Abstract
In 1988, the generalised HIV/AIDS epidemic in Thailand began and in the same year the first HIV-exposed infant in Thailand was born at King Chulalongkorn Memorial Hospital, Bangkok. From the early to mid-1990s, an epidemic wave of HIV-infected women and infants occurred. Heterosexual HIV transmission, as described in the Asian Epidemic Model, was the major mode of spread in Thailand, causing an increasing number of HIV-infected pregnant women. The early and concerted multi-sectoral response of Thai society reduced the prevalence of HIV infection in pregnant women from 2% in the mid-1990s to 0.6% in 2015 and mother-to-child transmission of HIV (MTCT) from an estimated 20–40% to 1.9%. Thus, Thailand became the first Asian country to achieve the World Health Organization’s (WHO) targets for the elimination of MTCT. In this narrative review, the key historic evolutions of the science and policy of prevention of mother-to-child transmission of HIV (PMTCT) in Thailand that addressed the four prongs of the recommended WHO PMTCT strategy are described, and the lessons learned are discussed.
Abbreviations:
- ANC, antenatal care
- ART, anti-retroviral therapy
- AEM, Asian Epidemic Model
- CMR, child mortality rate
- CDC, communicable disease control
- EID, early infant diagnosis
- EPP, Estimation and Projection Package
- FSW, female commercial sex worker
- HSM, heterosexual men
- HAART, highly active anti-retroviral therapy
- IDU, intravenous drug users
- MOPH, Ministry of Public Health
- NGO, non-government organisation
- PACTG, Paediatric AIDS Clinical Trials Group
- PLWHA, people living with HIV/AIDS
- PHIMS, Perinatal HIV Intervention Monitoring System
- PHOMS, Perinatal HIV Outcome Monitoring System
- PCR, polymerase chain reaction
- PROM, premature rupture of membranes
- STI, sexually transmitted infection
- TRCS, Thai Red Cross Society
- TDR, triple-drug regimen
- WLWHA, women living with HIV/AIDS
Introduction
Acquired immune deficiency syndrome (AIDS) was initially recognised in 1981 in homosexual men in North America, thereafter becoming a global epidemic. The first AIDS case in Thailand was reported in a Thai man in September 1984. After that, the generalised human immunodeficiency virus (HIV) epidemic occurred with a chain of transmission causing a rapid increase of HIV-infected infants by 1991 [Citation1, 2]. The first documented case of a Thai infant born to an HIV-infected mother was at King Chulalongkorn Memorial Hospital, Bangkok in July 1988, and the first Thai infant with AIDS was reported in the same year at Ramathibodi Hospital, Bangkok. After that, the HIV seropositivity rate amongst pregnant women rose from 0.2% in 1990 to 2.06% in 1994 [Citation3, 4].
The chain of transmission to infants
A chain of transmission in Thailand eventually leading to mother-to-child transmission of HIV (MTCT) to an infant is demonstrated by the epidemiological surveys conducted on the HIV epidemic in Thailand from 1988 to 1997. These describe some of the sexual practices in Thai culture relating to MTCT.
In 1992, the first national survey of risk behaviour was conducted by the Programme on AIDS of the Thai Red Cross Society (TRCS) and Chulalongkorn University funded by the World Health Organization’s (WHO) global Programme on AIDS, subsequently used in advocacy with the Thai government. Of 2801 Thai men and women aged 15–49 selected by stratified random sampling nationwide, 28% of men reported having had either premarital or extramarital sex in the last year, and three-quarters of them had paid for sex in the past year; 40% of men aged 20–24 had paid for sex. Amongst single men, 47% reported having had sex in the last year compared with 4% of single women, while 17% of married men reported extramarital sex compared with less than 1% of women [Citation5]. These data and other studies [Citation2] suggested a high prevalence of male patronage of female commercial sex workers (FSW) in 1992. They also suggested a gender difference in sexual behaviour. Traditionally, Thai heterosexual men (HSM) may have premarital intercourse with multiple partners and extramarital intercourse with FSW because they are commonly believed to require sexual variety. However, Thai women are not expected to engage in premarital or extramarital intercourse [Citation6].
The gender difference in sexual demand and supply has caused a large commercial sex industry in Thailand. An annual census of commercial sex work by the Thai Department of Communicable Disease Control (CDC) reported 86,494 FSW working in 6160 establishments nationwide in 1990 although other estimates suggest around 500,000 [Citation2].
Non-commercial extramarital and premarital sex is thought to play a role in spreading HIV amongst Thai women. Some wealthier Thai men traditionally support one or more minor wives, and unmarried men may have casual non-commercial or premarital sex with a long-term partner [Citation7–9].
At the beginning of the Thai HIV epidemic, low rates of condom use may have promoted the rapid spread of infection in Thai women in the early 1990s. Only 30% of married men reported consistent condom use, while urban unmarried men were twice as likely to use condoms as those in rural areas (48 vs. 24%, respectively). Increased, consistent use of condoms was associated with men with secondary or higher education compared with men who without (47 vs. 23%, respectively) [Citation5].
Thai women living with HIV/AIDS (WLWHA) have suffered from negative perceptions and attitudes in Thai society because physical and moral appearances are important. One of the barriers to prevention of mother-to-child transmission of HIV (PMTCT) is the stigmatisation and discrimination associated with Thai WLWHA. Qualitative studies of Thai WLWHA using interviews report anticipated stigmatisation on disclosure of their serostatus to family and friends, employers and co-workers as well as society. Reasons given included fear of being thought promiscuous, unsympathetic treatment by family and friends, unfair dismissal from work or ostracisation from the local community, all of which have been reported. Thus, Thai WLWHA refuse to return to the hospital where they tested positive or to receive home visits by health care workers. They fear intentional or unintentional disclosure of their serostatus by health care workers. Thai WLWHA have often not disclosed their serostatus to sexual partners, family and friends, and some have even migrated away from their local communities. The stigma may have been caused by the mass media and public health promotion campaigns using scare tactics to alter behaviour in the 1980s. However, recent studies have indicated that reactions towards Thai WLWHA vary greatly socially and geographically and that perceptions and attitudes are becoming more positive [Citation10, 11].
