, Puri R. Role of endoscopic ultrasound in non-small cell lung cancer. Int J Gastrointest Interv. [2016 Oct 31];5(3):187–192. doi: 10.18528/gii160014
- DiMagno EP, Buxton JL, Regan PT, et al. Ultrasonic endoscope. Lancet Lond Engl. [1980 Mar 22];1(8169):629–631. doi: 10.1016/S0140-6736(80)91122-8
- Pedersen BH, Vilmann P, Folke K, et al. Endoscopic ultrasonography and real-time guided fine-needle aspiration biopsy of solid lesions of the mediastinum suspected of malignancy. Chest. 1996 Aug;110(2):539–544. doi: 10.1378/chest.110.2.539
- Skovgaard Christiansen I, Kuijvenhoven JC, Bodtger U, et al. Endoscopic ultrasound with bronchoscope-Guided fine needle aspiration for the diagnosis of paraesophageally located lung lesions. Respir Int Rev Thorac Dis. 2019;97(4):277–283.
- Varadarajulu S, Hoffman BJ, Hawes RH, et al. EUS-guided FNA of lung masses adjacent to or abutting the esophagus after unrevealing CT-guided biopsy or bronchoscopy. Gastrointest Endosc. 2004 Aug;60(2):293–297. doi: 10.1016/S0016-5107(04)01680-3
- Korevaar DA, Colella S, Spijker R, et al. Esophageal endosonography for the diagnosis of intrapulmonary tumors: A systematic review and meta-Analysis. Respiration. 2017;93(2):126–137. doi: 10.1159/000452958
- Bodtger U, Vilmann P, Clementsen P, et al. Clinical Impact of endoscopic ultrasound-fine needle aspiration of left adrenal masses in established or suspected lung cancer. J Thorac Oncol. 2009 Dec;4(12):1485–1489. doi: 10.1097/JTO.0b013e3181b9e848
- Crombag LMMJ, Szlubowski A, Stigt JA, et al. EUS-B-FNA vs conventional EUS-FNA for left adrenal gland analysis in lung cancer patients. Lung Cancer (Amst Neth). 2017 Jun;108:38–44. doi: 10.1016/j.lungcan.2017.02.011
- Kuijvenhoven JC, Livi V, Morandi L, et al. The expanding role of endobronchial ultrasound in patients with centrally located intrapulmonary tumors. Lung Cancer (Amst Neth). 2019 Aug;134:194–201. doi: 10.1016/j.lungcan.2019.06.006
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013 May;143(5 Suppl):e211–e250. doi: 10.1378/chest.12-2355
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2014 May;45(5):787–798. doi: 10.1093/ejcts/ezu028
- Larsen SS, Vilmann P, Krasnik M, et al. Endoscopic ultrasound guided biopsy performed routinely in lung cancer staging spares futile thoracotomies: preliminary results from a randomised clinical trial. Lung Cancer (Amst Neth). 2005 Sep;49(3):377–385. doi: 10.1016/j.lungcan.2005.04.005
- Larsen SS, Vilmann P, Krasnik M, et al. Endoscopic ultrasound guided biopsy versus mediastinoscopy for analysis of paratracheal and subcarinal lymph nodes in lung cancer staging. Lung Cancer (Amst Neth). 2005 Apr;48(1):85–92. doi: 10.1016/j.lungcan.2004.10.002
- Annema JT, Versteegh MI, Veseliç M, et al. Endoscopic ultrasound added to mediastinoscopy for preoperative staging of patients with lung cancer. JAMA. [2005 Aug 24];294(8):931–936. doi: 10.1001/jama.294.8.931
- Yang L, Gu Y, Wang H, et al. Novel ProCore 25-gauge needle for endobronchial ultrasound-guided transbronchial needle aspiration reduces the puncture time and frequency, with comparable diagnostic rate for mediastinal and hilar lymphadenopathy. Thorac Cancer. 2020 Mar;11(3):748–753. doi: 10.1111/1759-7714.13332
