 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
We investigated the duration of suppression-induced forgetting (SIF), and the extent to which retrieval suppression differs between negative and neutral memories. We further examined if SIF was differently affected by sleep versus wake during the delay interval between retrieval suppression and re-test. Fifty participants first learned to associate neutral words with either neutral or negative images. Then, a subset of the words was shown again, and participants were asked to either recall (Think), or to suppress retrieval of (No-Think) the associated images. Finally, a memory test for all items was performed either immediately after the Think/No-Think (T/NT) phase (No Delay), or after a 3.5 h delay interval containing either sleep or wake. Results revealed a SIF effect only in the No Delay group, indicating that this forgetting effect dissipates already after a 3.5 h delay interval. Negative items were experienced as more intrusive than neutral ones during the T/NT phase.
Introduction
Sometimes, we are reminded of an event that we would prefer not to think about. This could be when remembering causes a high degree of emotional distress, for example memories of a traumatic experience, or a situation that has caused embarrassment or a threat to our self-image (e.g. a failed exam). One strategy for preventing the negative memory from entering awareness and causing distress is to suppress its retrieval. This is especially motivated when retrieving the negative memory is not associated with constructively processing it, but rather distracts us from the task currently at hand.
Attempts to suppress retrieval of a memory, in the face of a reminder, has indeed previously been demonstrated to lead to a poorer recall of it at a later unexpected memory test (Anderson & Green, Citation2001; Anderson & Huddleston, Citation2012). This phenomenon is referred to as suppression-induced forgetting (SIF). Making the unwanted memory less likely to be reactivated in the future may serve an adaptive function because it would allow for the emotional stress associated with the retrieval of the memory to be avoided.
One method for studying SIF is the Think/No-Think (T/NT) paradigm (Anderson & Green, Citation2001). In this paradigm, participants first learn associations between cues (in the present study, words) and associates (in the present study, pictures; most often both the cues and the associates have been words but other stimuli, for example faces and pictures, have been used as well). Then, in the T/NT phase, participants are repeatedly presented with only the cues. In half of the trials, participants are instructed to think of the associate that the cue was previously paired with (Think items). During the other half of trials, participants are instructed to suppress all thoughts of the associate (No-Think items). At a subsequent, unexpected, memory test, performance for No-Think items is typically impaired. This impairment is not just seen in comparison to Think items, but also to Baseline items (items that are encoded during the learning phase but not present during the T/NT phase). The amount of SIF is quantified as the decrease in memory performance for No-Think compared to Baseline items. This decrease indicates forgetting due to repeated suppression. This effect has been reliably replicated (but see also for example Bergström, Velmans, de Fockert, and Richardson-Klavehn (Citation2007) and Bulevich, Roediger, Balota, and Butler (Citation2006), for studies not demonstrating such an effect).
A growing body of evidence suggests that cognitive control mechanisms are recruited during memory suppression to prevent the cued memory from entering awareness (for reviews see Anderson & Hanslmayr, Citation2014; Engen & Anderson, Citation2018), and recent studies suggest that this cognitive control network may be domain general (Castiglione, Wagner, Anderson, & Aron, Citation2019; Depue, Orr, Smolker, Naaz, & Banich, Citation2016; Guo, Schmitz, Mur, Ferreira, & Anderson, Citation2018). Memory suppression engages cognitive control areas in the right dorsolateral prefrontal cortex which downregulates the hippocampus to prevent memory retrieval (for review see Anderson, Bunce, & Barbas, Citation2016), and this reduction in hippocampal activity during the T/NT phase has been shown to correlate with subsequent SIF in the final memory test (Depue, Curran, & Banich, Citation2007; Levy & Anderson, Citation2012). An alternative strategy for avoiding to think about an unwanted memory is to block retrieval by thinking about something else (referred to as thought substitution). Thought substitution has also been shown to induce subsequent forgetting, but this strategy is related to a different pattern of brain activity than memory suppression, suggesting that SIF cannot be explained by interference from alternative memories alone (Benoit & Anderson, Citation2012; Bergström, de Fockert, & Richardson-Klavehn, Citation2009; but see Tomlinson, Huber, Rieth, & Davelaar, Citation2009, for an interference account of SIF).
It remains to be specified how durable this forgetting phenomenon is over time. Typically, there will be longer periods of time between retrieval-suppression and the moment when we encounter a reminder of the suppressed memory. It is therefore important to examine if retrieval-suppression is affected differently by how this time is spent; for example if it has contained sleep or not. Furthermore, in real life situations, we are also more likely to want to suppress negative memories than neutral ones. Whereas previous studies have examined the effects of delay, sleep, and emotion on memory suppression separately, no study has incorporated all these factors into the same design. Motivated by this, the present study is, to the best of our knowledge, the first to examine SIF that both manipulates the emotion of the material, and examines the effects of sleep versus wake in the delay interval between retrieval-suppression and the re-test. Given that these factors have never been studied in combination before, we will first go through what previous research has found regarding the effect of emotion on SIF, the duration of SIF, sleep and forgetting, and sleep and emotional memory when studied separately.
Effects of emotion on suppression-induced forgetting
It has been suggested that it is more difficult to suppress negative material because of its more intrusive nature, which makes it more likely to capture our attention (for a review on emotion and attention, see Compton, Citation2003). Further, emotional memories are often better remembered than neutral ones (LaBar & Cabeza, Citation2006).
It has however also been suggested that the facilitated retrieval of emotional material would make it more susceptible to SIF (Depue, Banich, & Curran, Citation2006). According to this view, emotional memories are more deeply encoded, and consequently more likely to be involuntary reactivated during early suppression attempts. Memory suppression is only thought to be recruited when the memory is reactivated, given that there is no need to suppress the memory if it is not. Memories that are more likely to be reactivated, like emotional memories, would therefore be suppressed to a greater extent, and thus be more prone to SIF.
Support for the view that suppression is recruited when the memory is reactivated has come from neuroimaging studies using non-emotional material. One study showed that the hippocampus was downregulated to a greater extent when participants reported that the associate was reactivated (i.e. when experiencing a memory intrusion) during the T/NT phase compared to when it was not (Levy & Anderson, Citation2012). Another study showed higher activity in the dorsolateral prefrontal cortex during memory intrusions, indicating that they trigger the activation of inhibitory control mechanisms (Benoit, Hulbert, Huddleston, & Anderson, Citation2015). In addition, electrophysiological correlates of reactivation of an intruding memory has been related to the forgetting of that memory, further supporting the view that reactivation signals the need for cognitive control (Hellerstedt, Johansson, & Anderson, Citation2016).
Another potential reason for why negative memories may be more susceptible to SIF is that there might be higher motivation to suppress them because of their more distressing nature (Anderson & Huddleston, Citation2012).
Previous studies manipulating the emotion of the material in the T/NT paradigm have revealed highly contrasting results. Studies have found both increased (Depue et al., Citation2006; Marzi, Regina, & Righi, Citation2014; Noreen & MacLeod, Citation2013) and decreased (Chen et al., Citation2012; Nørby, Lange, & Larsen, Citation2010; Sakaki, Kuhbandner, Mather, & Pekrun, Citation2014) forgetting of negative items compared to neutral or positive ones. Other studies have found no differences in forgetting depending on the emotionality of the material (Joormann, Hertel, Brozovich, & Gotlib, Citation2005; Murray, Anderson, & Kensinger, Citation2015; Murray, Muscatell, & Kensinger, Citation2011; van Schie, Geraerts, & Anderson, Citation2013). Considering the contrasting findings of the previous literature, we did not have a directed hypothesis regarding how the emotionality of the material would affect the degree of SIF.
In order to examine if negative items were more difficult to suppress than neutral ones, we also collected introspective reports of memory intrusions. An intrusion was defined as the failure to avoid retrieval during a No-Think trial during the T/NT phase. The intention was to extend previous findings and examine if negative items were experienced as more intrusive than neutral ones. This has previously been studied by Gagnepain, Hulbert, and Anderson (Citation2017), who found no such effect.
van Schie et al. (Citation2013) found that participants reported that negative material was easier to suppress than neutral material, and that participants who in retrospect reported to have had more success in suppression during the T/NT phase also showed a larger SIF effect. This was however only the case for the forgetting of negative items. This study measured the participants’ overall experience of suppression difficulty in retrospect, after completion of the final memory test.
In the present study, we instead measured the participants’ experience of intrusions as they occurred during the T/NT phase on a trial by trial basis. This was done to get a more direct test of potential differences in intrusiveness between negative and neutral memories, just as in Gagnepain et al. (Citation2017).
We furthermore wanted to see if we could replicate previous studies finding that forgetting during the re-test can be predicted by participants’ decrease of intrusion frequency during the T/NT phase (Hellerstedt et al., Citation2016; Levy & Anderson, Citation2012), and to examine if this would be affected by the emotionality of the material to be suppressed.
The duration of suppression-induced forgetting
To further understand the potential adaptive function of retrieval suppression, it is important to determine if the reduction in accessibility of the unwanted memory is transient or more long lasting. The behavioural findings suggest that the memory is less accessible not only when retrieval suppression is applied, but also a few minutes after the suppression when the participants actively try to retrieve the previously suppressed memory (i.e. in the final test). It is however unclear how long lasting the effect is. To the best of our knowledge, only one study has found persisting forgetting effects after an increased delay between the T/NT phase and the memory test. Hotta and Kawaguchi (Citation2009) found SIF at both an immediate test, as well as when the memory test took place 24 h after the T/NT phase. This effect was however only present in participants who reported having substituted the associate word of the No-Think items with another item during the T/NT phase, and may therefore be due to interference from the substitute rather than suppression.
Several studies have found the SIF effect to have disappeared at re-tests taking place after three or eight hours (Fischer, Diekelmann, & Born, Citation2011), a week (Nørby et al., Citation2010; Meier, König, Parak, & Henke, Citation2011) and after several months to a year (Noreen & MacLeod, Citation2014). Two of these studies have even found rebound effects at the delayed re-test, with better memory for No-Think compared to Baseline items (Meier et al., Citation2011; Noreen & MacLeod, Citation2014). Fischer et al. (Citation2011) found no SIF effect after an eight hour delay containing either sleep or wake. In a second experiment, they found a rebound effect after a delay interval containing three hours of sleep late in the night, but not after an equivalent delay containing early sleep.
Based on these previous studies, we expected a reduced SIF effect after a delay interval, compared to at a memory test performed immediately after the T/NT phase. We were also interested in if the temporal profile of SIF would be affected by the emotional value of the material to be suppressed.
