ABSTRACT
Choice blindness (failure to notice when our choices are switched unexpectedly) suggests people are often unaware of reasons underlying their intentions/preferences. Some argue, however, that research revealing choice blindness simply reflects social-demand characteristics in participant-experimenter interactions. To address this, we compared autistic adults (a population less susceptible to social demands), to non-autistic adults on a computer-based choice blindness task. Sixteen autistic and 21 non-autistic adults chose between faces, based on preference, and justified their choices. On one fifth of trials, participants were presented with the face they did not choose (manipulation). Finally, previously presented face pairs were re-presented to assess choice stability. Choice blindness was seen for both groups, at equivalent rates. Autistic participants showed less stability of their choices compared to non-autistic participants. Our findings suggest that social-demand characteristics do not influence choice blindness, and that—in this situation—introspective ability does not differ between autistic and non-autistic participants.
KEYWORDS:
1. Introduction
In our everyday lives we make numerous choices, and are often required to offer explanations for them. Research has shown, however, that we are surprisingly susceptible to manipulation of these choices: failing to notice when a choice is switched (using, for example, sleight of hand) and proceeding to justify the new choice as if it was our original preference (Johansson et al., Citation2005). For example, participants were shown a series of female face photograph pairs and asked to choose which of each pair they found most attractive. Participants were then re-presented with their “chosen” photograph and asked to verbally justify the reason(s) for their choice. On 20% of trials a manipulation was introduced: participants were re-presented with the photograph that they did not choose using a sleight of hand double-card trick. In the majority of cases (74% of trials) participants failed to notice they had been given their non-preferred face. Further, participants confabulated by justifying choices they did not actually make; giving reasons that could only be applied to the non-chosen face, for example, saying “I preferred this option because I prefer blondes”, when they originally selected a brunette.
This phenomenon has been termed choice blindness, is considered a form of introspection illusion and has been demonstrated across a variety of situations, including moral statements (Hall et al., Citation2012), political attitudes (Hall et al., Citation2013; Strandberg et al., Citation2018), the tastes of jams and tea (Hall et al., Citation2010), preferences for risk (Kusev et al., Citation2022), eyewitness identifications (Sagana et al., Citation2013) and new classroom furniture in children and adolescents (Sauerland et al., Citation2014).
In addition, the choice blindness methodology has been used to study how preferences are influenced by the process of choice, a phenomenon known as choice-induced preference change (Brehm, Citation1956). Replicating the original choice blindness study but also adding a second round of choices for the same face pairs, Johansson et al. (Citation2014) showed that acceptance of the manipulation led to an increased selection of initially rejected faces the second time. This version of the choice-induced preference change effect has been replicated in a number of studies (e.g. Izuma & Murayama, Citation2013; Luo & Yu, Citation2017; Taya et al., Citation2014), and the effect has been found to last up to a week for political attitudes (Strandberg et al., Citation2018). This robust demonstration of choice blindness, along with accompanying confabulatory responses, has been used as empirical support for Nisbett and Wilson’s (Citation1977) claim for a lack of introspective ability in our own choices (Johansson et al., Citation2006).
Critics, however, have raised issues with this interpretation of choice blindness research. Moore and Haggard (Citation2006) argue instead that choice blindness emerges due to social demand characteristics in the participant-experimenter interaction, preventing participants from reporting when they detect false feedback. This is in line with research claiming confabulations are a response to social pressures to please others (Hirstein, Citation2004). Indeed, higher rates of confabulation seen by children and adolescents (e.g. in eye-witness experiments, Ackil & Zaragoza, Citation1998) have been suggested to reflect a social demand characteristic where children tell the experimenter what they want to hear, rather than being due to false memories (Bjorklund et al., Citation2000). As such, investigating choice blindness and confabulatory responses in populations with differing responses to social demands can help elucidate whether participants do indeed fail to detect manipulations within choice blindness paradigms.
The present study took this approach, comparing neurotypical adults to a population characterised by altered social interactions, and reduced social conformity: those on the autistic spectrum (Yafai et al., Citation2014).