Early responses in the prevention of mother-to-child transmission of HIV (1988–1999)
Early epidemiological surveys of perinatal HIV infection in Thailand
The HIV epidemic waves in FSW and HSM in the early 1990s was predicted to cause a further epidemic wave in women of reproductive age and consequently in children by the mid-1990s [Citation2]. Although biannual sentinel surveillance, including pregnant women in antenatal care (ANC) clinics, had been undertaken since 1989, more accurate epidemiological data on HIV in Thai women and their children was required to inform decision-making about PMTCT. Thus, a collaborative group of six Thai hospitals nationwide with extensive experience of HIV infection in children calculated the perinatal HIV transmission rate in Thailand between 1989 and 1994, estimating MTCT rates to be 25–42%, depending on the region [Citation12].
With seroprevalence rates rising from <1% in 1989 to a peak of >7% in 1995, upper northern Thailand, which includes the six provinces of Chiang Mai, Chiang Rai, Lampang, Phayao, Lamphun and Mae Hong Son, was considered to be the epicentre of the generalised Thai HIV epidemic during the early years from 1988 to 1992. Indeed, the highest perinatal transmission rate of 42% was in Chiangrai Hospital, a tertiary hospital in the provincial capital of Chiangrai, possibly reflecting an earlier start of the epidemic wave in Thai women who were not FSW and became immunocompromised in greater numbers before those in other regions [Citation12].
The reasons for the severity of the HIV epidemic in the early 1990s in upper northern Thailand are controversial because the data on intravenous drug users (IDU) in the region were limited. It has been suggested that the cause was an estimated large number of Thai IDUs in the region, some of whom may have transmitted HIV to FSWs. The high prevalence of IDUs may have been caused by the clearance of growing operations of opium for smoking in upper northern Thailand, leading to increased heroin imports from Myanmar and Laos to satisfy demand for opiates amongst Thai users [Citation13].
National public health promotion campaign and the 100% condom programme
Before 1990, the Thai general public thought that HIV/AIDS only affected certain populations such as men who have sex with men, male commercial sex workers, foreigners and IDUs. Because of this and the need to promote the tourist industry during the 1980s, government officials played down the potential for an HIV epidemic in Thailand [Citation14]. However, the first national sentinel surveillance in 1990 reported a dramatically increasing seroprevalence in some high-risk populations, prompting government action.
Under the advocacy and leadership of the minister for AIDS, Mechai Viravaidya, an intensive public health information promotion campaign was launched. During 1991–1992, national radio and television broadcast mandatory hourly 1-min HIV/AIDS education items. These addressed HIV/AIDS not only as a health issue but also a social one, emphasising prevention through behavioural change including condom use. Many government ministries provided educational initiatives sensitive to Thai cultural needs. Because communication about sexual behaviour between parents and adolescents is often limited, the Ministry of Education launched peer education programmes amongst students, causing education about sexual behaviour to be better received through friends. Private initiatives by non-government (NGO) and community-based organisations also played roles in educating and promoting behavioural change, for example, the Thai Business Coalition on AIDS that promotes education and prevention about HIV/AIDS in the workplace [Citation14, 15].
Low use of condoms was reported in the late 1980s and early 1990s [Citation16]. Thus, a national programme to promote condom use by commercial sex workers was established in 1991/92. After a successful innovative pilot project by Dr Rojanapithayakorn, the regional director of the Thai CDC in Ratchaburi Province, a national ‘100% Condom Programme’ was developed.
The 100% Condom Programme was supervised at the provincial government level, involving pragmatic multi-sectoral collaboration between local public health officials, sex establishment owners, local police and sex workers to promote 100% condom use by male patrons of commercial sex workers. Monitoring was undertaken by an extensive network of sexually transmitted infection (STI) clinics to identify HIV infection in male clients of sex establishments or FSW. This would be taken as evidence of violation by the sex establishment owner of the programme by their FSW. The owner would then receive a warning and ultimately could face permanent closure of the establishment [Citation2].
Poor quality and the relatively high cost of condoms were seen as an obstacle to the campaign’s success. To address this, the Thai Ministry of Public Health (MOPH) instructed distributors and vendors to store condoms appropriately in environments with low humidity and no exposure to sunlight. The MOPH provided around 60 million condoms which were distributed through STI clinics to FSW (typically a box of 100 condoms) and male clients as well as in workplaces, hotels and to NGOs [Citation17].
The 100% Condom Programme contributed to increased use of condoms by male clients of FSW (from 25% in 1989 to 94% in 1993), as well as decreased rates of premarital or extramarital sex in HSM (28–15%) and of visiting FSW from 22% in 1990 to 10% in 1993 [Citation18]. After the financial crisis of 1997/98, the distribution of free condoms was dramatically curtailed, and a social marketing policy was adopted instead. In 2008, the national Behavioral Surveillance Survey [Citation19] reported 50–76% consistent condom use by male students, military recruits and male factory workers when visiting FSW, while 20–40% of students had used a condom during the last sexual contact with their partner. The incidence of HIV infection amongst pregnant women visiting ANC clinics rose from 0.05% per year in 2005 to 0.18% per year in 2008 and was the greatest amongst females <20 years [Citation20].
Infant formula for HIV-infected mothers
Breast-feeding by HIV-infected mothers has been discouraged by the MOPH since 1993 because the risk of MTCT was estimated to be 20–40% [Citation21]. This was based on the WHO/UNICEF Consensus Statement on HIV Transmission and Breast-feeding in 1992 [Citation22] and a decision analysis model by Heymann [Citation23].
The WHO/UNICEF Consensus Statement on HIV Transmission and Breast-feeding stated that in settings where infectious diseases are not the primary cause of death during infancy, pregnant women known to be HIV-infected should be advised not to breastfeed but to use a safe alternative [Citation22].