The role of sleep in suppression-induced forgetting
Sleep, as compared to wake, has in a large body of studies been shown to have a beneficial effect on memory consolidation (for review, see Rasch & Born, Citation2013). Sleep has further been suggested to prioritise the strengthening of certain memories over others in a different manner than wake. The synaptic homeostasis hypothesis (Tononi & Cirelli, Citation2014) suggests that this is because of synaptic downscaling during sleep, where synapses that have been built up during the day are weakened so that only the stronger synapses (i.e. only more strongly encoded memories) survive, whereas the others are erased, increasing the signal to noise ratio. Support for this account comes from studies showing sleep to be more beneficial for memories for which a re-test is expected, or for which a reward is expected for successful remembering (Fischer & Born, Citation2009; van Dongen, Thielen, Takashima, Barth, & Fernández, Citation2012; Wilhelm et al., Citation2011). However, there are also several studies that have not found such an effect (Baran, Daniels, & Spencer, Citation2013; Oudiette, Antony, Creery, & Paller, Citation2013; Tucker, Tang, Uzoh, Morgan, & Stickgold, Citation2011; Wamsley, Hamilton, Graveline, Manceor, & Parr, Citation2016). It has also been reported that sleep has a larger benefit for weakly encoded memories (Drosopoulos, Schulze, Fischer, & Born, Citation2007; see also Diekelmann, Wilhelm, & Born, Citation2009).
Although the paradigms used in the studies above are quite different from the retrieval suppression method used in the present study, the results still suggest that sleep does not benefit all memories equally. Based on this, we wanted to examine if sleep would have a different effect on memories depending on if they had been subjected to retrieval suppression or not.
Beyond just not strengthening certain memories, sleep has further been suggested to actively promote forgetting of information that is not deemed relevant (e.g. Feld & Born, Citation2017; Langille, Citation2019; Poe, Citation2017). Poe (Citation2017) suggested that the epochs during sleep with low adrenergic tone, i.e. REM sleep and sleep spindles, allow for de-potentiation, which enables forgetting and reversal learning. The empirical support for these theories has however been highly varied.
Several studies have examined the effect of sleep on memories that can be expected to be remembered compared with memories that can be expected to be forgotten due to direct forgetting instructions. Similarly to SIF, inhibitory control has been proposed to be the mechanism underlying directed forgetting as well (e.g. Anderson & Hanslmayr, Citation2014), but the inhibition in this paradigm is occurring during or after encoding rather than during retrieval.
One study using this paradigm found sleep to benefit items cued to be remembered, but not items cued to be forgotten (Saletin, Goldstein, & Walker, Citation2011). This was not replicated by Rauchs et al. (Citation2011), even though they found the sleep group to have a more lenient response criteria for the items cued to be forgotten. Alger, Chen, and Payne (Citation2019) found that sleep, as compared to wake, specifically increased the difference between negative items cued to be remembered and negative items cued to be forgotten. No such difference was found for neutral items. Sleep has further been found to both increase (Hupbach, Citation2018) and decrease (Abel & Bäuml, Citation2013), as well as to have no effect on (Blaskovich, Szőllősi, Gombos, Racsmány, & Simor, Citation2017), the degree of list-method directed forgetting.
Another paradigm where inhibitory control has been proposed to cause forgetting is the retrieval-practice paradigm (Anderson, Bjork, & Bjork, Citation1994; Storm & Levy, Citation2012). In this paradigm, inhibitory control is proposed to be recruited during retrieval as well, similarly to the Think/No-think paradigm, but a difference to memory suppression is that the recruitment of inhibition is theorised to be unintentional and recruited automatically during selective memory retrieval, rather than an intentional strategy. Studies using this paradigm have shown contrasting results, with sleep resulting in both more (Abel & Bäuml, Citation2012; Racsmány, Conway, & Demeter, Citation2010), and less (Baran, Wilson, & Spencer, Citation2010), retrieval-induced forgetting.
The only previous study examining the effect of sleep using the T/NT paradigm (Fischer et al., Citation2011) showed no difference in SIF between the sleep and wake group, and instead only a main effect of group, with sleep having a beneficial effect on memory performance regardless of item type.
Given the contrasting results of the previous studies, we did not have a directed hypothesis regarding the role of sleep on SIF. If weak memories are erased during sleep due to synaptic downscaling (Tononi & Cirelli, Citation2014), this would mean that the weakening of the No-Think item caused by repeated retrieval suppression would make them benefit less from sleep, or perhaps even be actively erased. This would increase the SIF effect. However, the contrary could also be expected, with sleep decreasing SIF through the de-potentiation of the inhibitory processes suppressing the recall of them.
Studies examining which mechanisms during sleep that are responsible for consolidating certain memories over others, have yielded similarly contrasting findings. Theoretical accounts have previously suggested Rapid Eye Movement (REM) sleep to be involved in “repairing” memories that would otherwise be forgotten, and in removing unwanted learning (Crick & Mitchison, Citation1983; Norman, Newman, & Perotte, Citation2005), potentially through the lack of adrenergic tone during this stage allowing for de-potentiation, as suggested by Poe (Citation2017).
A second experiment reported in the Fischer et al. (Citation2011) study using a split night design (comparing sleep during the early half of the night, which is dominated by Slow Wave Sleep (SWS), with sleep during the second half of the night, which is dominated by REM), showed a rebound effect for the No-Think items only after late sleep. No such effect was present after early sleep. Early sleep however did benefit memory for the Think items more than late sleep.
REM duration has further been found to be associated with selectively increasing memory performance for items for which a low reward was expected, but not items for which a higher reward was expected (Oudiette et al., Citation2013), decreasing the retrieval-induced forgetting effect (Baran et al., Citation2010) and in decreasing performance on a task consisting of riding a bicycle with a reversed steering device (something that would require the inhibition of the “normal” way of riding a bicycle; Hoedlmoser et al., Citation2015). These results could be viewed as support for REM sleep having a role in decreasing inhibition, and increasing memory performance for items that would otherwise be forgotten. Based on these findings, we expected the duration of REM sleep to be negatively correlated with SIF.
Sleep and emotional memory
Beyond examining the potential effect of sleep on SIF, we also wanted to examine the often suggested role of sleep in the selective strengthening of emotional memories over neutral ones, and if such an effect would also be present for items subjected to retrieval suppression. Several studies have found sleep to have a larger effect on memory performance for emotional compared to neutral stimuli. (e.g. Payne, Stickgold, Swanberg, & Kensinger, Citation2008; Wagner, Gais, & Born, Citation2001), although an equally large body of studies has not found such an effect (e.g. Ackermann, Hartmann, Papassotiropoulos, de Quervain, & Rasch, Citation2015; Baran, Pace-Schott, Ericson, & Spencer, Citation2012).
The present study is the first to examine the interaction between sleep, emotion and SIF to see if the potential effect of sleep on SIF would be stronger for emotional items. A possible reason for the lack of an effect of sleep on SIF in the previous study (Fischer et al., Citation2011) could perhaps be because they only included neutral material. Given that sleep has previously been found to interact with both emotion and different forms of forgetting inductions, it is of interest to see what effect sleep has on unwanted emotional memories. For this reason, we varied the valence of the stimulus material used in this study.
It has often been suggested that REM sleep in particular would be especially beneficial for the consolidation of emotional memories, because of the high degree of activity of the hippocampus and amygdala during this stage (Hennevin, Hars, Maho, & Bloch, Citation1995; Maquet et al., Citation1996). Several studies using split night or selective REM deprivation designs have found REM sleep to be selectively beneficial for emotional memories (Groch, Wilhelm, Diekelmann, & Born, Citation2013; Groch, Zinke, Wilhelm, & Born, Citation2015; Wagner et al., Citation2001; Wiesner et al., Citation2015; but see also Morgenthaler et al., Citation2014 for a null result). However, only very few studies have found an actual correlation between REM duration and emotional memory performance (Nishida, Pearsall, Buckner, & Walker, Citation2009; Payne, Chambers, & Kensinger, Citation2011; Wiesner et al., Citation2015). The absolute majority of studies that have included polysomnography have reported no correlation between duration of REM and emotional memory performance (e.g. Ackermann et al., Citation2015; Baran et al., Citation2012).
A research question in the present study was whether sleep helps to strengthen the inhibition of these memories that is believed to occur from repeated suppression, or if sleep instead helps to “repair” the accessibility of these memories. Studying the link between sleep and SIF using both neutral and negative material is important considering that sleep disturbances, dysregulation of REM sleep and failure to inhibit thoughts of unwanted emotional memories are common features of both depression and post-traumatic stress disorder (PTSD; Brewin, Citation1998; Germain, Citation2013; Palagini, Baglioni, Ciapparelli, Gemignani, & Riemann, Citation2013). Thus, increased knowledge of the potential role of REM sleep in making unwanted memories more accessible could have important clinical implications.
It has further been shown that sleep after directed forgetting instructions during the encoding of emotional film clips increased physiological stress responses while watching images from these films during a subsequent re-test, without affecting explicit memory performance (Kuriyama, Honma, Yoshiike, & Kim, Citation2013). This indicates that emotional processing during sleep differs depending on if participants are instructed to remember or to suppress during encoding.
Research questions and hypotheses
Three main research questions were of interest in this study:
Would SIF remain after the delay interval? Most previous studies on this topic have used longer delay intervals. Here we wanted to examine if SIF effects would have diminished already after 3.5 h. No previous study has tested if the SIF effect is still present after a delay interval of only 3.5 h spent awake.
Would there be more or less SIF for negative material, would negative material be experienced as more intrusive during the T/NT phase, and would the effect of emotion interact with the duration of the delay interval? Considering that previous studies have shown contrasting results, with both more and less SIF for emotional items, we did not have a directed hypothesis, given that the result could be expected to go in both directions. We had an exploratory approach when it came to how the emotion of the material would affect the preservation of SIF in the two groups with the delayed re-test.
By including the intrusion measurement, we were also able to test the model that predicts that a larger degree of intrusions for emotional material during the T/NT-phase would lead to more inhibitory control, which would then result in increased forgetting of these memories during the re-test.
(3) Would sleep and wake affect the duration of SIF differently, and if so, would this potential effect be larger for stimuli with negative compared to neutral valence? Given the contrasting results in the previous literature regarding the role of sleep in the consolidation of memories subjected to inhibition during either encoding or retrieval, we did not have a directed hypothesis. Sleep, as compared to wake could be expected to both increase and decrease the degree of SIF. Most of the previous studies however have only examined this using neutral material. Given the suggested role of sleep in primarily strengthening emotional memories, we wanted to examine if there was an interaction between SIF, sleep and emotion. Based on previous findings, we further predicted that in the sleep group, there would be a negative correlation between SIF and time spent in REM sleep.
Apart from these main objectives, we also wanted to examine if sleep would have a beneficial effect on memory consolidation, and if this benefit would be larger for negative items relative to neutral ones.
Method
Participants
Participants were recruited through advertisements put up around the Lund University campus. The power analysis for our main research question, the difference in SIF between the groups, was made in G*Power using an estimated effect size f of 0.25, an α of .05, and a power of .80. As mentioned above, no previous study has examined if there is an interaction between delay group and emotion for SIF. Therefore, we based our estimated effect size on similar studies. These were Payne et al. (Citation2008), who found an interaction between Group (Sleep/Wake) and Valence on general memory performance (rather than SIF) with an of .20, and Marzi et al. (Citation2014), who found an interaction between Group (a low and a high trait anxiety group) x Item Type (No-Think, Baseline, Think) x Emotion, with an
of .10. With three different groups (Sleep/Wake/No Delay), and two different measures (Neutral/Negative), this analysis revealed the need for a sample size of 42 participants.