Autistic peopleFootnote1 experience differences in communication and social interaction compared to non-autistic people, combined with restrictive and repetitive patterns of behaviours and interests, and altered sensory sensitivities (American Psychiatric Association, Citation2013). Recent research showed that 1.76% of children in the UK were autistic (Roman-Urrestarazu et al., Citation2021), although the true prevalence is likely to be higher due to undiagnosed/undisclosed individuals. Research has suggested that autistic people may be less susceptible to social pressures than non-autistic people. Of most relevance to the current study, autistic children have been shown to be less susceptible to the influence of incorrect information provided by another individual (Large et al., Citation2019). Autistic and non-autistic children were asked to report on the direction of motion in an ambiguous visual illusion. Non-autistic children over the age of 12 were influenced by (incorrect) advice they received from an “advisor”, even when this directly contradicted what they could see in front of them. This was not the case for the autistic children, who were more resistance to social influence. Similarly, adults with a diagnosis of Asperger Syndrome (now considered part of the autistic spectrum, rather than a distinct condition, Happe, Citation2011) were shown to conform less with others’ opinions (Bowler & Worley, Citation1994) and higher levels of autistic traits—even within a non-autistic group of children—have been shown to correlate with reduced conformity (Yafai et al., Citation2014).
Here, we investigate whether autistic participants report more manipulation detections on choice blindness experiments (because they have reduced social constraints in reporting them) and subsequently make fewer confabulations compared to non-autistic participants. This pattern of results would suggest that social demands are involved in the choice blindness phenomenon. Conversely, if the autistic participants demonstrate equivalent (or even greater) choice blindness, this would be indicative of introspective underpinnings to the phenomenon. Indeed, while no consensus exists, systematic reviews on the topic suggest a likely reduction in introspective awareness for this group (DuBois et al., Citation2016).
A related secondary aim is to use the choice blindness paradigm as a tool to study choice-induced preference change in autistic participants. This is a well-documented and much discussed effect in cognitive psychology (Bem, Citation1972; Brehm, Citation1956; Chen & Risen, Citation2010; Egan et al., Citation2010; Festinger, Citation1962), but has, to the best of our knowledge, never been studied in autistic participants. This will be measured by introducing a second round of choices in the standard choice blindness task, allowing us to record preference consistency (the extent to which participants choose the same face when re-presented with a previously seen pair of images) in both manipulated and non-manipulated trials. Autistic participants have previously been shown to be more consistent in some decision tasks compared to neurotypical adults, and are less impacted by framing effects and sunk costs when making choices (De Martino et al., Citation2008; Farmer et al., Citation2017; Fujino et al., Citation2020). This is perhaps linked to a reduced impact of prior experience that allows autistic people to perceive the world more accurately than non-autistic people (Pellicano & Burr, Citation2012). This suggests that preference-change on a choice blindness task might be diminished for autistic people. We therefore predict that choices will remain more consistent for autistic participants.
2. Methods
2.1. Participants
Adult participants were recruited via the organisers of social groups for young autistic adults, teachers in higher education institutions and notice boards in those institutions.
A total of 50 individuals took part: 18 autistic adults (aged 17–42 years, mean age = 28.3, standard deviation (SD) = 5.9, 14 male, 4 female) and 32 non-autistic adults (aged 17–30 years, mean age = 22.1, SD = 3.9, 21 male, 11 female). While the challenges of recruiting clinical participants limited the sample size of the autistic group, we included a larger comparison group to increase the power of the study (see Oldfield, Citation2016).
All autistic participants had a formal diagnosis of autism. The autism diagnosis was then confirmed prior to taking part in the study by assessment with Module 4 of the Autism Diagnostic Observational Schedule (ADOS) (Lord et al., Citation2000) or when this was not possible due to participants’ time constraints, by completing the Social Communication Questionnaire (SCQ; Rutter et al., Citation2003) or Social Responsiveness Scale (Constantino & Gruber, Citation2012). All autistic participants scored above the threshold for a diagnosis of autism. Participants in both groups were assessed for nonverbal and verbal IQ using the Wechsler Abbreviated Scale for Intelligence, with all participants having IQ scores above 70 (Wechsler, Citation1999). This was to ensure any differences in performance were not due to a difference in cognitive abilities. Autistic and non-autistic participant groups were equivalent with respect to IQ and gender (both p > .368), but differed in age. The autistic group was slightly older (t = 4.44, df = 48, p < .001). See for demographic information.
Table 1. Demographic information for study participants.