The decision-analysis model looked at the risk of child mortality from breast-feeding by HIV-infected mothers related to the under-5 child mortality rate (CMR). An under-5 CMR of <60/1000 live births was estimated as appropriate for recommending bottle-feeding because the benefit of preventing transmission of HIV by breast-feeding would outweigh the risk of death from other causes, especially infectious diseases. The under-5 CMR in Thailand has been <60/1000 live births since 1981 [Citation23].
A safe and economically feasible supply of infant formula was also required. The government therefore provided a free infant formula for children of HIV-infected mothers. Thereafter, the MTCT rates without anti-retroviral therapy (ART) decreased from 31% in 1994 to 19% in 1997 [Citation24–27].
Free infant formula continues to be provided by the national PMTCT policy despite the introduction of free, point-of-care WHO option B + in 2014. The MOPH maintains this policy to achieve <1% perinatal transmission by 2030, and Thailand has an established programme to support infant formula replacement feeding. Perinatal transmission may still be possible in a breast-feeding mother taking the WHO option B+. A study amongst infants and mothers on lifelong ART estimated a rate of transmission at 18 months of 4.1% (95% CI 2.2–7.6) [Citation28]. The vast majority in Thailand also have access to safe drinking water, good sanitation and a guaranteed free supply of infant formula, and mothers and caregivers are usually competent to safely bottle-feed infants and have access to comprehensive child health services.
Thai Red Cross zidovudine donation programme
In 1994, the Paediatric AIDS Clinical Trials Group (PACTG) protocol 076 demonstrated that providing zidovudine to women during the second and third trimesters of pregnancy during labour and to newborns could reduce MTCT by 67% [Citation29]. This was confirmed by several subsequent studies, showing that infection rates in infants whose mothers received the PACTG 076 zidovudine regimen were 3.4–8.6% [Citation30].
The PACTG 076 zidovudine regimen, avoidance of breast-feeding and delivery by Caesarean section were adopted as the standard of care in most high-income countries, which thereafter reported significant reduction in paediatric cases of HIV infection and AIDS. However, because these strategies are expensive and complex, their implementation in most of low- and some middle-income countries is not possible.
The HIV epidemic in pregnant women in Thailand peaked at an HIV prevalence of 2.5% in 1995 [Citation31]. However, there was no national PMTCT programme at that time. Thus, under the patronage of Her Royal Highness Princess Soamsawali and with the support of the MOPH, the TRCS initiated a zidovudine donation campaign, ‘Save a Child’s Life from AIDS’ in February 1996. The key objectives were PMTCT by procuring zidovudine funded by donation for HIV-infected pregnant women and to test the feasibility and acceptability of zidovudine therapy. All interested hospitals nationwide with adequate supportive infrastructure could request free zidovudine from the TRCS. In 94 hospitals nationwide, over 7000 HIV-infected pregnant women have been provided with ART since 1996, and over 7700 HIV-free infants have been born [Citation32]. Donations are received from individuals, groups and organisations including the MOPH and UNICEF, through the TRCS’s annual fund-raising events, especially the nationwide Red Cross Red Ribbon Sale on 26 February each year, and through miscellaneous activities. This ‘community-to-community’ approach involves donations and activities by one section of the community to help another. Buddhist Thais believe that charitable acts ‘make merit’ that can bring oneself good fortune later. Furthermore, the donation campaign has stimulated public awareness, contributing to a better understanding of HIV/AIDS in Thailand. The programme was selected as one of the UNAIDS best practices in the year 2000 [Citation33].
The ART regimens for the ‘Save a Child’s Life from AIDS’ programme of the TRCS have also evolved with advances in prevention. At its inception in 1996, zidovudine was offered at any time from 14 to 34 weeks gestation until delivery along with peripartum 3 × 100 mg tablets zidovudine q3 h. A single dose of nevirapine for the mother during labour and the newborn within 72 h of birth was added in 2000 after the HIVNET 012 protocol demonstrated an almost 50% reduction in the risk of postpartum perinatal transmission of HIV during the first 14–16 weeks in breast-feeding women compared with zidovudine (transmission rates 9.4 and 18.9%, respectively) [Citation34]. In 2004, a triple-drug regimen (TDR) of zidovudine and lamivudine with one of nevirapine, efavirenz or lopinavir/ritonavir was introduced, achieving a transmission rate as low as 1.1% in 1832 pregnant women between 2004 and 2010 at a duration of TDR of 10.4 (7.3–13.4) weeks [Citation35]. Zidovudine for 6 weeks postpartum and free infant formula until 12 months of age have also been provided for all TRCS regimens.
National prevention of mother-to-child transmission of HIV policy (2000 to the present)
From 1997 to 1999, the MOPH implemented pilot PMTCT projects in Thailand to provide HIV testing for pregnant women and zidovudine for PMTCT together with a monitoring system [Citation36, 37]. In 2000, the MOPH announced the first national PMTCT policy and issued guidelines for all government hospitals to integrate PMTCT activities into routine maternal and child health services, including HIV counselling and voluntary testing of all pregnant women, ART for PMTCT and provision of infant formula for HIV-exposed infants. This programme covers all public and private health-care facilities. Since 2003, the government has funded PMTCT services for Thais under the universal health coverage policy. From 2007 to 2014, non-Thai HIV-infected pregnant women could access PMTCT services through a Global Fund project. These services can currently be accessed through hospital social welfare funds, the ‘Save a Child’s Life from AIDS’ project of the TRCS, government-sponsored migrant health insurance or other special projects.