Thirty-seven participants were recruited for the Sleep/Wake condition of the experiment and 21 participants were recruited for the No Delay group. Participants were recruited separately for these two different conditions of the experiment. Inclusion criteria for the study were; being between 18 and 35 years old, not diagnosed with any psychiatric or sleep disorders, not taking any medications known to affect sleep, having normal colour vision and having Swedish as native language, or the ability to speak it at an expert level.
Participants had to sleep for at least 6 h per night during the five nights preceding the experiment, and for at least 7 h during the final night before the experiment. The consumption of nicotine or caffeine was prohibited during the experimental day.
Three participants in the Sleep/Wake version withdrew their participation during the experiment, and one was excluded from further analysis after having reported not following instructions during the T/NT phase. Three participants in the No Delay group withdrew their participation during the experiment, and one participant was excluded from further analysis due to not following instructions during the re-test because of excessive sleepiness. The final sample included in the analysis consisted of 16 participants (eight female) in the Wake group, 17 participants (eight female) in the Sleep group and 17 participants (11 female) in the No Delay group.
Mean age for the participants in each group is shown in . A univariate ANOVA showed that the age of the participants did not differ between the groups, F(2,47) = 1.71, p = .19.
Table 1. Descriptive data for the different groups (Mean and SD).
Participants in the Sleep and Wake groups received two cinema tickets and lunch as compensation for taking part in the experiment. Given that the No Delay condition was less time consuming, participants in this condition received only one cinema ticket. The study followed the Helsinki declaration and was approved by the Lund University ethics review board (Lund; 2013/696).
Material
The stimulus material consisted of 57 word-image pairs. Six different sets of International Affective Picture System (IAPS) images (Lang, Bradley, & Cuthbert, Citation2008) were used, three neutral and three negative, containing eight images each. The assignment of set to item type (Think, Baseline, No-Think) was counterbalanced across participants. The neutral sets had a mean arousal rating of 5.40 (SD = 0.46) and a mean valence rating of 5.77 (SD = 0.57). The negative sets had a mean arousal rating of 5.31 (SD = 0.49), and a mean valence rating of 2.64 (SD = 0.37). There were also nine filler images that were neutral in both valence and arousal.
The words used were common neutral concrete Swedish nouns with a maximum of three syllables (e.g. hammer, lamp, table).
The Trait Anxiety part of the State Trait Anxiety Inventory (STAI-T; Spielberger, Citation1983) was completed online by the participants before the experimental day. This was done to ensure that there were no group differences in this variable given that previous studies have found trait anxiety to be associated with the degree of SIF (Marzi et al., Citation2014; Waldhauser, Johansson, Bäckström, & Mecklinger, Citation2011).
In order to assess sleepiness throughout the experimental day, we used the Karolinska Sleepiness Scale (KSS; Åkerstedt & Gillberg, Citation1990).
Procedure
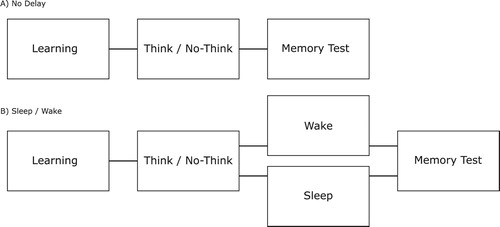
Participants in the Sleep/Wake version arrived at the lab at 10:00 am We kept the start time constant between the Sleep and the Wake group whereas the start time varied in the No Delay group for convenience reasons, considering that their participation was less time consuming. Most participants (13 out of 17) in the No Delay group chose to start the task in the morning (between 9:30–11), whereas the remaining four participants chose to start the task in the afternoon (between 13 and 14). An overview of the procedure is presented in .
Figure 1. (A) Participants in the No-Delay group first encoded words-image pairs. When they had learned these associations to criterion, they performed the Think/No-Think task and then completed the memory test after a five-minute break. (B) Participants in the Sleep and the Wake groups performed the learning and the Think/No-Think task in the same manner as the No Delay group, but performed the final memory test after a 3.5 h long delay interval. For the Sleep group, this delay interval contained a 2-hour nap opportunity, whereas the Wake group spent a similar amount of time passively resting.
Participants were first informed about the purpose of the study, and were told the cover story that we were interested in individual differences in the ability to focus one’s attention and ignore distractors. The participants in the Sleep/Wake groups were additionally told that the focus of the study was to see how sleep, as compared to wake, affected this ability. Participants were also told that we were interested in the relation between eye movements, eye blinks and attention.
Two Ag/AgCI electrodes were then placed below the right eyelid and one behind the right ear to measure EMG blink activity from the orbicularis oculi muscle.
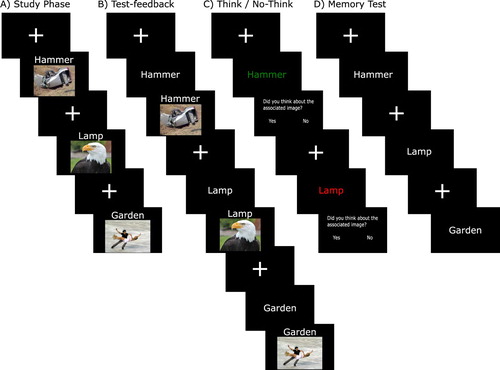
Participants then completed the Karolinska Sleepiness Scale for the first time (KSS1). The experiment then proceeded in nine different phases. The experiment was run on a computer using E-prime (Psychology Software Tools). The background colour of the screen was black and the words were presented in white font colour except for during the T/NT phase. The inter-trial interval (ITI) was 1750 ms, with a fixation cross appearing on the screen during the first 1500 ms. The experimenter sat next to the participant throughout the experiment and recorded the accuracy of their responses using the keyboard. An overview of the design is presented in .
Figure 2. Overview of the experimental procedure. (A) Study phase. Words-image pairs were presented on the screen, one by one, and participants were asked to form an association between the word and the image. (B) Test/feedback. During this phase, only the words appeared on the screen and participants were asked to say which image they had previously been associated with. After each trial, the correct image was shown on the screen as feedback. This stage was repeated until participants had learned the images to criterion. (C) Think / No-Think. Here, a subset of the words were presented on the screen again. If a word was written in Green, participants were asked to think of the image that it had previously been associated with (Think items). If the word was written in red, participants were asked to suppress all thoughts of the associated image (No-Think items). After each trial, participants were asked to use the keyboard to indicate if they had thought about the associated image or not. A subset of the images was never presented during this phase (Baseline items). (D) Memory Test. The final memory test took place either immediately after the Think / No Think phase or after a 3.5 h delay interval containing either sleep or wake. Participants were presented with all the words again and were asked to say which image they had previously been associated with, regardless of which colour they had been presented in during the Think / No-Think phase. The pictures are example images used under the Creative Commons license and obtained from maxpixel.net.
Phase 1 – Describing the images
In the first phase, all 57 images (24 neutral, 24 negative and 9 filler images) were shown on the screen, one at a time, in a randomised order. After the image had been shown for 3000 ms, a burst of white noise was played in order to elicit an eye blink. This was done to examine if sleep and Think/No Think instructions would affect emotional reactivity differently. The analysis of the eye blink data yielded no significant results however, due to enormous variation in habituation of responses across participants, and will therefore not be discussed further. 500 ms after the burst of white noise, the words “describe the image” appeared on the screen and participants were asked to describe the content of the image with three to four words. After the participant had described the image, they proceeded to the next image. There was no time limit, and each trial lasted until a description of the image had been given.
Phase 2 – Study phase 1
In this phase, each image was presented on the screen together with a word centred above it. Participants were asked to first read the word out loud, and then say the name of the image. They were allowed to name the image however they wished, but each image had to have a unique name which clearly distinguished it from the other images. If the name they chose was not specific enough, they were asked by the experimenter to provide a more specific description. They were then asked to stick with that name of the image for the rest of the experiment. Participants were instructed to try to create an association between the word and the image, and that these associations would be used in a subsequent attention task (the word memory was never used, to ensure that participants did not expect a memory test). The word-image pairs were shown in a randomised order for 3000 ms each.
Phase 3 – Test/Feedback 1
In this phase, each word was shown, without its associated image, at the centre of the screen in a randomised order. The participants were asked to verbally say the name of the image it had previously been associated with. Each word was shown for a maximum of 3000 ms, or until the participant had given a response. After that, both the word and the image were shown on the screen again as feedback for additionally 3000 ms. The participants were asked to use this time to further strengthen the association between the word and the image.
Phase 4 – Study phase 2
This phase was identical to Study Phase 1.
Phase 5 – Test/Feedback 2
This phase was identical to Test/Feedback 1. If the participant did not give accurate responses to at least 32 of the 48 trials (filler pairs not included), this phase was repeated until this criterion was reached.
Phase 6 – Criterion test
In this phase, all the words (including the filler words) were shown on the screen again in a randomised order, one at a time, and the participants were asked to say which image it had previously been associated with. Each word was shown until the participant had responded, or for a maximum of 4000 ms. Correct responses given after this time limit were scored as inaccurate. The correct image was not shown as feedback, to avoid additional learning during this phase. Only items correctly remembered during this test were used in the subsequent data analyses.
Phase 7 – Think/No-Think (T/NT)
In this phase, participants were told that the words would once again be individually presented on the screen. Unlike previous phases however, they were not supposed to say anything out loud. Instead, they were instructed to just focus on the word, and to do a task depending on what colour it was written in.
If the word was shown in green (Think items), they were asked to think about the image it had previously been associated with, and to keep the image in their mind for the entire duration that the word was being shown.
If the word was shown in red (No-Think items), participants were asked to avoid all thoughts of the associated image for the entire duration that the word was being shown. Participants were further told that if they came to think about the image, they were to push it out of mind as quickly as possible. They were instructed that it was very important that they read all the words written in red so that they understood their meaning, and to look at them during the entire duration of the trial. They were also instructed not to replace the associated image with another thought, word or image.
After each trial, participants were asked if the associated image had come to mind or not. They answered using the keyboard. Participants were further instructed not to think about the image while answering the question, and to not prepare their answer to the question while the word was still being displayed.
Participants then went through two practice phases of this procedure using the nine filler pairs. After each practice phase, participants answered a questionnaire which was included to ensure they had understood and were following the instructions.
Next, participants viewed 32 of the words they had seen before (16 previously associated with neutral images and 16 previously associated with negative ones). Half of the words were shown in green (Think items) and the other half were shown in red (No-Think items). Eight neutral and eight negative items were not shown at all during this phase (Baseline items). Each word was presented for 4000 ms, after which the question asking if they had thought about the previously associated image or not appeared on the screen. The question was shown for 1500 ms, or until the participant had responded. The words were shown in a pseudorandomised order so that no more than three of the same Item Type (Think or No-Think) could be shown in a row, and so there would be equally many presentations of each Item Type for each 16 items shown. When each item had been shown once, there was a one minute break before the next round started. After half of the rounds, participants once again answered the questionnaire to make sure they were following the instructions, and were then reminded about the instructions one last time. Each word was presented in the same colour throughout the entire T/NT phase.