- Wolters C, Darwiche K, Franzen D, et al. A Prospective, Randomized Trial for the Comparison of 19-G and 22-G endobronchial ultrasound-guided transbronchial aspiration needles; Introducing a novel end point of sample weight corrected for blood content. Clin Lung Cancer. 2019 May;20(3):e265–73. doi: 10.1016/j.cllc.2019.02.019
- Giri S, Pathak R, Yarlagadda V, et al. Meta-analysis of 21- versus 22-G aspiration needle during endobronchial ultrasound-guided transbronchial needle aspiration. J Bronchol Interv Pulmonol. 2015 Apr;22(2):107–113. doi: 10.1097/LBR.0000000000000159
- Xiang Y, Zhang F, Akulian J, et al. EBUS-TBNA by a new Fuji EBUS scope (with video). J Thorac Dis. 2013 Feb;5(1):36–39. doi: 10.3978/j.issn.2072-1439.2012.12.06
- Yarmus L, Akulian J, Ortiz R, et al. A randomized controlled trial evaluating airway inspection effectiveness during endobronchial ultrasound bronchoscopy. J Thorac Dis. 2015 Oct;7(10):1825–1832. doi: 10.3978/j.issn.2072-1439.2015.09.42
- Korevaar DA, Crombag LM, Cohen JF, et al. Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer: a systematic review and meta-analysis. Lancet Respir Med. 2016 Dec;4(12):960–968. doi: 10.1016/S2213-2600(16)30317-4
- Vilmann P, Clementsen P. Combined EUS and EBUS are complementary methods in lung cancer staging: Do not forget the esophagus. Endosc Int Open. [2015 Aug 31];3(04):E300–1. doi: 10.1055/s-0034-1392786
- Oki M, Saka H, Ando M, et al. Transbronchial vs transesophageal needle aspiration using an ultrasound bronchoscope for the diagnosis of mediastinal lesions: a randomized study. Chest. 2015 May;147(5):1259–1266. doi: 10.1378/chest.14-1283
- Ng J, Chan HP, Kee A, et al. Transitioning to Combined EBUS EUS-B FNA for Experienced EBUS Bronchoscopist. Diagn Basel Switz. [2021 Jun 2];11(6):1021. doi: 10.3390/diagnostics11061021
- Leong P, Deshpande S, Irving LB, et al. Endoscopic ultrasound fine-needle aspiration by experienced pulmonologists: a cusum analysis. Eur Respir J. 2017 Nov;50(5):1701102. doi: 10.1183/13993003.01102-2017
- Clementsen PF, Bodtger U, Konge L, et al. Diagnosis and staging of lung cancer with the use of one single echoendoscope in both the trachea and the esophagus: A practical guide. Endosc Ultrasound. 2021;10(5):325–334. doi: 10.4103/EUS-D-20-00139
- Hwangbo B, Lee GK, Lee HS, et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest. 2010 Oct;138(4):795–802. doi: 10.1378/chest.09-2100
- Crombag LMM, Dooms C, Stigt JA, et al. Systematic and combined endosonographic staging of lung cancer (SCORE study). Eur Respir J. 2019 Feb;53(2):1800800. doi: 10.1183/13993003.00800-2018
- Mondoni M, Gasparini S, Varone F, et al. Accuracy and Predictors of Success of EUS-B-FNA in the Diagnosis of Pulmonary Malignant Lesions: A Prospective Multicenter Italian Study. Respiration. 2022 Aug;101(8):775–783. doi: 10.1159/000524398
- Christiansen IS, Svendsen MBS, Bodtger U, et al. Characterization of Lung Tumors that the Pulmonologist can Biopsy from the Esophagus with Endosonography (EUS-B-FNA). Respiration. 2021;100(2):135–144. doi: 10.1159/000512074
- Heerink WJ, de Bock Gh, de Jonge Gj, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol. 2017 Jan;27(1):138–148. doi: 10.1007/s00330-016-4357-8
- Kagohashi K, Satoh H, Ishikawa H, et al. Liver metastasis at the time of initial diagnosis of lung cancer. Med Oncol Northwood Lond Engl. 2003;20(1):25–28. doi: 10.1385/MO:20:1:25
- Li J, Zhu H, Sun L, et al. Prognostic value of site-specific metastases in lung cancer: a population based study. J Cancer. 2019;10(14):3079–3086. doi: 10.7150/jca.30463