2.2. Materials
A computer-based choice blindness task was presented using MATLAB (MathWorks Inc., Citation2014), on a DELL laptop (LATITUDE E5540) with a 15-inch LED screen (resolution of 1024 × 768 pixels). Stimuli were 25 pairs of coloured images of female faces (Ozturk, Citation2004). On each trial, a pair of female faces were presented on playing cards for three seconds against a white background (see ). The playing cards then flipped so the back of two playing cards (red and black), and not the faces, were visible. Participants used the laptop trackpad to click on the playing card that corresponded to the face they preferred. Next, a confidence line (from 1-not sure to 7-completely certain) was presented at the bottom of the screen and participants clicked to rate how confident they were with their choice. Finally, the chosen face was shown in the centre of the screen and participants were prompted to justify reasons for their choice via a set of 20 tick boxes (hair; ears; skin; shape; eyebrows; cheeks; friendly; attractive; chin; teeth; familiar; features; neck; eyes; symmetry; personality; nose; mouth; age; I did not intend to choose this face).
Figure 1. Example of an experimental trial: (A) Two female photographs are shown to participants for three seconds. (B) Faces disappear and the participant selects the back of the card corresponding to the face they prefer. (C) Participants click on a 7-point scale of confidence to indicate their certainty. (D) Participants are shown their chosen face and 20 tick boxes to indicate the reasons for their choice. On non-manipulated trials, participants are shown the face they previously selected. On the manipulated trials, participants are shown the alternative face to their choice. [To view this figure in color, please see the online version of this journal.]
![Figure 1. Example of an experimental trial: (A) Two female photographs are shown to participants for three seconds. (B) Faces disappear and the participant selects the back of the card corresponding to the face they prefer. (C) Participants click on a 7-point scale of confidence to indicate their certainty. (D) Participants are shown their chosen face and 20 tick boxes to indicate the reasons for their choice. On non-manipulated trials, participants are shown the face they previously selected. On the manipulated trials, participants are shown the alternative face to their choice. [To view this figure in color, please see the online version of this journal.]](/cms/asset/fa8b39d0-0c67-4bbc-bf5c-7dff66682392/pecp_a_2356283_f0001_oc.jpg)
Overall, 35 trials were created. Across the first 25 trials, 20% of the trials (trial numbers 6, 10, 15, 19 and 23), involved a manipulation: participants were presented with the face they did not choose when being asked to justify reasons for their choice. Detections of manipulations were identified when participants ticked the box labelled “I did not intend to choose this face”. The final 10 trials (trials 26–35) were repetitions of previous face pairs (five previously presented manipulated trials and five previously presented non-manipulated trials). These trials allowed the investigation of whether choices remain stable or change after manipulation.
2.3. Procedure
All procedures were approved by the UCL IOE Ethics Committee (REC 783) and participants gave written, informed consent before beginning the study. Participants completed the tasks at UCL, and began with the IQ measures and—for the autistic participants—an autism diagnostic test/questionnaire (ADOS, SRS or SCQ). When embarking on the experimental task, participants were told that they would be presented with pairs of female faces, and were asked to decide which one they preferred. The researcher went through the first trial with the participant to ensure they understood the task. Participants were told they could tick as many or as few of the boxes as they wanted in order to justify their choice. The “I did not intend to choose this face” tick box was highlighted to participants in case they mistakenly chose the wrong face. After the experiment, participants were given a full debrief about the manipulation, and the purpose of the experiment.
2.4. Statistical analysis
Data are presented descriptively, below, and were analysed using frequentist statistics (Mann–Whitney U/Analysis of Variance) to determine whether there is a group difference (autistic vs. non-autistic) or an impact of trial type (manipulated vs. non-manipulated trials) on the various phenomena. Age was included as a covariate, to account for the baseline difference between the two groups on this factor. Given the small sample size, Bayesian analyses were also computed. Differences in the findings from these methods are outlined below (and see Supplementary Materials for full details of the Bayesian Analyses).
3. Results
3.1. Detection of manipulation
If a participant selected the option “I did not intend to choose this face” on the justification screen, the trial was coded as a “detected manipulation”. On average, individual participants did not detect more than two manipulations across the experiment (see ) and there was no significant difference between the groups (U = 300, p = .797) in the number of manipulations detected. The Bayesian non-parametric t-test revealed no difference between autistic and non-autistic group (U = 276.000, Rhat = 1.001).
Table 2. Average number of manipulations detected by each group.