Phase 8 – Delay interval
After the T/NT phase, participants in the Sleep/Wake condition were randomly allocated into either the Sleep or the Wake group. Participants in the Sleep group had the polysomnography put on, and participants in the Wake group had a 40-minute break in the lab during which they were allowed to read or use their phone or laptop. Participants were then served lunch after which the Sleep group had a two-hour sleep opportunity and the Wake group spent two hours quietly resting in a comfortable chair. During this session, participants in the Wake group were not allowed to use their phone or read. This was because we wanted them to be as passive and subjected to as little novel interference as the participants in the Sleep group. Every 15 min, the experimenter came to talk to the participants in the Wake group to make sure that they were feeling okay and that they had not fallen asleep. After two hours, participants in both groups had a 15 min break during which they were allowed to do what they wanted. This was done in order to give any potential sleep inertia in the Sleep group time to decrease. The experiment then resumed, approximately 3.5 h after the end of the T/NT phase.
Participants in the No Delay group had a five-minute break after the T/NT phase and then proceeded directly to the Re-test phase which started with participants completing the KSS for a second time (KSS2).
Phase 9 – Re-test
Participants were instructed that they would once again view all the words, one at a time, and say which image it had previously been associated with regardless of which font colour it had been shown in during the T/NT phase. Participants first practiced this using the nine filler items and were then tested on all the items in a procedure identical to the criterion test.
Polysomnography recordings
Polysomnography was recorded with a sampling rate of 256 Hz. EEG was measured with F3, F4, C3, C4, O1 and O2 referenced to the contralateral mastoid in accordance with the 10–20 montage system. The EEG data was filtered with a high-pass filter of 0.3 Hz, and a low-pass filter of 35 Hz. Electrooculography was measured with one electrode below the left ocular canthus and one above the right ocular canthus and electromyography with two submental electrodes. The equipment used was an Embla Titanium (Embla Systems).
Data analysis
When testing correlations with variables that were not normally distributed we used Spearman’s Rho. In case of non-significant effects, we also performed Bayesian statistics to calculate the strength of evidence for the null hypothesis. This was done using the JASP version 0.9.2. software (JASP Team, 2019), using the default settings. All confidence intervals (CI) reported for the effect sizes are 95% and Cohen’s δ, which is the default in JASP.
Memory performance
The SIF effect was defined as the difference in memory performance between No-Think and Baseline items (so that a negative value indicates that No-Think items were forgotten to a larger extent than Baseline items). This contrast is used to compare the effect of retrieval-suppression with passive forgetting over time. The Think effect was defined as the difference in memory performance between Think and Baseline items.
Intrusions
The No-Think trials were divided into intrusion- and non-intrusion trials based on the subjective reports collected during the No-Think phase. An intrusion was defined as when a participant responded that they had failed to keep the image associated with a No-Think word out of awareness during the T/NT phase. Data was averaged over two T/NT rounds at a time. To test if the decrease of intrusions during the T/NT phase was correlated with forgetting, we also calculated the slopes of the degree of intrusions during the different rounds throughout the T/NT phase. The number of intrusions during the first two rounds were set to 100% as a baseline, in accordance with previous studies (Levy & Anderson, Citation2012; Hellerstedt et al., Citation2016).
Sleep staging
Sleep was scored according to the manual of the AASM (Iber, Ancoli-Israel, Chesson, & Quan, Citation2007), by a professional sleep technician blind to the study design as well as by the first author who is a trained scorer. In order to be blind to the hypothesis, sleep was scored without knowledge of the participants’ memory performance. Disagreements between the sleep scorers were settled by following the interpretation of the more senior external sleep scorer. Epochs with an arousal lasting for the majority of the epoch were scored as wake.
Results
Sleepiness, learning performance and trait anxiety
Before moving on to the main analyses testing our hypotheses, we wanted to ensure that the groups did not differ in any of the control measures. Descriptive data for these variables is presented in .
Univariate ANOVAs revealed that the groups did not differ in either the number of test/feedback cycles needed to reach the criterion of 66% accuracy for word-image associations (counting the phase described as “Test/Feedback 2” in the methods description above as the first one), the number of correct responses during the criterion test, or in trait anxiety, all ps ≥ .14. One participant in the Wake group and one in the No Delay group did not complete the STAI-T questionnaire so the analysis for this measurement was based on n = 48.
The participants were significantly sleepier before the re-test compared to at the beginning of the experimental day, p < .001, = .26. This increase of sleepiness was however equivalent in all three groups, as evident by the lack of an interaction effect of Group (No Delay/Wake/Sleep) and Time (KSS1/KSS2), and there was no general difference in sleepiness as evident by the lack of a main effect of Group, both ps ≥ .51.
No differences between the Sleep and the Wake group
To test if sleep and wake would affect memory performance differently, we initially performed a 2 X 3 X 2 mixed ANOVA with Group (Sleep/Wake), Item Type (Think, Baseline, No-Think) and Emotion (Neutral/Negative). This revealed no main effect of Group, F(1, 31) < 0.001, p = .99, indicating no general memory benefit after sleep compared to after wake, and no main effect of Emotion, F(1, 31) = 0.34, p = .57, indicating that negative items were not better remembered than neutral ones. This further revealed that no SIF or Think effect was present after the delay interval, as evident by the lack of a main effect of Item Type, F(2, 62) = 0.03, p = .98. There was no support for the prediction that sleep and wake would differently affect the SIF or the Think effect, as evident by a lack of an interaction effect of Item Type and Group, F(2, 62) = 0.30, p = .74.
Furthermore, there was no interaction effect of Group and Emotion, and no three-way interaction between Group, Item Type and Emotion, both ps ≥ .40, indicating that sleep, compared to wake, did not have a stronger effect on negative items compared to neutral ones, regardless of item type.
We further calculated Bayesian statistics for all the contrasts of interest. The BF01 value for the group difference in the SIF effect (memory performance for Baseline items subtracted from memory performance for No-Think items) was 2.59 (CI: −0.42–0.78). For neutral items only it was 2.55 (CI: −0.42–0.78), and for negative items only, 2.91 (CI: −0.51–0.68). These values show that support for the null hypothesis was slightly below moderate, and rather of an anecdotal character.
For the Think effect, the BF01 for both emotions combined was 3.00 (CI: −0.61–0.57). The BF01 for the neutral items only was 3.00 (CI: −0.61–0.58), and for the negative items only it was 2.98 (CI: −0.62–0.56), indicating moderate support for the null hypothesis.
Given the lack of any differences between the Sleep and the Wake group in any memory performance variable, these two groups were collapsed into one when testing the effect of the delay interval on the SIF and the Think effect respectively. For descriptive data for memory performance for the Sleep and the Wake group separately, see Supplementary Table 1. SIF for the Sleep and the Wake group separately is displayed in Supplementary Figure 1.
SIF was only evident in the No-Delay group, and was equivalent for both negative and neutral items
Descriptive data for memory performance for the No Delay and the combined delay groups is displayed in . The effect of the delay interval on SIF was tested with a 2 × 2 mixed ANOVA with Emotion (Neutral/Negative) and Group (No-Delay/The combined delay groups).
Table 2. Memory performance (Mean and SD).
This revealed a main effect of Group, indicating a larger SIF effect in the No Delay groups compared to in the combined delay groups, F(1,48) = 11.86, p = .001, = .20. These results are displayed in . There was no main effect of Emotion, F(1,48) = 0.35, p = .58, and no interaction effect of Group and Emotion, F(1,48) = 1.69, p = .20, indicating that the increased SIF effect in the No Delay group was equivalent for neutral and negative items.
Figure 3. Suppression-induced forgetting in the final memory test for the No Delay and the combined delay groups (the sleep and the wake group). Suppression-induced forgetting was calculated by subtracting performance for the Baseline items from performance for the No-Think items. A negative value indicates that No-Think items were forgotten to a larger extent than Baseline items (lower values indicates more suppression-induced forgetting). The plot illustrates group means (larger black dots) together with 95% confidence intervals (error bars). Individual data points from every subject are shown in the dot plots and the distribution of the data for each group are depicted in split violin plots.
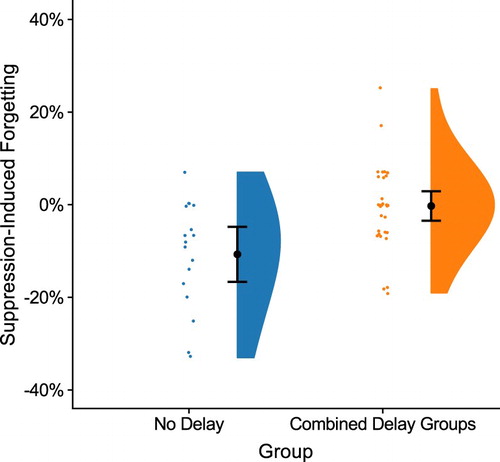
In the No Delay group, there were significant SIF effects for both emotions combined as well as for both neutral and negative items separately. See for statistics of these contrasts. The degree of the SIF effect did not differ between neutral and negative items, t(16) = 1.50, p = .15.
No significant SIF effects were evident in the combined delay groups for either both emotions combined, or for neutral or negative items separately, all ps ≥ .60. See the supplementary material for comparisons between the Think and the No-Think items.
Bayesian statistics for the lack of a SIF effect in the two delay groups combined showed moderate support for the null hypothesis for all emotions combined (BF01 = 5.32[CI: −0.29–0.35]) and for both neutral (BF01 = 4.71 [CI: −0.24–0.41]) and negative (BF01 = 5.15 [CI: −0.38–0.28]) items separately. Similar results were found for the Wake group separately for both emotions combined (BF01 = 3.75 [CI: −0.52–0.39]) as well as for both neutral (BF01 = 3.90 [CI: −0.47–0.42]) and negative (BF01 = 3.63 [CI: −0.55–0.36]) items separately. The same analysis in the Sleep group separately revealed moderate support for the null hypothesis for both emotions combined (BF01 = 3.37 [CI: −0.30–0.58]) and for negative items (BF01 = 4.02 [CI: −0.43–0.45]), but only just below moderate support for neutral items separately (BF01 = 2.77 [CI: −0.65–0.25]).
Delay did not affect the Think effect
The effect of the delay interval on the Think effect (memory performance for Think items minus memory performance for Baseline items) was tested with a 2 × 2 mixed ANOVA with Emotion (Neutral/Negative) and Group (No-Delay/The combined delay groups).
This revealed no main effect of Group, F(1,48) = 2.66, p = .11, indicating that the delay did not affect the size of the Think effect. There was no main effect of Emotion, F(1,48) = 1.79, p = .19, indicating that the Think effect did not vary depending on emotion, and no interaction effect of Group and Emotion, F(1,48) = 0.001, p = .98, indicating that the effect of emotion did not differ between the groups.
Memory performance for Think items did not significantly differ from Baseline items for either both emotions combined, or for neutral or negative items separately in either group, all ps > .13.
Bayesian statistics within each group separately revealed that in the No Delay group, there was only anecdotal support for the null hypothesis for both emotions combined (BF01 = 1.57 [CI: −0.13–0.79]) and the neutral items (BF01 = 1.39 [CI: −0.12–0.82]), but moderate support for the negative items (BF01 = 3.42 [CI: −0.32–0.57]).