ART regimens in the national PMTCT policy have changed as prevention has evolved. At inception in 2000, zidovudine starting at 34 weeks gestation was offered to HIV-positive pregnant women, and zidovudine for 4 weeks was given to newborns. In 2004, a single-dose of nevirapine (WHO option A) was added, and, since 2010, highly active anti-retroviral therapy (HAART) (WHO option B) has been offered during pregnancy, continuing post-partum based on CD4 count. Since 2014, HAART for life regardless of CD4 count (WHO option B+) has been offered to Thais universally. In the same year, modification of the national PMTCT guidelines classified infants on the basis of the risk of acquiring HIV. Those with a standard risk receive zidovudine for 4 weeks. Infants at high risk, namely maternal plasma HIV viral load >50 copies/mL or infants of mothers taking HAART for <4 weeks before delivery, are offered zidovudine, lamivudine and nevirapine for 6 weeks [Citation38]. Both standard and high-risk infants receive HIV DNA polymerase chain reaction (PCR) testing at 1, 2 and 4 months. All children born to HIV-positive mothers have confirmatory HIV antibody testing at 18 months [Citation39].
Other programme evolutions include HIV couples counselling and voluntary testing, infant HIV testing, a training curriculum for hospital personnel to reduce discrimination against people living with HIV/AIDS (PLWHA), monitoring systems which promote data use for improvement of the national programme and delivery service guidelines.
Since 2010, couples counselling and voluntary HIV testing have been offered. Couples HIV counselling should emphasise confidentiality, prevention of horizontal transmission, and psychosocial issues of serodiscordance and problem-solving. This is intended to promote mutual disclosure amongst couples to prevent transmission and stigmatisation. Pregnant women are offered voluntary HIV testing by rapid test and other screening tests including for syphilis, hepatitis B (HBsAg) and thalassaemia. In the case of a female pregnant partner carrier, men are offered screening for HIV, syphilis and thalassaemia. Pregnant women testing HIV-positive should be followed up with an immediate CD4 count and then every 6 months. ART is offered immediately, regardless of CD4 count. Further STI testing for gonorrhoea, chlamydia and other vaginal infections should be undertaken. The high-risk group of intravenous-drug-using, pregnant women should be screened for hepatitis C (anti-HCV). Tuberculosis screening is offered to those with a history of exposure or suspicious signs or symptoms, and they should also be checked for any signs or symptoms of opportunistic infections. Integration of the PMTCT service into family planning and reproductive health services has also been instrumental assuring confidentiality and retaining continuity of care.
Infant HIV-testing has been improved. From 2000 until 2006, antibody testing was undertaken at 12 and 18 months of age, while DNA PCR testing in research studies or other projects was performed in only some infants aged >2 months. Early infant diagnosis (EID) with HIV DNA PCR at 1, 2 and 4 months of age was introduced in 2014. Guidelines recommend that any infant testing positive by rapid test should receive a confirmatory PCR test as soon as possible and all HIV-exposed infants should also receive HIV antibody testing at 18 months of age regardless of PCR results. The cost and complexity of timely collection, transportation, testing biological samples and returning results to patients is challenging. However, Thailand has 15 nationwide laboratory networks for PCR testing. Under the universal health coverage policy, EID services are currently free for infants aged <12 months in all public and private hospitals (906 public and 45 private hospitals). Whole blood or dried blood spots can be tested by a standardised, low-cost in-house conventional PCR optimised for the predominant HIV strain in Thailand (CRF01_AE) [Citation40].
Training in HIV counselling and ART has improved. Prior to 2000, universities and research centres were responsible for HIV/AIDS health-care training. However, there was no integration of HIV counselling and ART knowledge into the regular curriculum of health-care programmes. Thus, 2-day courses on HIV medicine and programme implementation were held between 2000 and 2004, and approximately 8000 health-care professionals (physicians, nurses, counsellors, laboratory technicians and pharmacists) in 908 hospitals received training. University experts, public health programme managers at various levels, physicians, scientists, researchers, NGOs and PLWHA developed the curriculum with two components. The first described global and national policy, trends in ART, general HIV knowledge and HIV care including prevention and treatment of opportunistic infections, and ART management. The second focused on specific training in management of the drug supply chain for pharmacists, diagnostic and monitoring laboratory techniques for collecting and sending blood specimens and reagent supply chain management for laboratory technicians, and HIV and ARV counselling for adults and children and adherence issues for counsellors. Multi-sectoral collaboration and partnerships between the Departments of Health, Mental Health and Medical Services, the Thai AIDS Society, NGOs and PLWHA provided the training. Trained health-care professionals who had attended trainer training courses became consultants in their local areas [Citation41].
The monitoring of HIV/AIDS in Thailand has improved. Before the national PMTCT policy was introduced, the army and the Epidemiology Division of the MOPH began surveillance in high-risk groups in 1989. Initially, data on pregnant women were collected through ANC services in 14 provinces, expanding to all 73 provinces by 1990. In 2000, the MOPH introduced the Perinatal HIV Intervention Monitoring System (PHIMS) to monitor PMTCT services. PHIMS collects monthly reports from hospitals of HIV testing of pregnant women and their partners as well as ART coverage for PMTCT. PHIMS is integrated into routine hospital reporting activities. By 2015, PHIMS covered 92% of government hospitals (77% of total deliveries including Thais and non-Thais). To monitor perinatal HIV outcomes, the MOPH created the Perinatal HIV Outcome Monitoring System (PHOMS) in 2001. Initially, 64 public hospitals in four of the country’s 77 provinces submitted data on the number of infants born to HIV-positive mothers, the number of HIV-infected infants and the MTCT rate. This system expanded to 191 facilities in 14 provinces during 2004–2007. In 2008, the National AIDS Programme to monitor national HIV treatment and care services replaced PHOMS. Infant HIV DNA PCR test results reported by the National AIDS Programme are used to calculate MTCT rates. From 2001 to 2012, these rates included HIV-exposed infants who were not tested for HIV or whose test results were not reported. Adjusted MTCT rates from 2013 to 2015 have been calculated using SPECTRUM version 5.4 [Citation38].