3.2. Confidence ratings
The mean confidence level was calculated for each participant across each trial type (see ). A repeated measures ANCOVA indicated that there was no difference in confidence ratings between the groups (F (1,47) = .529, p = .471, η2 = .010) or between the manipulated and non-manipulated trials (F (1,47) = .336, p = .565, η2 = 2.897 × 10−4). The manipulation occurred after the confidence question was presented and therefore would not impact it, however the comparison allowed us to ensure that we had not inadvertently chosen to manipulate trials where the participants were less sure of their choices compared to the non-manipulation trials. There was no age effect (F (1,47) = 1.285, p = .263, η2 = .025), interaction between group and trial type (F (1,47) = .172, p = .680, η2 = 1.485 × 10−4) or between age and trial type (F (1,47) = .202, p = .655, η2 = 1.741 × 10−4). The Bayesian analysis showed the same trends.
Table 3. Confidence ratings for non-manipulated and manipulated trials by group.
3.3. Justification statements
As with the confidence levels, a repeated measures ANCOVA indicated that there was no difference in the average number of justification statements made by the two groups (F (1,47) = .0013, p = .911, η2 = 2.242 × 10−4) or between the number of statements made for manipulated and non-manipulated trials (F (1,47) = 1.417, p = .240, η2 = .002). There was also no interaction between group and trial type (F (1,47) = .451, p = .505, η2 = 5.021 × 10−4). See for mean number of statements made by each group for each trial type.
Table 4. Descriptive statistics displaying the mean number of justification statements made for non-manipulated and manipulated trials by autistic adults and non-autistic adults.
Finally, there was an age effect (F (1,47) = 5.770, p = .020, η2 = .103), which was positively correlated with both manipulated (r = .350, p < .05) and non-manipulated trials (r = .391, p < .01), but no interaction between age and trial type (F (1,47) = .853, p = .360, η2 = 9.511 × 10−4). The Bayesian analysis showed that the model including age had a slightly better fit to the null model (BFM = 4.060), with the age effect on confidence ratings being moderate (BF10 = 5.365) which is also explained by the moderate effect size reported in our ANCOVA model (η2 = .103). See supplementary materials for the full analysis.
3.4. Preference change
For the final 10 trials in the experiment, the participant’s preference was classified as “stable” (i.e. they chose the same face as they did when first presented with this face pair) or “changed” (i.e. they chose the alternative face, compared to when first presented with the face pair). The percentage of stable vs. changed choices for each group, and each trial type, are presented in and . A repeated measures ANCOVA indicated that there was a significant difference in the percentage preference change across the two groups (F (1,47) = 5.198, p = .027, η2 = .047). This reflected a higher level of change for the autistic participants. There was no main effect of trial type (F (1,47) = .722, p = .400, η2 = .008) or interaction between group and trial type (F (1,47) = .229, p = .635, η2 = .002). Similarly, no age effect (F (1,47) = .177, p = .676, η2 = .002) or interaction between age and trial type (F (1,47) = .329, p = .596, η2 = .004). Our Bayesian analysis revealed that the trial type + group model had the best fit (BFM = 2.896), but there was weak evidence supporting that trial type + group effect on preference change rates (BF10 = 2.282). Even individually, there was weak evidence for the effects of group (BF10 = 1.536) and trial type (BF10 = 1.371) which could also explain the small effect size of our ANCOVA (η2 = .047).
Figure 2. Mean number of trials where preference changed (out of five trials) for non-manipulated and manipulated trials by autistic adults and non-autistic adults.
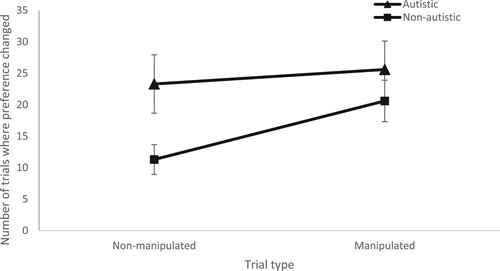
Table 5. Descriptive statistics displaying the percentage of trials where preference changed for non-manipulated and manipulated trials by autistic adults and non-autistic adults.
The Bayesian analyses could explain the trends seen in : non-autistic participants show greater preference change on manipulated trials compared to non-manipulated trials, whereby autistic participants show similar rates of preference change on manipulated and non-manipulated trial types, and appear to have less stable preferences overall.