In the two delay groups combined, there was moderate support for the null effect for both emotions combined, as well for neutral and negative items separately (BF01 = 5.03 [CI: −0.38–0.26], 4.21 [CI: −0.22–0.45], and 3.12 respectively [CI: −0.51–0.16]). This was also the case in the Wake group separately (BF01 = 3.85 [CI: −0.50–0.40], 3.58 [CI: −0.37–0.55] and 3.11 [CI: −0.61–0.30] respectively), as well as in the Sleep group separately (BF01 = 3.83 [CI: −0.50–0.38], 3.39 [CI: −0.31–0.56] and 3.04 [CI: −0.62–0.28] respectively). Bayesian statistics for comparing the No Delay group with the two delay groups combined revealed only anecdotal support for the null hypothesis for both emotions combined, as well as for neutral and negative items separately (BF01 = 1.12 [CI: −0.13–0.98], 2.20 [CI: −0.28–0.80] and 2.01 [CI: −0.26–0.83] respectively). This argues that although we had moderate support for the null finding regarding a lack of a Think effect within the Sleep and the Wake group, we did not have sufficient sensitivity to detect any differences for the Think items between the No Delay group and the combined the delay groups and thus, this will not be discussed further.
Intrusions decreased as a function of repetition during the No-Think phase and negative items were more intrusive
Intrusions of the images associated with the No-Think words during the T/NT phase were analysed with a mixed 2 X 5 ANOVA with Emotion (Negative/Neutral) and Trial (Trial block 1-5). These results are displayed in . Results revealed a main effect of Trial, F(2.45, 120) = 37.99, p < .001, = .44. Post hoc polynomial contrasts revealed that this effect was linear, FLinear(1, 49) = 64.22, p < .001,
= .57, showing that the number of intrusions decreased throughout the T/NT phase, replicating previous findings (Gagnepain et al., Citation2017; Hellerstedt et al., Citation2016; Levy & Anderson, Citation2012; van Schie & Anderson, Citation2017).
Figure 4. Percentage of intrusions of neutral and negative items (Mean ± S.E.M) respectively throughout the different blocks of No-Think trials during the T/NT phase.
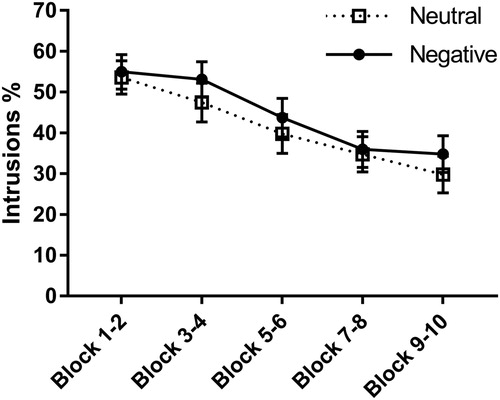
Regarding the research question if negative images would be more intrusive, there was, unlike the results of Gagnepain et al. (Citation2017), a main effect of Emotion, F(1, 49) = 4.88, p = .032, = .09, revealing that there was more intrusions for negative than for neutral items. There was no interaction effect of Trial and Emotion, F(4, 196) = 1.14, p = .34, indicating that the decrease of intrusions across trials was similar regardless of emotion.
Unlike previous studies (Levy & Anderson, Citation2012; Hellerstedt et al., Citation2016), we found no correlations between the slope of the decrease of intrusions throughout the T/NT phase and the SIF effect, not for all participants combined, or for any of the groups separately, all ps >. 32. There were no correlations between the slope of decreases of intrusions and the size of the SIF effect when testing negative and neutral items separately, not for all participants combined, both ps ≥ .32, or for any group separately, all ps ≥ .23.
Analysing participants’ responses from the Think trials revealed the same main effect of Emotion, F(1,49) = 5.98, p = .018, =.11, indicating that participants thought of the negative items to a larger extent than the neutral items. There was also a main effect of Trial, F(2.34,196) = 4.67, p = .001,
=.09. Post hoc polynomial contrasts revealed that this effect was linear, FLinear(1,49) = 7.71, p = .008,
= .14, indicating that the extent to which the participants thought of the images during the Think-trials decreased as a function of Trial block. There was no interaction between Trial and Emotion, F(3.15,196) = 1.72, p = .15, indicating that this decrease was equivalent for negative and neutral items. For descriptive data of responses to Think trials divided by emotion and trial block, see Supplementary Table 2.
To compare the respective main effects of Trial and Emotion between Think and No-Think items, we conducted a 2 X 2 X 5 mixed ANOVA with Item Type (Think/No-Think), Emotion (Neutral/Negative) and Trial (Trial block 1-5). This revealed a main effect of Item Type, F(1,149) = 135.66, p < .001, = .74, indicating that participants more often thought of the associate during Think trials than during No-Think trials. There was no interaction of Item Type and Emotion, F(1,49) = 0.30, p = .86, indicating that the increased retrieval of negative items was equivalent for Think and No-Think trials. There was also a significant interaction effect of Item Type and Trial, F(2.69, 131.55) = 20.14, p < .001,
= .29. Post hoc polynomial contrasts revealed this effect to be linear, FLinear(1,49) = 38.28, p < .001,
= .44, indicating a sharper decrease for No-Think trials than for Think trials. There was no three-way interaction of Item Type, Emotion and Trial, F(4,196) = 1.46, p = .22, indicating that the interaction effect of Item Type and Trial was equivalent for both emotions.
Polysomnography results
Due to technical errors, polysomnography data was missing from two participants and thus, all these results are based on n = 15. Descriptive sleep data is presented in .
Table 3. Sleep statistics (Mean and SD).
Contrary to our expectations, there was no correlation between the duration of REM sleep and the size of the SIF effect, not for both emotions combined, rs = −0.39, p = .15, or for neutral, rs = −0.35, p = .20, or negative, rs = −.21, p = .45, items separately. Further, we also tested if time spent in REM sleep was associated with the consolidation of memory for negative items. Results revealed no such correlations between REM sleep and memory of negative items for either Baseline, Think or No-Think items, all ps ≥ .15.
There was no correlation between time spent in SWS with the Think effect for both emotions combined, or for negative or neutral items separately, all ps ≥ .25. There was no correlation between SWS and the SIF effect for all items combined, or for neutral items only, both ps ≥ .15. There was a significant negative correlation between time spent in SWS and the SIF effect for negative items, rs = .53, p = .043, so that more time spent in SWS predicted less SIF. This seems to have been driven by a negative correlation specifically between SWS and memory performance for negative Baseline items, rs = −.58, p = .024, and there was also a correlation between SWS and negative and neutral Baseline items combined, rs = −.52, p = .047. Neither of these correlations were however significant after correcting for multiple comparisons. SWS duration was not correlated with memory performance for any other item type, regardless of emotion.
There were no correlations between total sleep time (TST) and the SIF effect, either for both emotions combined, or for either emotion separately, all ps > .10. There were no correlations between TST and the Think effect for either both emotions combined, or for negative items, both ps ≥ .43. There was a tendency towards a negative correlation between TST and the Think effect for neutral items rs = −.47, p = .079.
Trait Anxiety and SIF
For all participants combined, there was no correlation between the degree of SIF and Trait Anxiety for either both emotions combined, or for neutral or negative items separately, all ps ≥ .60. In the No Delay group however, there was a significant correlation between Trait Anxiety and SIF for negative items so that higher Trait Anxiety was associated with less below baseline-forgetting of the No-Think items, rs = .51, p = .044. This correlation was however no longer significant after applying Bonferroni corrections. No such correlations were present for neutral items or for both emotions combined, both ps > .30.
There were no correlations between Trait Anxiety and SIF in either the Sleep or the Wake group for either neutral or negative items separately, or for both emotions combined, all ps ≥ .31.
Discussion
Suppression-induced forgetting was only present in the No Delay group
A SIF effect was only present in the group that took the re-test immediately after the T/NT phase, and not in the groups that were tested after an additional delay interval of 3.5 h. This is similar to previous findings examining the duration of SIF. Most of these studies have used a delay interval of a week or more (Meier et al., Citation2011; Nørby et al., Citation2010; Noreen & MacLeod, Citation2014), and only one used shorter time periods, either 8 h in the first experiment or approximately 3.5 h in the second (Fischer et al., Citation2011; the second experiment however did not have a wake group but instead an early and a late sleep group). The present study is the first to demonstrate reduced SIF after such a short prolonged delay interval containing time spent awake. The pattern of results suggests that retrieval-suppression leads to a temporary reduction in the accessibility of the suppressed memories. This reduction extends beyond the time when cognitive control is actively applied, as indicated by forgetting in an immediate test. The suppressed memories do however regain accessibility over time when cognitive control is no longer actively exerted over them.
The only study that has found a preserved SIF effect at a delayed re-test was Hotta and Kawaguchi (Citation2009), who found such an effect still 24 h after the T/NT phase. However, they only found this effect in participants who afterwards reported to have used a thought substitution strategy when suppressing the No-Think items from awareness during the T/NT phase. Their results might therefore be due to interference from the substitute rather than suppression, and thus not represent inhibition. In the present study, we used direct suppression instructions that explicitly instructed the participants not to use a substitution strategy. In line with the present results, no SIF effect was found in the Hotta and Kawaguchi (Citation2009) study after a 24 h delay when the participants reported having used a suppression strategy.
The finding that the forgetting effects caused by retrieval-suppression seem to dissipate quite rapidly in laboratory studies does not necessarily mean that retrieval suppression cannot have more durable consequences in everyday life. As Anderson and Huddleston (Citation2012) point out, retrieval suppression of negative autobiographical memories is likely to be attempted repeatedly during extensive periods of time. In our study, retrieval was only suppressed during ten trials with a duration of four seconds each. As suggested by Anderson and Huddleston (Citation2012), future studies should examine if repeated suppression distributed over a longer period of time produces more long-lasting effects. Further, when suppressing retrieval of actual memories that cause emotional distress, people are likely to be more motivated to engage in retrieval suppression and do so with more effort.
Even if retrieval suppression causes only short-term forgetting of the suppressed memory, it may still be an effective strategy to avoid distress from an intruding negative memory in the present moment. This could serve an important function even if the memory needs to be suppressed de novo each time a reminder of it is encountered.
A potential limitation to keep in mind when interpreting these results is that given that the experimental procedure was shorter in the No Delay group, they did not complete the task at the same times as the Sleep and the Wake group. Instead, participants in the No Delay group could choose for themselves which time they wanted to come to the lab and start the task. An interesting question for future studies will definitely be if suppression ability varies depending on time of day. There were however too few participants that came in the afternoon (only 4 of 17) to make any meaningful statistical comparisons regarding this in the present study.
Equivalent SIF for negative and neutral items in the No Delay group
The SIF effect found in the No Delay group was similar for both negative and neutral items. This finding is in line with several previous studies that have not found an effect of emotion on SIF (Joormann et al., Citation2005; Murray et al., Citation2015; Murray et al., Citation2011; van Schie et al., Citation2013). Other studies have found increased SIF for negative items (Depue et al., Citation2006; Marzi et al., Citation2014; Noreen & MacLeod, Citation2014), whereas others have found significant results in the opposite direction, with less SIF for negative items (Chen et al., Citation2012; Nørby et al., Citation2010; Sakaki et al., Citation2014). In our opinion, there is no clear factor that systematically varies between studies that have found effects in the different directions that could explain these contrasting findings. Several different factors could explain why emotional material is remembered to a greater extent than neutral material. Such factors could include arousal, valence, personal relevance and intrusiveness. In the present study, we found that negative images were experienced as more intrusive, using a material where the negative images differed from the neutral ones in valence, and not in arousal. Hopefully, future studies systematically varying and measuring these kinds of variables will contribute to further the understanding of the relationship between SIF and emotion. A hypothesis for future research is that it is perhaps arousal, and not valence, that determines the role of emotion on SIF.