Elective Caesarian section is recommended at 38 weeks of confirmed gestation for HIV-infected women in a setting that is safe and feasible with the following indications: viral load >1000 copies/mL at 36 weeks or previously unknown viral load, poor compliance with ART or late ANC with <4 weeks of ART, no ANC and no ART as well as according to general obstetric indications for Caesarian section. For vaginal deliveries, invasive procedures such as foetal scalp electrodes, forceps extraction, vacuum extraction and artificial membrane rupture are not recommended, unless indicated. This is because MTCT risk is increased by premature rupture of the membrane (PROM)>4 h prior to delivery. If PROM has occurred, labour should be induced to reduce delivery time. Episiotomy should be performed carefully at the correct time to reduce the risk of exposing the newborn to maternal blood and body fluid [Citation42].
From the early multi-sectoral response to the HIV epidemic to the evolution of an effective national PMTCT policy under a strong national health care service, Thailand has successfully eliminated MTCT. In 2015, 98.3% of pregnant women attended an ANC clinic at least once (WHO elimination target >95%). The percentage of pregnant women tested for HIV has increased from 61.9% in the pilot PMTCT projects in 1998 to 99.6% under the national PMTCT policy in 2015 (WHO elimination target >95%). HIV prevalence in pregnant women has decreased from 2% in the mid-1990s to 0.6% in 2015, and MTCT has fallen from 20–40% to 1.9% (WHO elimination target <2% in non-breast-feeding populations). The use of ART for PMTCT has increased from 64.6% in 1998 to 95.6% in 2015 (WHO elimination target >90%) [Citation38].
Lessons learned
The successful elimination of MTCT was made possible by an effective monitoring system. This enabled modelling of the HIV epidemic in Thailand which was instrumental in bringing about a massive, well-focused multi-sectoral response across Thai society with the support of various foreign NGOs. The four prongs of WHO’s recommended PMTCT strategy have been successfully addressed.
Monitoring systems
In 1989, soon after suspicions of a silent epidemic were raised, the Epidemiology Division of the MOPH began nationwide bi-annual HIV sentinel surveillance in high-risk groups including pregnant women. The Thai army and MOPH identified and described the epidemic waves in FSW and HSM. This enabled a massive national response by the government and other sectors of society. Epidemiological data were also useful in focusing HIV prevention measures in high-risk groups, on high-risk behaviour and in the worst affected regions. Knowing where to allocate resources is crucial in resource-limited settings. The evolution and expansion of monitoring systems to the national level including PHIMS and PHOMS providing monthly data further enhanced PMTCT efforts by quantifying the need and efficacy of interventions. Data from PHOMS (2001–2007) and the National AIDS Programme (from 2008 to the present) have been used to monitor the performance of PMTCT and to improve missed opportunities for prevention, and the latest evaluation was undertaken in 2011 [Citation43].
The Asian epidemic model
Modelling has attempted to predict the future course of HIV in Thailand. Early modelling attempts since 1994 by the National Economic and Social Development Board using EPIMODEL projected the numbers of new infections in men, women and children separately and were successfully used in advocacy to the government and NGOs [Citation3]. However, because of its theoretical nature, the accuracy of this model was limited. Developed in collaboration between the TRCS and the East West Centre, the Asian Epidemic Model (AEM) reflects the infections, behaviour and movement of primary groups and transmission modes driving HIV transmission in Thailand and has been used since 1990 (Figure ).
Figure 1. HIV transmission dynamics in Thailand Citation45. FSW, female commercial sex worker; IDU, intravenous drug user; MSM, men who have sex with men.
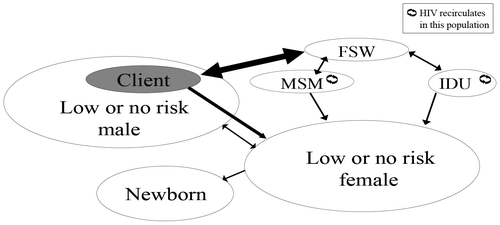
The user adjusts AEM fitting parameters to create HIV prevalence outputs matching observed epidemiological trends. This semi-empirical approach produces more accurate predictions with a large number of inputs from national monitoring of infections and outcomes as well as national social and behavioural survey data. Data from AEM and other inputs are then used in SPECTRUM 5.4 integrated with the Estimation and Projection Package to model MTCT (Figure ) [Citation44].
Figure 2. The structure of SPECTRUM 5.4 integrated with EPP to model MTCT in Thailand [Citation44].
![Figure 2. The structure of SPECTRUM 5.4 integrated with EPP to model MTCT in Thailand [Citation44].](/cms/asset/bdefacb3-e775-4f73-9e6b-c75601f47b07/ypch_a_1281873_f0002_b.gif)
AEM predictions correlate well with the observed data and are used to inform decision-making about HIV/AIDS policy in Thailand [Citation44, 45]. This emphasises the importance of a diverse range of high-quality monitoring and survey data for successful elimination of MTCT.
Addressing the four prongs of the WHO PMTCT strategy
Prong 1: Primary prevention of HIV in women of childbearing age. WHO recommendations include condom use, information and counselling about the risk of sexual HIV transmission, testing and counselling for pregnant and post-partum women as a part of routine reproductive health services and management of STI in women of reproductive age.
The highly successful Thai Population Programme for family planning services was introduced in the early 1970s and has increased contraception rates from 14.8% in the early 1970s to79.6% in 2009 [Citation46, 47].
The 100% Condom Programme and public health education campaigns to promote safe sex and discourage high-risk behaviour have been crucial in preventing HIV infection in women of reproductive age by breaking the chain of transmission by HIV-infected FSW to HSM and consequently to non-FSW Thai women.