4. Discussion
In the present study, we used a computer-based face preference task to examine the choice blindness phenomenon for a group of autistic and non-autistic adults. Choice blindness was evident for both groups, and to a similar extent. Likewise, there were no significant group differences in the confidence ratings, or number of justification statements chosen. This absence of group differences suggests that choice blindness is not solely a reflection of social demands. Instead, it is likely that choice blindness results from failures of introspection. Indeed, it has been reported that autistic people may have reduced metacognitive abilities with respect to decision making (see van der Plas et al., Citation2023 for a review). As such, we may have expected to see even greater choice blindness in the clinical group. The minimal number of switches that were detected by either group in the present study, however, suggests that a floor effect might be present that prevents any observation of further group differences. Interestingly, the present findings also highlight a dissociation between our tendency to miss changes in the external environment (change blindness) versus in one’s own choices (choice blindness). Autistic people have been widely shown to have resistance to change blindness: showing superior ability to detect—for example—changes in visual scenes or continuity errors in videos (Fletcher-Watson et al., Citation2012; Smith & Milne, Citation2009). Our findings suggest this does not confer an advantage on choice blindness paradigms.
The second aim of the present study was to examine choice-induced preference change for autistic individuals. As noted above, existing research indicates that autistic people show a more rational approach to decision making, with seemingly less influence of prior experience/context on the decision in hand (De Martino et al., Citation2008). We therefore predicted that the autistic participants in our study may show reduced choice-induced preference change compared to our non-autistic participants. Our hypothesis was, in part, confirmed: there was evidence of choice-induced preference change in our non-autistic group, but not in our autistic group. In contrast to our predictions, however, the lack of preference change was driven by high levels of preference inconsistency (i.e. choosing the face that was not previously chosen) for both manipulated and non-manipulated trials in the autistic group. While initially unexpected, this pattern of behaviour may echo findings from recent research that highlights an “extreme switching phenomenon”, whereby autistic people show a more extensive sampling strategy on lab-based experimental tasks such as the Iowa Gambling Task (Zeif et al., Citation2023). This, together with the apparent absence of choice-induced preference change, might offer insight into challenges that autistic people report regarding decision making. For example, it has been suggested that for autistic people (compared to non-autistic people) decision-making is associated with anxiety, exhaustion, and a tendency to avoid the process where possible (Luke et al., Citation2012; Vella et al., Citation2018). As noted above, and of particular relevance to the present study, differences in metacognition have also been noted: variation in how autistic and non-autistic people weight up the value of potential choices and subsequently evaluate their own decisions (van der Plas et al., 2022). These findings also map onto autobiographical accounts of decision making, written by autistic people, that highlight the negative impact of indecisiveness (Autism Guide, Citation2019).
4.1. Limitations
While our findings offer a preliminary insight into choice blindness and choice-induced preference change for autistic people, the limitations of our data should also be acknowledged. First, given the demands and verbal nature of the task, our study was only accessible for intellectually able participants, and only a small number of autistic people took part. This may limit the generalisability of the conclusions drawn. Similarly, we did not systematically record certain demographic factors (e.g. education level, employment status) so we are unable to assess whether the autistic and non-autistic groups differed on aspects—unrelated to neurotype—that might have impacted on task performance. Second, while scores on the diagnostic tests indicated that the autistic participants had different social communication behaviours compared to neurotypical norms, we did not directly assess whether the groups of participants in the present study differed on this factor. Relatedly, the use of face stimuli may have introduced a confounding factor into the results, given the known differences in face processing between autistic and non-autistic individuals (Singleton et al., Citation2014). It is important to note, however, that we saw no difference in the reported confidence levels, or number of justification statements made when choosing a preferred face, suggesting that both groups engaged with the task in a similar way. Future research could seek to replicate the findings with non-social stimuli, and to explore whether individual differences in choice blindness vary according to social communication behaviours.
5. Conclusions
In the current study, by considering the decision-making behaviour of autistic and non-autistic adults, we present evidence in support of choice blindness as a phenomenon of introspection, not social demands. Further, in the autistic group we highlight a lack of choice-induced preference change, and less stable preferences overall, when compared to the non-autistic group. This may have implications for our understanding of challenges surrounding decision-making that are experienced by autistic people.