- Fally M, Nessar R, Behrendt N, et al. Endoscopic Ultrasound-Guided Liver Biopsy in the Hands of a Chest Physician. Respiration. 2016;92(1):53–55. doi: 10.1159/000446924
- Christiansen IS, Bodtger U, Naur TMH, et al. EUS-B-FNA for Diagnosing Liver and Celiac Metastases in Lung Cancer Patients. Respiration. 2019;98(5):428–433. doi: 10.1159/000501834
- Johnson KD, Laoveeravat P, Yee EU, et al. Endoscopic ultrasound guided liver biopsy: Recent evidence. World J Gastrointest Endosc. [2020 Mar 16];12(3):83–97. doi: 10.4253/wjge.v12.i3.83
- Parekh PJ, Majithia R, Diehl DL, et al. Endoscopic ultrasound-guided liver biopsy. Endosc Ultrasound. 2015;4(2):85–91. doi: 10.4103/2303-9027.156711
- Jeffus S, Quiroga EF, Hasan Z, et al. The yield and impact of pulmonologist-performed EUS-B-FNA of subdiaphragmatic lesions—an institutional experience. J Am Soc Cytopathol. 2023 May;S2213294523000522. 2023;12(5):362–367. doi: 10.1016/j.jasc.2023.05.004
- Chapman GS, Kumar D, Redmond J, et al. Upper abdominal computerized tomography scanning in staging non-small cell lung carcinoma. Cancer. [1984 Oct 15];54(8):1541–1543. doi: 10.1002/1097-0142(19841015)54:8<1541:AID-CNCR2820540812>3.0.CO;2-N
- Ettinghausen SE, Burt ME. Prospective evaluation of unilateral adrenal masses in patients with operable non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 1991 Aug;9(8):1462–1466. doi: 10.1200/JCO.1991.9.8.1462
- Meena N, Hulett C, Jeffus S, et al. Left adrenal biopsy using the convex curvilinear ultrasound scope. Respir Int Rev Thorac Dis. 2015;89(1):57–61. doi: 10.1159/000368370
- Christiansen IS, Ahmad K, Bodtger U, et al. EUS-B for suspected left adrenal metastasis in lung cancer. J Thorac Dis. 2020 Mar;12(3):258–263. doi: 10.21037/jtd.2020.01.43
- Moretti A, Kovacevic B, Vilmann P, et al. Performance of EUS-FNA and EUS-B-FNA for the diagnosis of left adrenal glands metastases in patients with lung cancer: A systematic review and meta-analysis. Lung Cancer (Amst Neth). 2023 Dec;186:107391. doi: 10.1016/j.lungcan.2023.107391
- Eloubeidi MA, Beydoun M, Jurdi N, et al. Transduodenal EUS-guided FNA of the right adrenal gland to diagnose lung cancer where percutaneous approach was not possible. J Med Liban. 2011;59(3):173–175.
- Bibi R, Bodtger U, Nessar R, et al. Endoscopic ultrasound-guided pleural biopsy in the hands of the pulmonologist. Respirol Case Rep. 2020 Mar;8(2):e00517. doi: 10.1002/rcr2.517
- Ray A, Jain SR, Narwal A, et al. Endoscopic ultrasound fine-needle aspiration with an echobronchoscope (EUS-B-FNA) from a difficult-to-access paraspinal lesion. Lung India Off Organ Indian Chest Soc. 2019;36(5):457–458. doi: 10.4103/lungindia.lungindia_226_19
- Nessar R, Toennesen LL, Bodtger U, et al. Endoscopic ultrasound-guided ascites aspiration in the hands of the chest physician using the EBUS endoscope in the oesophagus. Respir Med Case Rep. 2020;29:100998. doi: 10.1016/j.rmcr.2020.100998
- Christiansen IS, Clementsen PF, Petersen JK, et al. Aspiration of Pericardial Effusion Performed with EUS-B-FNA in Suspected Lung Cancer. Respiration. 2020;99(8):686–689. doi: 10.1159/000508844
- Issa MA, Sidhu JS, Tehrani SG, et al. Endoscopic ultrasound-guided pancreas biopsy in the hands of a chest physician. Respir Med Case Rep. 2023;43:101833. doi: 10.1016/j.rmcr.2023.101833