The success of these interventions resulted from strong political leadership and commitment, heavy government investment and a pragmatic multi-sector response by Thai society. In 1991, the national AIDS policy of Thailand was transferred from the MOPH to the Office of the Prime Minister by Prime Minister Anand Panyarachun following advocacy by the then Minister of AIDS, Mechai Viravaidya. A National AIDS Prevention and Control Committee, which included the MOPH as the secretariat and was chaired by the prime minister, was formed and met quarterly until 1999. The positioning of the policy signalled to all government and non-government sectors to respond. After 1991, the government greatly increased investment in the national HIV/AIDS programme. In 1988, the government spent only US$684,000 on HIV/AIDS, while most funds came from NGO donations. By 1997, government spending on the programme had increased to US$82 million with only 4% being donations. This political commitment and increased investment included the provision of 60 million free condoms in the 100% Condom Programme and mass media campaigns. These were effective in increasing condom use and decreasing high-risk sexual behaviour amongst men [Citation31].
School-based education on the risk of HIV transmission has been provided by the MOPH since 1990, and conventional HIV/AIDS education has evolved from Thai studies of risky behaviour and its determinants. These studies found that many mistakenly believed that they only had a transmission risk of HIV from interaction with certain identifiable persons in high-risk groups such as IDUs or brothel-based FSWs at the beginning of the epidemic. For example, a non-brothel-based FSW with a clean and tidy appearance was often incorrectly assumed to be a low HIV-transmission risk because she was assumed to have had fewer sexual partners and to be hygienic. Thus, the educational message was evolved to address the underlying social, cultural and economic forces driving the epidemic to create a proper understanding of the causes and risks for transmission. The new peer education approach was developed from a pilot workshop programme organised by the Paediatric Society of Thailand from 1995 until 2006. This involved a 2-day course for 11–15-year-old secondary school pupils and HIV/AIDS educationalists. The aims were increased awareness of HIV/AIDS, and its physical and social effects as well as behaviour practices considered to be risky. A combination of educationalist-led and participant-led lessons and activities was used. Participating students were asked to disseminate the information learned to their peers [Citation48]. This fostered life-skills empowerment rather than behaviour modification so that their culture, peer pressure and norms would promote safer sexual behaviour [Citation49]. A national survey in 2009 of 19–49-year-old Thai women reported 85.2% of 15–24-year-old females had received some formal instruction in sex education, family planning and reproductive tract infection [Citation47]. Despite previous and current measures to address Prong 1, recent data suggest a possible resurgence of MTCT in the younger generation of Thais who were not of reproductive age in the 1990s when the public health campaign was most intensive [Citation19, 20].
In 2016, the Thai PMTCT programme includes counselling and voluntary HIV testing as part of a comprehensive package of ANC services. Couples counselling and voluntary testing is recommended to encourage mutual disclosure to encourage communication between sexual partners about contraception. This is suitable in Thailand because of the very high rate of ANC (98.9% in 2009) [Citation50]. ANC services also include testing for hepatitis B, syphilis and other STIs if suspected, and referral to STI services is recommended [Citation42]. The interventions addressing Prong 1 have decreased the estimated number of new HIV infections in adult women from 34,710 in 1992 to 2226 in 2016 [Citation45].
Prong 2: Prevention of unintended pregnancies in women living with HIV. Because of its high ANC rates, Thailand has included prevention of unintended pregnancy in women with HIV as part of the PMTCT counselling service at ANC clinics. The Thai national PMTCT guidelines recommend dual methods of contraception for HIV-infected women and their partners to avoid unintended pregnancy as recommended by the WHO based on studies which show that 14–21% of people who use condoms only become pregnant in the first year of use [Citation51]. This should include consistent condom use plus another form of birth control (e.g. sterilisation, hormonal implant, hormonal injection or intrauterine device). Women should also be advised of potential interaction between the oral contraceptive pill and ART [Citation42].
The use of dual contraception is low. A recent survey of PLWHA undertaken mainly in tertiary hospitals in Bangkok reported that only 202 (29.6%) of 898 used two or more methods of contraception, one of which was condoms, 683 (96.3%) used at least one method, 422 (87.7%) of whom used condom, 37 (7.7%) had been sterilised and 765 (82.5%) had disclosed their serostatus to their partner. Female gender and knowledge of HIV status for 1–5 or >5 years were associated with dual contraception. An intervention of a brief communication or referral to family planning services caused 66 of 317 (20.8%) of those who only used one form of contraception to change to dual contraception at a 12-month follow-up [Citation52]. To date, no data on national rates of unintended pregnancy in HIV-infected women are available. However, a national reproductive health survey in 2009 reported that 16.2% of ever-married women aged 19–49 years had had an unintended last pregnancy [Citation47], which is less than the global estimated prevalence (38% in the year 2010) [Citation53].
Prong 3: Prevention of HIV transmission from a woman living with HIV to her infant and Prong 4: Provision of appropriate treatment, care and support to women and children living with HIV and their families. Thailand has had great success in addressing prongs 3 and 4. In 2015, PHIMS data reported 95.6% of HIV-positive pregnant women and 99.5% of HIV-infected infants received free WHO Option B + through the national PMTCT programme [Citation38]. The Thai 2014 national PMTCT guidelines are in accordance with WHO recommendations for Prongs 3 and 4, and these overlap in interventions aimed at them. Prong 3 includes testing pregnant women for HIV, providing HIV-infected pregnant women with ART as soon as possible and measures to reduce the risk of MTCT. Prong 4 includes HIV testing of women, male partners and children, CD4 testing and clinical staging to determine eligibility for ART in both pregnant women and infants, screening for and treatment of opportunistic infections and linkages to longitudinal child, reproductive and HIV health services [Citation53]. The high ANC rates in Thailand are key as it is the first part of a ‘cascade’ leading to appropriate ART for mother and infant and access to other health services. Thai guidelines recommend pre-test counselling and voluntary HIV rapid test at the first ANC visit with same-day results and re-testing later in the pregnancy for HIV-negative women and their partner if possible. Guidance for women presenting late to ANC, at labour, at delivery or post-partum recommends voluntary testing immediately with same-day results. CD4 testing and referrals and consultations with other health care services are offered to any seropositive pregnant woman [Citation42].