Supplemental Material
Download MS Word (37.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data from this study are available from the authors on request. Participants of this study did not agree for their data to be shared publicly.
Notes
1 In the present paper, we use the identity-first language broadly preferred by the autistic community and autistic advocates. We acknowledge, however, that there is no single way to describe autism and individuals may elect other ways of doing so Botha et al. (Citation2023); Kenny et al. (Citation2016).
References
- Ackil, J. K., & Zaragoza, M. S. (1998). Memorial consequences of forced confabulation: Age differences in susceptibility to false memories. Developmental Psychology, 34(6), 1358–1372. https://doi.org/10.1037/0012-1649.34.6.1358
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5.
- Bem, D. J. (1972). Advances in experimental social psychology. Self-perception Theory, 6, 1–62.
- Bjorklund, D., Cassel, W., Bjorklund, B., Brown, R., Park, C., Ernst, K., & Owen, F. (2000, September 1). Social demand characteristics in children's and adults’ eyewitness memory and suggestibility: The effect of different interviewers on free recall and recognition. Applied Cognitive Psychology, 14(5), 421–433. https://doi.org/10.1002/1099-0720(200009)14:5<421::AID-ACP659>3.0.CO;2-4
- Botha, M., Hanlon, J., & Williams, G. L. (2023, February 1). Does language matter? Identity-first versus person-first language use in autism research: A response to Vivanti. Journal of Autism and Developmental Disorders, 53(2), 870–878. https://doi.org/10.1007/s10803-020-04858-w
- Bowler, D. M., & Worley, K. (1994). Susceptibility to social influence in adults with Asperger's syndrome: A research note. Journal of Child Psychology and Psychiatry, 35(4), 689–697. PM:8040221 (NOT IN FILE).
- Brehm, J. W. (1956, May). Postdecision changes in the desirability of alternatives. The Journal of Abnormal and Social Psychology, 52(3), 384–389. https://doi.org/10.1037/h0041006
- Chen, M. K., & Risen, J. L. (2010, October). How choice affects and reflects preferences: Revisiting the free-choice paradigm. Journal of Personality and Social Psychology, 99(4), 573–594. https://doi.org/10.1037/a0020217
- Constantino, J. N., & Gruber, C. P. (2012). Social responsiveness scale: SRS-2. Western psychological services.
- De Martino, B., Harrison, N. A., Knafo, S., Bird, G., & Dolan, R. J. (2008). Explaining enhanced logical consistency during decision making in autism. The Journal of Neuroscience, 28(42), 10746–10750. https://doi.org/10.1523/JNEUROSCI.2895-08.2008
- DuBois, D., Ameis, S. H., Lai, M. C., Casanova, M. F., & Desarkar, P. (2016, August). Interoception in autism spectrum disorder: A review. International Journal of Developmental Neuroscience, 52(1), 104–111. https://doi.org/10.1016/j.ijdevneu.2016.05.001
- Egan, L. C., Bloom, P., & Santos, L. R. (2010, January 1). Choice-induced preferences in the absence of choice: Evidence from a blind two choice paradigm with young children and capuchin monkeys. Journal of Experimental Social Psychology, 46(1), 204–207. https://doi.org/10.1016/j.jesp.2009.08.014
- Farmer, G. D., Baron-Cohen, S., & Skylark, W. J. (2017). People with autism spectrum conditions make more consistent decisions. Psychological Science, 28(8), 1067–1076. https://doi.org/10.1177/0956797617694867
- Festinger, L. (1962). A theory of cognitive dissonance (Vol. 2). Stanford University Press.