We did observe a correlation between trait anxiety and SIF (so that higher trait anxiety predicted less SIF) in the No Delay group for the negative items, but this correlation was no longer significant after correcting for multiple comparisons. Although this correlation did not survive the Bonferroni correction, it is worth noting that it is in line with Marzi et al. (Citation2014), who found that high trait anxiety specifically predicted less SIF for negative items, and Waldhauser et al. (Citation2011) who found such a correlation using neutral material only.
One potential limitation of our study is that some of the emotional tone of the images could potentially have been lost when they were being transformed to words. The fact that we did have an effect of emotion on intrusions, as well as for voluntary recall during Think trials, does however argue for that the manipulation of emotion had been effective even though participants answered using words.
More intrusions for negative items
Our study found more intrusions for negative items compared to neural ones. One aim of this study was to test a model suggesting that the higher degree of intrusiveness of emotional items would also make them more susceptible to SIF. Such a result would have been in line with EEG and fMRI studies, suggesting that inhibitory control is recruited as a reaction to the reactivation of a memory (Benoit et al., Citation2015; Hellerstedt et al., Citation2016; Levy & Anderson, Citation2012). Negative memories would be more likely to be reactivated, and therefore more retrieval suppression would be needed in order to purge them from awareness, which would lead to a reduced availability of these items, and ensuing SIF. However, despite finding increased intrusions for negative items, the lack of a difference for SIF between neutral and negative items in the present study, does not support such an interpretation.
In a recent study, Gagnepain et al. (Citation2017) found no difference in intrusiveness between negative and neutral items. One reason for why the present study did find such a difference could be because we had more intrusions in general. This could perhaps be because of stronger encoding of the material due to higher requirements during the criterion test (cued recall instead of forced choice). If the material is more deeply encoded, it is also likely to be more intrusive.
Another study by van Schie et al. (Citation2013) employed a different strategy for measuring intrusiveness. They asked participant in retrospect, after the conclusion of the experiment, rather than after each trial, and found that participants considered negative items easier to suppress, contrary to our results. To the best of our knowledge, no study so far has compared intrusion ratings made immediately after each trial with an overall rating following the entire experiment, and it is possible that the conflicting results are due to differences in these measures. Interestingly, the increased retrieval of negative items during the T/NT phase was not unique for No-Think items, but also the Think items showed this same pattern. This indicates that emotion has similar effects on both voluntary and involuntary memory retrieval.
Although there was a decrease of the extent to which participants thought about the associated images as a function of trial block also for the Think trials, it should be mentioned that the decrease for No-Think items was significantly steeper with a large effect size. Furthermore, even though there was also a decrease for retrieval of the Think items, the minimal value for which participants thought about a Think item during a trial block was 90.03%, indicating good compliance with the Think instructions (in comparison, the lowest value for the No-Think items was 29.78%).
The previously reported relationship between a reduction in intrusion frequency over repetitions during the T/NT phase and the degree of SIF (Hellerstedt et al., Citation2016; Levy & Anderson, Citation2012) was, contrary to our expectations, not replicated in the present study.
Suppression-induced forgetting diminished similarly after both sleep and wake
The present study did not find any support for the expectation that sleep, as compared to wake, would differently affect the degree of SIF (either by “repairing” these memories by selectively strengthening the suppressed items, or by consolidating the inhibition they had been subjected to). Instead, sleep and wake did not differently affect memory for any item type. Even though the Bayesian statistics showed below moderate support for the null hypothesis, which means this null finding should be interpreted with caution, our findings are consistent with the only previous study testing the effect of sleep on SIF (Fischer et al., Citation2011). That study found no difference between a group that spent a day awake and a group that had a night of sleep during the delay interval between the T/NT phase and the re-test. Neither did they find significant SIF in either of these groups, even though the sleep group showed improved memory performance for Baseline items.
The result of the second experiment reported by Fischer et al. (Citation2011), showing an increased rebound effect after REM rich late sleep was not replicated here, as evident by the lack of a correlation between REM duration and the SIF effect. One reason for this could be that we used a design with a daytime nap, which normally contains quite little REM. There are, however, studies that have found REM sleep to be correlated with forgetting in other kind of paradigms after similarly short sleep durations (e.g. Oudiette et al., Citation2013; Hoedlmoser et al., Citation2015).
In previous work examining the role of sleep in the consolidation of memories subjected to inhibition, sleep has been found to both increase (Abel & Bäuml, Citation2012; Hupbach, Citation2018; Racsmány et al., Citation2010; Saletin et al., Citation2011), decrease (Abel & Bäuml, Citation2013; Baran et al., Citation2010) and have no effect (Blaskovich et al., Citation2017; Rauchs et al., Citation2011) on forgetting of these memories. In summary, the effect of sleep on memories subjected to inhibition during either encoding or retrieval remains to be determined.
Determining what happens with unwanted memories during sleep is a very important research question. If sleep is reliably found to help make these memories less accessible and less likely to be automatically retrieved in face of a reminder, this could be of great clinical importance. This would be a further argument to pay additional attention to sleep difficulties in disorders associated with problems regulating unwanted negative thoughts and memories, such as depression and PTSD. If sleep can be improved in these conditions, perhaps so could symptoms related to the involuntary recall of distressing memories. If sleep is found to make unwanted memories more accessible on the other hand, this could also be of potential clinical use. This could perhaps help explain why certain subgroups of people suffering from depression have been found to actually experience an improvement in mood following sleep deprivation (for a meta-analysis, see Boland et al., Citation2017), although this is just speculation at the moment.
No effect of sleep on emotional memory
We did not find any support for sleep having a stronger effect on the memory for negative items compared to neutral ones, regardless of item type. This contrasts several previous studies that have found full or partial support for sleep to be especially beneficial for emotional memories. However, half of the published studies have not reported such an effect, and thus this is an effect that is replicated in far from every study. It could be argued that our design was not sensitive to detect memory differences caused by sleep to begin with, especially considering that memory performance was high, and near ceiling in both the Sleep and the Wake group. This was also supported by the Bayesian statistics that indicated slightly below moderate support for the null hypothesis regarding differences between the Sleep and the Wake group, which means that also this null finding should be interpreted with caution. This is also a probable explanation for why we did not find a main effect of sleep on memory performance for all items combined regardless of item type and emotion.
By using cued recall instead of forced choice to assess memory performance, we made sure that no correct answers in the test/feedback cycles or the criterion test were based on chance. We consider this methodological change a strength compared to previous studies because it helped to ensure that the items had been properly encoded before they were subjected to retrieval suppression. This strong encoding can also however have contributed to some of the ceiling effects.
Other potential explanations for why no interaction between sleep and emotion was found could be the slight methodological differences in our design compared to what has been the most commonly used. These differences include the memory task used, that the neutral and negative images differed in valence and not in arousal, and that the Wake group was not allowed to go on with their normal everyday activities during the delay interval.
Only one previous study has examined the effect of sleep on emotional memory using a cued recall task, and it did not find sleep to be more beneficial for emotional memories than neutral ones (Lehmann, Seifritz, & Rasch, Citation2016).
Another potential reason for the lack of an interaction between sleep and emotion could be that our negative and neutral items only varied in valence. To the best of our knowledge, this is the first study to control for arousal when comparing memory performance for neutral and negative material between a sleep and a wake group. Previous studies (that have reported valence and arousal ratings), have varied the material in either arousal only or in both valence and arousal, with negative items being more arousing than the neutral ones. This suggests that the supposed beneficial effect of sleep on emotional memory is perhaps driven more by the arousal of the stimuli than by its valence. This should be systematically examined in future studies.
We attempted to minimise the outside interference that our wake participants were subjected to by having them quietly rest in the lab rather than to go on with their daily activities. Few studies have used a similar design. There are, however, studies that have compared sleep with both active and passive wake that have found increased memory performance after sleep even compared to a passive wake group (McDevitt, Duggan, & Mednick, Citation2015; Schönauer, Pawlizki, Köck, & Gais, Citation2014). Further support for this was found by Payne et al. (Citation2015), who used a sleep group that first took a nap and then spent a similar amount of time awake as the wake group had done. This study still found a beneficial effect of sleep on emotional memory, even though more time had passed between learning and re-test for the sleep group.
It is of course possible that although there were no effects of sleep, item type and emotion on explicit recall, there could be an effect in emotional reactivity as shown in the study by Kuriyama et al. (Citation2013). However, because our measurement of this did not yield any usable data, we are not able to say anything about this in the present study.
Another factor that might have potentially affected the results is that the re-test took place only 15 min after the end of the sleep/wake stage. It is possible that this was not a sufficient amount of time for the Sleep group to recover from sleep inertia, which could have affected their performance on the memory test. Even though giving the Sleep group more time to recover would have been ideal, there was, however, no group difference in sleepiness right before the memory test. No participant in the Wake group reported having fallen asleep during the delay interval, neither when the experimenter checked on them, nor in retrospect. We did however not have any objective measurement of this, which should be added in future studies.
One reviewer pointed out that a potential reason for why we did not find a difference in memory performance between the Sleep and the Wake group could be that some previous work (van Dongen et al., Citation2012; Wilhelm et al., Citation2011), has found that sleep only increases memory performance when participants are expecting a re-test (but see also Wamsley et al., Citation2016). Given that the T/NT paradigm per definition have the participants not expect a memory test, it could perhaps be less sensitive to detect effects of sleep. There are, however, several studies that have found an effect of sleep on memory even when no re-test has been expected. All studies using the emotional trade-off paradigm with sleep (e.g. Payne et al., Citation2008) have for example used incidental encoding, and yet found effects of sleep. Considering the mixed findings in the previous literature and the already discussed ceiling effects, we do not believe that a lack of re-test expectancy was a major contributor to the absence of an effect of sleep on memory in the present study.
Our prediction that the duration of REM sleep would be correlated with memory performance for negative items was not supported. There are several studies reporting such an effect of REM sleep on emotional memory, but most of them have used selective REM deprivation paradigms or split night designs. Few studies have reported a correlation between REM duration and emotional memory consolidation, with studies not reporting such an effect being considerably more common. The largest study to date, with 929 participants (Ackermann et al., Citation2015), found no correlation between emotional memory performance and time spent in any sleep stage. No other sleep related variables correlated with either the SIF or the Think effect after controlling for multiple comparisons.
Future research on whether sleep has a stronger effect on emotional memories than neutral ones is important because it could help us determine whether it is adaptive or not to sleep right after a negative experience. If sleep mainly strengthens the negative aspects of an event, there is a risk that sleep could cause the experience to be remembered as more negative than it actually was. Additionally, as mentioned above, mapping out exactly which sleep stages or other sleep related variables that contribute to consolidating different kinds of memories becomes increasingly important considering the improvements in methods that allow us to manipulate sleep. This means that it might one day be possible to custom design sleep so that an emotional experience is processed in an optimal way.