Reducing the cost of providing ART is crucial to PMTCT programmes in resource-limited settings. Initially in 1996, the expense of the PACTG 076 protocol regimen was prohibitive, and donations from Thai society and foreign NGOs were necessary. Thus, studies establishing the efficacy of shorter, less expensive ART regimens were conducted to reduce the cost of therapy. Legal battles were also fought with pharmaceutical companies because of the World Trade Organization’s Agreement on Trade-Related Aspects of Intellectual Property Rights that limited the scope of generic drug production in Thailand since compliant Thai government regulations were introduced in 1998 under threat of sanctions from the United States of America. The solution to prohibitive costs was compulsory licensing of non-commercial (government) use and patent challenges. Thailand’s Government Pharmaceutical Organisation has been producing generic ART since 1995, and the price difference between these generic and patented ART is large. For example, a generic fixed-dose triple combination of stavudine, lamivudine and nevirapine cost US$360/patient/per year compared with US$4376 for the patented equivalent in 2007 [Citation54].
The future challenges and evolution of PMTCT in Thailand
Despite significant achievements, challenges remain for Thai PMTCT. Coverage of couple counselling (60% of service outlets in 2014) and testing (only 42% of couples in ANC services) is low. More innovations, training, staff incentives and outcome monitoring are needed to increase levels of service delivery. Non‐Thai pregnant women do not universally access PMTCT services in Thailand, possibly because the service is not free to them through the national PMTCT programme [Citation55]. Thus, funding must be found for this population. Pooled funding from multiple domestic sources has been proposed [Citation56]. More interventions are required to encourage mothers who are HIV-positive to access post-partum care services because 40% do not. The PHIMS monitoring system needs to be expanded to include private hospitals and large hospitals outside the MOPH system [Citation55].
Conclusion
The prevalence of HIV in pregnant women has decreased substantially during the past two decades. MTCT has been drastically reduced by early and concerted efforts in many sectors. The shared commitment, decision-making, resources and efforts by all sectors of Thai society to achieve the goals of elimination of MTCT have been necessary. The government, NGOs, businesses and Thai communities, including community opinion leaders and PLWHA, have all played a part in an effective multi-sectoral response. Leadership has also been required because the cost of indecision and delay would be high. Every additional HIV-infected child would increase the ultimate economic and social cost to the country. Children are the future. The country’s response to their problems indicates how highly Thailand values its future.
Disclosure statement
No potential conflict of interest was reported by the author.
Notes on contributor
Usa Thisyakorn is a professor of Paediatrics, Chulalongkorn University, Bangkok, Thailand. She graduated from Chiangmai University, Thailand. Her research interests include Dengue, HIV and Vaccines.
References
- Brown T, Sittitrai W, Vanichseni S, et al. The recent epidemiology of HIV and AIDS in Thailand. AIDS. 1994;8(Suppl 2):S131–141.
- Weigner BG, Limpakarnjanarat K, Ungchusak K, et al. The epidemiology of HIV infection and AIDS in Thailand. AIDS. 1991;5(Suppl 2):S71–85. Erratum in: AIDS. 1993;7.
- Brown T, Sittitrai W. The impact of HIV on children in Thailand. Program on AIDS, Thai Red Cross Society. Report No. 16; 1995.
- Thisyakorn U, Paupanwatana S, Kanchanamayul V, et al. Perinatal transmission of HIV and pediatric AIDS in Thailand. Presented at the International Symposium on Biomedical Research Issues of HIV Infection in Thailand. Bangkok; 1994. Available from: http://www.popline.org/node/298259
- Sittitrai W, Phanuphak P, Barry J, et al. Thai sexual behavior and risk of HIV infection: a report on the 1990 survey of partner relations and risk of HIV infection in Thailand. Program on AIDS, Thai Red Cross Society; 1992.
- Sittitrai W, Brown T. Risk factors for HIV infection in Thailand. AIDS. 1994;8(Suppl 2):S143–153.
- Phongpaichit P. From peasant girls to Bangkok masseuses. Geneva: International Labour Office; 1982. p. 12.
- Maticka-Tyndale E, Kiewying M, Haswell-Elkins M, et al. Knowledge, attitudes and beliefs about HIV/AIDS among women in northeastern Thailand. AIDS Educ Prev. 1994;6:205–218.
- Xenos P, Pitaktepsombati P, Sittitrai W. Partner patterns in the sexual behavior of unmarried, rural Thai men. Asian Pac Popul Forum. 1993;6:104–117.
- Liamputtong P, Haritavorn N, Kiatying-Angsulee N. HIV and AIDS, stigma and AIDS support groups: perspectives from women living with HIV and AIDS in central Thailand. Soc Sci Med. 2009;69:862–868.10.1016/j.socscimed.2009.05.040
- Yokota F, van Landingham MJ. Gender differences in stigma and community support among people living with HIV/AIDS in Thailand. In: Liamputtong P, editor. Stigma, discrimination and living with HIV/AIDS: a cross-cultural perspective. Dordrecht: Springer; 2013. p. 399–415.10.1007/978-94-007-6324-1
- Srison D, Thisyakorn U, Paupunwatana S, et al. Perinatal HIV infections in Thailand. Southeast Asian J Trop Med Public Health. 1995;26:659–663.
- Celentano D. HIV prevention among drug users: an international perspective from Thailand. J Urban Health. 2003;80(Suppl 3):97–105.
- Cohen E. Tourism and AIDS in Thailand. Ann Tourism Res. 1988;15:465–622.
- Lyttleton C. Messages of distinction: the HIV/AIDS media campaign in Thailand. Med Anthropol. 1996;16:363–389.
- Charumilind S, Jain SH, Rhatigan J. HIV in Thailand: The 100% Condom Program. Boston, MA: Harvard Business Publishing; 2011.