- Fletcher-Watson, S., Leekam, S. R., Connolly, B., Collis, J. M., Findlay, J. M., McConachie, H., & Rodgers, J. (2012). Attenuation of change blindness in children with autism spectrum disorders. British Journal of Developmental Psychology, 30(Pt 3), 446–458. PM:22882373http://onlinelibrary.wiley.com/store/10.1111j.2044-835X.2011.02054.x/asset/j.2044-835X.2011.02054.x.pdf?v=1&t=hu42waon&s=9ecd5c951b9156b534f9c6e996a50a3653aecf4c (NOT IN FILE)
- Fujino, J., Tei, S., Itahashi, T., Aoki, Y. Y., Ohta, H., Kubota, M., Hashimoto, R.-i., Nakamura, M., Kato, N., & Takahashi, H. (2020). Impact of past experiences on decision-making in autism spectrum disorder. European Archives of Psychiatry and Clinical Neuroscience, 270(8), 1063–1071. https://doi.org/10.1007/s00406-019-01071-4
- Guide, A. (2019). Autism and the difficultly of making simple decisions. Retrieved June 1, 2023, from https://autismguide.co.uk/emotional-issues/autism-and-the-difficultly-of-making-simple-decisions-part-two/
- Hall, L., Johansson, P., & Strandberg, T. (2012). Lifting the veil of morality: Choice blindness and attitude reversals on a self-transforming survey. PLoS One, 7(9), e45457. https://doi.org/10.1371/journal.pone.0045457
- Hall, L., Johansson, P., Tärning, B., Sikström, S., & Deutgen, T. (2010, October). Magic at the marketplace: Choice blindness for the taste of jam and the smell of tea. Cognition, 117(1), 54–61. https://doi.org/10.1016/j.cognition.2010.06.010
- Hall, L., Strandberg, T., Pärnamets, P., Lind, A., Tärning, B., & Johansson, P. (2013). How the polls can be both spot on and dead wrong: Using choice blindness to shift political attitudes and voter intentions. PLoS One, 8(4), e60554. https://doi.org/10.1371/journal.pone.0060554
- Happe, F. (2011). Why fold Asperger syndrome into autism spectrum disorder in the DSM-5? Spectrum. https://www.spectrumnews.org/opinion/viewpoint/why-fold-asperger-syndrome-into-autism-spectrum-disorder-in-the-dsm-5/
- Hirstein, W. (2004). Brain fiction: Self-deception and the riddle of confabulation. The MIT Press. https://doi.org/10.7551/mitpress/1660.001.0001.
- Izuma, K., & Murayama, K. (2013). Choice-induced preference change in the free-choice paradigm: A critical methodological review. Frontiers in Psychology, 4, https://doi.org/10.3389/fpsyg.2013.00041
- Johansson, P., Hall, L., Sikström, S., & Olsson, A. (2005, October 7). Failure to detect mismatches between intention and outcome in a simple decision task. Science, 310(5745), 116–119. https://doi.org/10.1126/science.1111709
- Johansson, P., Hall, L., Sikström, S., Tärning, B., & Lind, A. (2006, December). How something can be said about telling more than we can know: On choice blindness and introspection. Consciousness and Cognition, 15(4), 673–692; discussion 693–679. https://doi.org/10.1016/j.concog.2006.09.004
- Johansson, P., Hall, L., Tärning, B., Sikström, S., & Chater, N. (2014). Choice blindness and preference change: You will like this paper better if you (believe you) chose to read it! Journal of Behavioral Decision Making, 27(3), 281–289. https://doi.org/10.1002/bdm.1807
- Kenny, L., Hattersley, C., Molins, B., Buckley, C., Povey, C., & Pellicano, E. (2016, May). Which terms should be used to describe autism? Perspectives from the UK autism community. Autism, 20(4), 442–462. https://doi.org/10.1177/1362361315588200
- Kusev, P., van Schaik, P., Teal, J., Martin, R., Hall, L., & Johansson, P. (2022). How false feedback influences decision-makers' risk preferences. Journal of Behavioral Decision Making, 35(5), e2278. https://doi.org/10.1002/bdm.2278
- Large, I., Pellicano, E., Mojzisch, A., & Krug, K. (2019, February 12). Developmental trajectory of social influence integration into perceptual decisions in children. Proceedings of the National Academy of Sciences, 116(7), 2713–2722. https://doi.org/10.1073/pnas.1808153116
- Lord, C., Risi, S., Lambrecht, L., Cook, E. H., Leventhal, B. L., Dilavore, P. C., Pickles, A., & Rutter, M. (2000). The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of autism and developmental disorders, 30(3), 205–223.