Conclusions
The present study suggests that SIF diminishes over time. SIF was present in the group that performed the memory test immediately after having repeatedly suppressed memory-retrieval, but not in the groups that did the memory test after a 3.5 h delay interval. This was the case regardless of whether the groups had spent this delay interval awake or asleep, suggesting that sleep and wake reduced this forgetting phenomenon to an equal degree (even though the Bayesian statistics showed that we were not able to reliably reject the null hypothesis regarding the difference between the Sleep and the Wake group). Another important finding is that it was more difficult to suppress the retrieval of negative memories than neutral ones, as evident by the larger degree of intrusions for the negative items. The SIF effect was however equivalent for both neutral and negative items.
Supplemental Material
Download Zip (87 KB)Acknowledgments
The authors gratefully acknowledge the Lund University Humanities Lab. We also gratefully acknowledge Stuart Smith for help with scoring the sleep data.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Per Davidson http://orcid.org/0000-0001-9943-9862
Additional information
Funding
References
- Abel, M., & Bäuml, K. H. T. (2012). Retrieval-induced forgetting, delay, and sleep. Memory (Hove, England), 20(5), 420–428. doi: 10.1080/09658211.2012.671832
- Abel, M., & Bäuml, K. H. T. (2013). Sleep can eliminate list-method directed forgetting. Journal of Experimental Psychology-Learning Memory and Cognition, 39(3), 946–952. doi: 10.1037/a0030529
- Ackermann, S., Hartmann, F., Papassotiropoulos, A., de Quervain, D., & Rasch, B. (2015). No associations between interindividual differences in sleep parameters and episodic memory consolidation. Sleep, 38(6), 951–959. doi: 10.5665/sleep.4748
- Åkerstedt, T., & Gillberg, M. (1990). Subjective and objective sleepiness in the active individual. International Journal of Neuroscience, 52(1-2), 29–37. doi: 10.3109/00207459008994241
- Alger, S. E., Chen, S., & Payne, J. D. (2019). Do different salience cues compete for dominance in memory over a daytime nap? Neurobiology of Learning and Memory, 160, 48–57. doi: 10.1016/j.nlm.2018.06.005
- Anderson, M. C., Bjork, R. A., & Bjork, E. L. (1994). Remembering can cause forgetting: Retrieval dynamics in long-term memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 20(5), 1063–1087. doi: 10.1037/0278-7393.20.5.1063
- Anderson, M. C., Bunce, J. G., & Barbas, H. (2016). Prefrontal–hippocampal pathways underlying inhibitory control over memory. Neurobiology of Learning and Memory, 134, 145–161. doi: 10.1016/j.nlm.2015.11.008
- Anderson, M. C., & Green, C. (2001). Suppressing unwanted memories by executive control. Nature, 410(6826), 366–369. doi: doi:10.1038/35066572
- Anderson, M. C., & Hanslmayr, S. (2014). Neural mechanisms of motivated forgetting. Trends in Cognitive Sciences, 18(6), 279–292. doi: 10.1016/j.tics.2014.03.002
- Anderson, M. C., & Huddleston, E. (2012). Towards a cognitive and neurobiological model of motivated forgetting. In R. F. Belli (Ed.), True and false recovered memories: Toward a reconciliation of the debate (pp. 53–120). New York, NY: Springer New York.
- Baran, B., Daniels, D., & Spencer, R. M. (2013). Sleep-dependent consolidation of value-based learning. PLoS ONE, 8(10), e75326. doi: 10.1371/journal.pone.0075326
- Baran, B., Pace-Schott, E. F., Ericson, C., & Spencer, R. M. C. (2012). Processing of emotional reactivity and emotional memory over sleep. The Journal of Neuroscience, 32(3), 1035–1042. doi: 10.1523/jneurosci.2532-11.2012
- Baran, B., Wilson, J., & Spencer, R. M. C. (2010). REM-dependent repair of competitive memory suppression. Experimental Brain Research, 203(2), 471–477. doi: 10.1007/s00221-010-2242-2
- Benoit, R. G., & Anderson, M. C. (2012). Opposing mechanisms support the voluntary forgetting of unwanted memories. Neuron, 76(2), 450–460. doi: 10.1016/j.neuron.2012.07.025
- Benoit, R. G., Hulbert, J. C., Huddleston, E., & Anderson, M. C. (2015). Adaptive top–down suppression of hippocampal activity and the purging of intrusive memories from consciousness. Journal of Cognitive Neuroscience, 27(1), 96–111. doi: 10.1162/jocn_a_00696
- Bergström, Z. M., de Fockert, J. W., & Richardson-Klavehn, A. (2009). ERP and behavioural evidence for direct suppression of unwanted memories. Neuroimage, 48(4), 726–737. doi: 10.1016/j.neuroimage.2009.06.051
- Bergström, Z. M., Velmans, M., de Fockert, J., & Richardson-Klavehn, A. (2007). ERP evidence for successful voluntary avoidance of conscious recollection. Brain Research, 1151, 119–133. doi: 10.1016/j.brainres.2007.03.014
- Blaskovich, B., Szőllősi, Á, Gombos, F., Racsmány, M., & Simor, P. (2017). The benefit of directed forgetting persists after a daytime nap: The role of spindles and Rapid Eye movement sleep in the consolidation of relevant memories. Sleep, 40(3), zsw076-zsw076. doi: 10.1093/sleep/zsw076
- Boland, E. M., Rao, H., Dinges, D. F., Smith, R. V., Goel, N., Detre, J. A., … Gehrman, P. R. (2017). Meta-analysis of the antidepressant effects of acute sleep deprivation. The Journal of Clinical Psychiatry, 78(8), e1020–e1034. doi: 10.4088/jcp.16r11332
- Brewin, C. R. (1998). Intrusive autobiographical memories in depression and post-traumatic stress disorder. Applied Cognitive Psychology, 12(4), 359–370. doi:10.1002/(SICI)1099-0720(199808)12:4<359::AID-ACP573>3.0.CO;2-5
- Bulevich, J. B., Roediger, H. L., Balota, D. A., & Butler, A. C. (2006). Failures to find suppression of episodic memories in the think/no-think paradigm. Memory & Cognition, 34(8), 1569–1577. doi: 10.3758/bf03195920
- Castiglione, A., Wagner, J., Anderson, M., & Aron, A. R. (2019). Preventing a thought from coming to mind elicits increased right frontal beta just as stopping action does. Cerebral Cortex, 29(5), 2160–2172. doi: 10.1093/cercor/bhz017
- Chen, C., Liu, C., Huang, R., Cheng, D., Wu, H., Xu, P., … Luo, Y.-J. (2012). Suppression of aversive memories associates with changes in early and late stages of neurocognitive processing. Neuropsychologia, 50(12), 2839–2848. doi: 10.1016/j.neuropsychologia.2012.08.004
- Compton, R. J. (2003). The interface between emotion and attention: A review of evidence from psychology and neuroscience. Behavioral and Cognitive Neuroscience Reviews, 2(2), 115–129. doi: 10.1177/1534582303002002003
- Crick, F., & Mitchison, G. (1983). The function of dream sleep. Nature, 304(5922), 111–114. doi: 10.1038/304111a0
- Depue, B. E., Banich, M. T., & Curran, T. (2006). Suppression of emotional and nonemotional content in memory: effects of repetition on cognitive control. Psychological Science, 17(5), 441–447. doi: 10.1111/j.1467-9280.2006.01725.x
- Depue, B., Curran, T., & Banich, M. (2007). Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science, 317(5835), 215–219. doi: 10.1126/science.1139560
- Depue, B., Orr, J., Smolker, H., Naaz, F., & Banich, M. (2016). The organization of right prefrontal networks reveals common mechanisms of inhibitory regulation across cognitive, emotional, and motor processes. Cerebral Cortex, 26, 1634–1646. doi: 10.1093/cercor/bhu324
- Diekelmann, S., Wilhelm, I., & Born, J. (2009). The whats and whens of sleep-dependent memory consolidation. Sleep Medicine Reviews, 13(5), 309–321. doi: 10.1016/j.smrv.2008.08.002
- Drosopoulos, S., Schulze, C., Fischer, S., & Born, J. (2007). Sleep's function in the spontaneous recovery and consolidation of memories. Journal of Experimental Psychology: General, 136(2), 169–183. doi: 10.1037/0096-3445.136.2.169
- Engen, H. G., & Anderson, M. C. (2018). Memory control: A fundamental mechanism of emotion regulation. Trends in Cognitive Sciences, doi: 10.1016/j.tics.2018.07.015
- Feld, G. B., & Born, J. (2017). Sculpting memory during sleep: Concurrent consolidation and forgetting. Current Opinion in Neurobiology, 44, 20–27. doi: 10.1016/j.conb.2017.02.012
- Fischer, S., & Born, J. (2009). Anticipated reward enhances offline learning during sleep. Journal of Experimental Psychology: Learning, Memory, and Cognition, 35(6), 1586–1593. doi: 10.1037/a0017256
- Fischer, S., Diekelmann, S., & Born, J. A. N. (2011). Sleep’s role in the processing of unwanted memories. Journal of Sleep Research, 20(2), 267–274. doi: 10.1111/j.1365-2869.2010.00881.x
- Gagnepain, P., Hulbert, J., & Anderson, M. C. (2017). Parallel regulation of memory and emotion supports the suppression of intrusive memories. The Journal of Neuroscience, doi: 10.1523/jneurosci.2732-16.2017
- Germain, A. (2013). Sleep disturbances as the Hallmark of PTSD: Where are we now? American Journal of Psychiatry, 170(4), 372–382. doi: 10.1176/appi.ajp.2012.12040432
- Groch, S., Wilhelm, I., Diekelmann, S., & Born, J. (2013). The role of REM sleep in the processing of emotional memories: Evidence from behavior and event-related potentials. Neurobiology of Learning and Memory, 99, 1–9. doi: 10.1016/j.nlm.2012.10.006
- Groch, S., Zinke, K., Wilhelm, I., & Born, J. (2015). Dissociating the contributions of slow-wave sleep and rapid eye movement sleep to emotional item and source memory. Neurobiology of Learning and Memory, 122, 122–130. doi: 10.1016/j.nlm.2014.08.013
- Guo, Y., Schmitz, T. W., Mur, M., Ferreira, C. S., & Anderson, M. C. (2018). A supramodal role of the basal ganglia in memory and motor inhibition: Meta-analytic evidence. Neuropsychologia, 108, 117–134. doi: 10.1016/j.neuropsychologia.2017.11.033
- Hellerstedt, R., Johansson, M., & Anderson, M. C. (2016). Tracking the intrusion of unwanted memories into awareness with event-related potentials. Neuropsychologia, 89, 510–523. doi: 10.1016/j.neuropsychologia.2016.07.008
- Hennevin, E., Hars, B., Maho, C., & Bloch, V. (1995). Processing of learned information in paradoxical sleep: Relevance for memory. Behavioural Brain Research, 69(1–2), 125–135. doi: 10.1016/0166-4328(95)00013-J
- Hoedlmoser, K., Birklbauer, J., Schabus, M., Eibenberger, P., Rigler, S., & Mueller, E. (2015). The impact of diurnal sleep on the consolidation of a complex gross motor adaptation task. Journal of Sleep Research, 24(1), 100–109. doi: 10.1111/jsr.12207
- Hotta, C., & Kawaguchi, J. (2009). Self-initiated use of thought substitution can lead to long term forgetting. Psychologia, 52(1), 41–49. doi: 10.2117/psysoc.2009.41
- Hupbach, A. (2018). Long-term effects of directed forgetting. Memory (Hove, England), 26(3), 321–329. doi: 10.1080/09658211.2017.1358748
- Iber, C., Ancoli-Israel, S., Chesson, A., & Quan, S. (2007). The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications (1st ed). Winchester, IL: American Academy of Sleep Medicine.