- Ahmad AK, Arshad A, Laursen CB, et al. Endoscopic ultrasound-guided fine-needle aspiration using the bronchial ultrasound scope (EUS-B-FNA) for diagnosing pancreatic metastasis in a lung cancer patient case report. Eur Clin Respir J. 2024;11(1):2294545. doi: 10.1080/20018525.2023.2294545
- Hashemi SMS, Dahele M, Daniels JMA, et al. Complications of endoscopic ultrasound-guided needle aspiration. Acta Oncol Stockh Swed. 2014 Sep;53(9):1265–1268. doi: 10.3109/0284186X.2014.887855
- Ito T, Oki M, Saka H, et al. Respiratory failure patient with lung cancer diagnosed by transesophageal bronchoscopic ultrasound-guided aspirates. Respirol Case Rep. 2018 May;6(4):e00309. doi: 10.1002/rcr2.309
- Prasad KT, Sehgal IS, Gupta N, et al. Endoscopic ultrasound (with an echobronchoscope)-guided fine-needle aspiration for diagnosis of a mediastinal lesion in a mechanically ventilated patient: A case report and systematic review of the literature. Indian J Crit Care Med Peer-Rev Off Publ Indian Soc Crit Care Med. 2016 Oct;20(10):608–612. doi: 10.4103/0972-5229.192057
- Christiansen IS, Bodtger U, Nessar R, et al. Safety and feasibility of oesophageal ultrasound for the work-up of thoracic malignancy in patients with respiratory impairment. J Thorac Dis. 2023 Jul 31;151:3965–3973. doi: 10.21037/jtd-22-1705
- Nakashima K, Umeda Y, Demura Y, et al. Safety and utility of Endoscopic Ultrasound with Bronchoscope-guided Fine Needle Aspiration (EUS-B-FNA) in suspected lung cancer patients with poor respiratory or general conditions: a prospective three-center observational study. BMC Pulm Med. [2023 Jun 14];23(1):206. doi: 10.1186/s12890-023-02508-2
- Cintrão Samouco GA, De Santis M, Matos P, et al. Feasibility and patient satisfaction with transnasal diagnostic EUS-b under conscious sedation. Interventional pulmonology [Internet] European Respiratory Society. 2020 [cited 2023 Feb 6]:2855. Available from doi:10.1183/13993003.congress-2020.2855
- Samouco G, De Santis M, Matos P, et al. Nasal Insertion Overall Satisfaction with EUS-B (NOSE): Evaluating the Feasibility of Transnasal Diagnostic EUS-B. Adv Respir Med. [2022 Jan 1];90(2):184–190. doi: 10.5603/ARM.a2022.0023
- Steinfort DP, Irving LB. Patient satisfaction during endobronchial ultrasound-guided transbronchial needle aspiration performed under conscious sedation. Respir Care. 2010 Jun;55(6):702–706.
- Karewicz A, Faber K, Karon K, et al. Evaluation of patients’ satisfaction with bronchoscopy procedure. PLoS One. 2022 Oct 6;17(10):e0274377. doi: 10.1371/journal.pone.0274377
- Konge L, Clementsen PF, Ringsted C, et al. Simulator training for endobronchial ultrasound: a randomised controlled trial. Eur Respir J. 2015 Oct;46(4):1140–1149. doi: 10.1183/13993003.02352-2015
- Farr A, Clementsen P, Herth F, et al. Endobronchial ultrasound: launch of an ERS structured training programme. Breathe Sheff Engl. 2016 Sep;12(3):217–220. doi: 10.1183/20734735.013116
- Konge L, Annema J, Vilmann P, et al. Transesophageal ultrasonography for lung cancer staging: learning curves of pulmonologists. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2013 Nov;8(11):1402–1408. doi: 10.1097/JTO.0b013e3182a46bf1
- Konge L, Vilmann P, Clementsen P, et al. Reliable and valid assessment of competence in endoscopic ultrasonography and fine-needle aspiration for mediastinal staging of non-small cell lung cancer. Endoscopy. 2012 Oct;44(10):928–933. doi: 10.1055/s-0032-1309892