- Luke, L., Clare, I. C., Ring, H., Redley, M., & Watson, P. (2012). Decision-making difficulties experienced by adults with autism spectrum conditions. Autism, 16(6), 612–621. https://doi.org/10.1177/1362361311415876
- Luo, J., & Yu, R. (2017). The spreading of alternatives: Is it the perceived choice or actual choice that changes our preference? Journal of Behavioral Decision Making, 30(2), 484–491. https://doi.org/10.1002/bdm.1967
- MathWorks Inc (2014). MATLAB. In The MathWorks Inc. http://www.mathworks.com
- Moore, J. W., & Haggard, P. (2006). Commentary on ‘how something can be said about telling more than we can know: On choice blindness and introspective report’. Consciousness and Cognition, 15(4), 693–696. https://doi.org/10.1016/j.concog.2006.09.003
- Nisbett, R. E., & Wilson, T. D. (1977). Telling more than we can know: Verbal reports on mental processes. Psychological Review, 84(3), 231–259. https://doi.org/10.1037/0033-295X.84.3.231
- Oldfield, M. (2016). Unequal sample sizes and the use of larger control groups pertaining to power of a study. Dstl, 1(1).
- Ozturk, M. (2004). Faces of tomorrow. http://ultrafeel.tv/face-future/
- Pellicano, E., & Burr, D. (2012). When the world becomes ‘too real’: A Bayesian explanation of autistic perception. Trends in Cognitive Sciences, 16(10), 504–510. https://doi.org/10.1016/j.tics.2012.08.009
- Roman-Urrestarazu, A., van Kessel, R., Allison, C., Matthews, F. E., Brayne, C., & Baron-Cohen, S. (2021, June 1). Association of race/ethnicity and social disadvantage with autism prevalence in 7 million school children in England. JAMA Pediatrics, 175(6), e210054. https://doi.org/10.1001/jamapediatrics.2021.0054
- Rutter, M., Bailey, A., & Lord, C. (2003). The social communication questionnaire. Western Psychological Services.
- Sagana, A., Sauerland, M., & Merckelbach, H. (2013). Witnesses’ blindness for their own facial recognition decisions: A field study. Behavioral Sciences & the Law, 31(5), 624–636. https://doi.org/10.1002/bsl.2082
- Sauerland, M., Sagana, A., Otgaar, H., & Broers, N. J. (2014). Self-relevance does not moderate choice blindness in adolescents and children. PLoS One, 9(6), e98563. https://doi.org/10.1371/journal.pone.0098563
- Singleton, C., Ashwin, C., & Brosnan, M. (2014). Attention to non-social and social details in adults with high and low degrees of autistic traits: A change blindness study.
- Smith, H., & Milne, E. (2009). Reduced change blindness suggests enhanced attention to detail in individuals with autism. Journal of Child Psychology and Psychiatry, 50(3), 300–306. PM:19309329. http://onlinelibrary.wiley.com/store/10.1111j.1469-7610.2008.01957.x/asset/j.1469-7610.2008.01957.x.pdf?v=1&t=hu443y0f&s=183711bc96658e1d96e4659989f2ec9b7983b847 (NOT IN FILE).
- Strandberg, T., Sivén, D., Hall, L., Johansson, P., & Pärnamets, P. (2018, September). False beliefs and confabulation can lead to lasting changes in political attitudes. Journal of Experimental Psychology: General, 147(9), 1382–1399. https://doi.org/10.1037/xge0000489
- Taya, F., Gupta, S., Farber, I., & Mullette-Gillman, O. D. A. (2014). Manipulation detection and preference alterations in a choice blindness paradigm. PLoS One, 9(9), e108515. https://doi.org/10.1371/journal.pone.0108515
- van der Plas, E., Mason, D., & Happé, F. (2023). Decision-making in autism: A narrative review. Autism, 27(6), 1532–1546. https://doi.org/10.1177/13623613221148010
- Vella, L., Ring, H. A., Aitken, M. R., Watson, P. C., Presland, A., & Clare, I. C. (2018). Understanding self-reported difficulties in decision-making by people with autism spectrum disorders. Autism, 22(5), 549–559. https://doi.org/10.1177/1362361316687988
- Wechsler, D. (1999). Wechsler abbreviated scale for intelligence. Psychological Corporation.
- Yafai, A. F., Verrier, D., & Reidy, L. (2014, November). Social conformity and autism spectrum disorder: A child-friendly take on a classic study. Autism, 18(8), 1007–1013. https://doi.org/10.1177/1362361313508023
- Zeif, D., Yakobi, O., & Yechiam, E. (2023). Choice behavior in autistic adults: What drives the extreme switching phenomenon? PLoS One, 18(3), e0282296. https://doi.org/10.1371/journal.pone.0282296