- Joormann, J., Hertel, P. T., Brozovich, F., & Gotlib, I. H. (2005). Remembering the good, forgetting the bad: Intentional forgetting of emotional material in depression. Journal of Abnormal Psychology, 114(4), 640–648. doi: 10.1037/0021-843X.114.4.640
- Kuriyama, K., Honma, M., Yoshiike, T., & Kim, Y. (2013). Memory suppression trades prolonged fear and sleep-dependent fear plasticity for the avoidance of current fear. Scientific Reports, 3, doi: 10.1038/srep02227
- LaBar, K. S., & Cabeza, R. (2006). Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience, 7, 54. doi: 10.1038/nrn1825
- Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual technical report A-8. Gainesville, FL: University of Florida, The Center for Research in Psychophysiology.
- Langille, J. J. (2019). Remembering to forget: A dual role for sleep oscillations in memory consolidation and forgetting. Frontiers in Cellular Neuroscience, 13, 71–71. doi: 10.3389/fncel.2019.00071
- Lehmann, M., Seifritz, E., & Rasch, B. (2016). Sleep benefits emotional and neutral associative memories equally. Somnologie, 20(1), 47–53. doi: 10.1007/s11818-015-0034-4
- Levy, B. J., & Anderson, M. C. (2012). Purging of memories from conscious awareness tracked in the human brain. The Journal of Neuroscience, 32(47), 16785–16794. doi: 10.1523/JNEUROSCI.2640-12.2012
- Maquet, P., Péters, J.-M., Aerts, J., Delfiore, G., Degueldre, C., Luxen, A., & Franck, G. (1996). Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature, 383(6596), 163–166. doi: 10.1038/383163a0
- Marzi, T., Regina, A., & Righi, S. (2014). Emotions shape memory suppression in trait anxiety. Frontiers in Psychology, 4, 1001. doi: 10.3389/fpsyg.2013.01001
- McDevitt, E. A., Duggan, K. A., & Mednick, S. C. (2015). REM sleep rescues learning from interference. Neurobiology of Learning and Memory, 122, 51–62. doi: 10.1016/j.nlm.2014.11.015
- Meier, B., König, A., Parak, S., & Henke, K. (2011). Suppressed, but not forgotten. Swiss Journal of Psychology, 70, 5–11. doi: 10.1024/1421-0185/a000033
- Morgenthaler, J., Wiesner, C. D., Hinze, K., Abels, L. C., Prehn-Kristensen, A., & Göder, R. (2014). Selective REM-sleep deprivation does not diminish emotional memory consolidation in young healthy subjects. PLoS ONE, 9(2), e89849. doi: 10.1371/journal.pone.0089849
- Murray, B. D., Anderson, M. C., & Kensinger, E. A. (2015). Older adults can suppress unwanted memories when given an appropriate strategy. Psychology and Aging, 30(1), 9–25. doi: 10.1037/a0038611
- Murray, B. D., Muscatell, K. A., & Kensinger, E. A. (2011). Effects of emotion and age on performance during a think/no-think memory task. Psychology And Aging, 26(4), 940–955. doi: 10.1037/a0023214
- Nishida, M., Pearsall, J., Buckner, R. L., & Walker, M. P. (2009). REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cerebral Cortex, 19(5), 1158–1166. doi: 10.1093/cercor/bhn155
- Nørby, S., Lange, M., & Larsen, A. (2010). Forgetting to forget: On the duration of voluntary suppression of neutral and emotional memories. Acta Psychologica, 133(1), 73–80. doi: 10.1016/j.actpsy.2009.10.002
- Noreen, S., & MacLeod, M. D. (2013). It's all in the detail: Intentional forgetting of autobiographical memories using the autobiographical think/no-think task. Journal of Experimental Psychology: Learning, Memory, and Cognition, 39(2), 375–393. doi: 10.1037/a0028888
- Noreen, S., & MacLeod, M. D. (2014). To think or not to think, that is the question: Individual differences in suppression and rebound effects in autobiographical memory. Acta Psychologica, 145, 84–97. doi: 10.1016/j.actpsy.2013.10.011
- Norman, K. A., Newman, E. L., & Perotte, A. J. (2005). Methods for reducing interference in the Complementary learning Systems model: Oscillating inhibition and autonomous memory rehearsal. Neural Networks, 18(9), 1212–1228. doi: 10.1016/j.neunet.2005.08.010
- Oudiette, D., Antony, J. W., Creery, J. D., & Paller, K. A. (2013). The role of memory reactivation during wakefulness and sleep in determining which memories endure. The Journal of Neuroscience, 33(15), 6672–6678. doi: 10.1523/JNEUROSCI.5497-12.2013
- Palagini, L., Baglioni, C., Ciapparelli, A., Gemignani, A., & Riemann, D. (2013). REM sleep dysregulation in depression: State of the art. Sleep Medicine Reviews, 17(5), 377–390. doi: 10.1016/j.smrv.2012.11.001
- Payne, J., Chambers, A., & Kensinger, E. (2011). Sleep promotes lasting changes in selective memory for emotional scenes. Frontiers in Integrative Neuroscience, 6, 108–108. doi: 10.3389/fnint.2012.00108
- Payne, J. D., Kensinger, E. A., Wamsley, E. J., Spreng, R. N., Alger, S. E., Gibler, K., … Stickgold, R. (2015). Napping and the selective consolidation of negative aspects of scenes. Emotion, 15(2), 176–186. doi: 10.1037/a0038683
- Payne, J. D., Stickgold, R., Swanberg, K., & Kensinger, E. A. (2008). Sleep Preferentially Enhances memory for emotional Components of Scenes. Psychological Science, 19(8), 781–788. doi: 10.1111/j.1467-9280.2008.02157.x
- Poe, G. R. (2017). Sleep Is for forgetting. The Journal of Neuroscience, 37(3), 464–473. doi: 10.1523/jneurosci.0820-16.2017
- Racsmány, M., Conway, M. A., & Demeter, G. (2010). Consolidation of episodic memories during sleep long-term effects of retrieval practice. Psychological Science, 21(1), 80–85. doi: 10.1177/0956797609354074
- Rasch, B., & Born, J. (2013). About sleep's role in memory. Physiological Reviews, 93(2), 681–766. doi: 10.1152/physrev.00032.2012
- Rauchs, G., Feyers, D., Landeau, B., Bastin, C., Luxen, A., Maquet, P., & Collette, F. (2011). Sleep contributes to the strengthening of some memories over others, depending on hippocampal activity at learning. The Journal of Neuroscience, 31(7), 2563–2568. doi: 10.1523/JNEUROSCI.3972-10.2011
- Sakaki, M., Kuhbandner, C., Mather, M., & Pekrun, R. (2014). Memory suppression can help people “unlearn” behavioral responses—but only for nonemotional memories. Psychonomic Bulletin & Review, 21(1), 136–141. doi: 10.3758/s13423-013-0480-6
- Saletin, J. M., Goldstein, A. N., & Walker, M. P. (2011). The role of sleep in directed forgetting and remembering of human memories. Cerebral Cortex, 21(11), 2534–2541. doi: 10.1093/cercor/bhr034
- Schönauer, M., Pawlizki, A., Köck, C., & Gais, S. (2014). Exploring the effect of sleep and reduced interference on different forms of declarative memory. Sleep, 37(12), 1995–2007. doi: 10.5665/sleep.4258
- Spielberger, C. D. (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press.
- Storm, B. C., & Levy, B. J. (2012). A progress report on the inhibitory account of retrieval-induced forgetting. Memory & Cognition, doi: 10.3758/s13421-012-0211-7
- Tomlinson, T. D., Huber, D. E., Rieth, C. A., & Davelaar, E. J. (2009). An interference account of cue-independent forgetting in the no-think paradigm. Proceedings of the National Academy of Sciences, 106(37), 15588–15593. doi: 10.1073/pnas.0813370106
- Tononi, G., & Cirelli, C. (2014). Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and Integration. Neuron, 81(1), 12–34. doi: 10.1016/j.neuron.2013.12.025
- Tucker, M. A., Tang, S. X., Uzoh, A., Morgan, A., & Stickgold, R. (2011). To sleep, to strive, or both: How best to optimize memory. PLoS ONE, 6(7), e21737. doi: 10.1371/journal.pone.0021737
- van Dongen, E. V., Thielen, J.-W., Takashima, A., Barth, M., & Fernández, G. (2012). Sleep supports selective retention of associative memories based on relevance for future utilization. PLoS ONE, 7(8), e43426. doi: 10.1371/journal.pone.0043426
- van Schie, K., & Anderson, M. C. (2017). Successfully controlling intrusive memories is harder when control must be sustained. Memory (Hove, England), 25(9), 1201–1216. doi: 10.1080/09658211.2017.1282518
- van Schie, K., Geraerts, E., & Anderson, M. C. (2013). Emotional and non-emotional memories are suppressible under direct suppression instructions. Cognition & Emotion, 27(6), 1122–1131. doi: 10.1080/02699931.2013.765387
- Wagner, U., Gais, S., & Born, J. (2001). Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learning & Memory, 8(2), 112–119. doi: 10.1101/lm.36801
- Waldhauser, G. T., Johansson, M., Bäckström, M., & Mecklinger, A. (2011). Trait anxiety, working memory capacity, and the effectiveness of memory suppression. Scandinavian Journal of Psychology, 52(1), 21–27. doi: 10.1111/j.1467-9450.2010.00845.x
- Wamsley, E. J., Hamilton, K., Graveline, Y., Manceor, S., & Parr, E. (2016). Test expectation enhances memory consolidation across both sleep and wake. PLoS ONE, 11(10), e0165141. doi: 10.1371/journal.pone.0165141
- Wiesner, C. D., Pulst, J., Krause, F., Elsner, M., Baving, L., Pedersen, A., … Göder, R. (2015). The effect of selective REM-sleep deprivation on the consolidation and affective evaluation of emotional memories. Neurobiology of Learning and Memory, 122, 131–141. doi: 10.1016/j.nlm.2015.02.008
- Wilhelm, I., Diekelmann, S., Molzow, I., Ayoub, A., Mölle, M., & Born, J. (2011). Sleep selectively enhances memory expected to be of future relevance. The Journal of Neuroscience, 31(5), 1563–1569. doi: 10.1523/jneurosci.3575-10.2011