- Rojanapithayakorn W. One hundred percent condom programme. Poster session presented at the 8th International Conference on AIDS. Amsterdam; 1992 [abstract PoD 5654]. Available from: http://quod.lib.umich.edu/c/cohenaids/5571095.0050.029/708?page=root;rgn=subject;size=100;view=image;q1=abstracts++summaries
- Hanenberg RS, Rojanapithayakorn W, Kunasol P, et al. Impact of Thailand’s HIV-control programme as indicated by the decline of sexually transmitted diseases. Lancet. 1994;344:243–245.10.1016/S0140-6736(94)93004-X
- Bureau of Epidemiology, Thailand. Behavioural Surveillance Survey Report 2008. Nonthaburi: Department of Disease Control, Ministry of Public Health; 2008.
- UNGASS. National AIDS Prevention and Alleviation Committee. UNGASS Country Progress Report 2010: Thailand reporting period January 2008–December 2009. Nonthaburi: Ministry of Public Health; 2010.
- Kanshana S, Simonds RJ. National program for preventing mother-child HIV transmission in Thailand: successful implementation and lessons learned. AIDS. 2002;16:953–959.10.1097/00002030-200205030-00001
- Global Program on AIDS Consensus statement from the WHO/UNICEF consultation on HIV and breastfeeding. Wkly Epidmiol Rec. 1992;67:177–179.
- Heymann SJ. HIV Transmission via breastfeeding: perspectives on prevention. Presented at the International Workshop on Pediatric AIDS in Thailand: Public Health and Social Dilemma; Bangkok; 1995. Available from: http://www.popline.org/node/298263
- Sriminipurn S. Maternal-infant human immunodeficiency virus transmission and breast feeding in Phayao Hospital. Buddhachinaraj Med J. 1996;13:41–47.
- Trirattanapa K, Puapanwattana S, Hansuttivejakul R, et al. Perinatal HIV transmission rate by polymerase chain reaction in Chiang Rai Hospital. Thai J Pediatr. 1997;36:119–127.
- Gulgolgarn V, Songpanich P, Chaiyabuttra K, et al. CD4 T cell count in infants born to mothers infected with HIV. Thai J Pediatr. 1999;38:128–134.
- Shaffer N, Chuachoowong R, Mock PA, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet. 1999;353:773–780.10.1016/S0140-6736(98)10411-7
- Chikhungu L, Bispo S, Newell ML. Postnatal HIV transmission rates at age 6 and 12 months in infants of HIV-positive women on antiretroviral therapy initiating breastfeeding: a systematic review. In: Updates on HIV and infant feeding guideline. Geneva: WHO; 2016.
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180.10.1056/NEJM199411033311801
- Fiscus S, Adimora AA, Schoenbach VJ, et al. Can zidovudine monotherapy continue to reduce perinatal HIV transmission? The North Carolina experience 1993–1997. Presented at the 12th International Conference on AIDS. Geneva, 1998 [abstract 33162]. Available from: http://quod.lib.umich.edu/c/cohenaids/5571095.0140.073/634?page=root;size=100;view=image
- World Bank. Thailand’s response to AIDS: building on success, confronting the future. Washington, DC: Thailand Social Monitor V; 2000. p. 6.
- Charity Under Royal Patronage. The Thai Red Cross AIDS Research Centre 2012. Available from: http://en.trcarc.org/Homepage/?page_id=1066
- Rongkavilit C, Thisyakorn U, Phanuphak P. Prevention of mother-to-child transmission of HIV: Thai Red Cross zidovudine donation programme. Joint United Nation Programme on HIV/AIDS Best Practice Collection; 2000 Sep; p. 11–40. Available from http://en.trcarc.org/Homepage/wp-content/uploads/2012/12/TRC-ARC_UNAIDS_Best_Practice_in_PMTCT.pdf
- Jackson JB, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–868.10.1016/S0140-6736(03)14341-3
- Phanuphak N. Efficacy and safety of maternal triple-drug ARV regimens: Thai Red Cross PMTCT Program, 2004–2010. Presented at the 18th Conference on Retroviruses and Opportunistic Infections. Boston, MA, 2011 [abstract 742].
- Thaineua V, Sirinirund P, Tanbanjong A, et al. From research to practice: use of short course zidovudine to prevent mother-to-child HIV transmission in the context of routine health care in Northern Thailand. Southeast Asian J Trop Med Public Health. 1998;29:429–442.
- Kanshana S, Thewanda D, Teeraratkul A, et al. Implementing short-course zidovudine to reduce mother-infant HIV transmission in a large pilot program in Thailand. AIDS. 2000;14:1617–1623.10.1097/00002030-200007280-00018
- Lolekha R, Boonsuk S, Plipat T, et al. Elimination of mother-to-child transmission of HIV — Thailand. MMWR Morbid Mortal Wkly Rep. 2016;65:562–566.10.15585/mmwr.mm6522a2
- Manosuthi W, Ongwandee S, Bhakeecheep S, et al. Guidelines for antiretroviral therapy in HIV-1 infected adults and adolescents 2014, Thailand. AIDS Res Ther. 2015;12:12.10.1186/s12981-015-0053-z
- Naiwatanakul T, Voramongkol N, Punsuwan N, et al. Uptake of early infant diagnosis in Thailand’s national program for preventing mother-to-child HIV transmission and linkage to care, 2008–2011. J Int AIDS Soc. 2016;19:20511.
- Chasombat S, Lertpiriyasuwat C, Thanprasertsuk S, et al. The National Access to Antiretroviral Program for PHA (NAPHA) in Thailand. Southeast Asian J Trop Med Public Health. 2006;37:704–715.
- Thailand. Bureau of AIDS, TB and STIs, Department of Disease Control, Ministry of Public Health. [Essentials of HIV/AIDS Treatment and Prevention; 2014]. Available from: http://thaiaidssociety.org/images/PDF/essentials_of_hiv_aids_treatment_and_prevention_2014_thailand.pdf
- Collins IJ, Cairns J, Ngo-Giang-Huong N, et al. Cost-effectiveness of early infant HIV diagnosis of HIV-exposed infants and immediate antiretroviral therapy in HIV-infected children under 24 months in Thailand. PLoS One. 2014;9:e91004.10.1371/journal.pone.0091004
- Thailand Working Group on HIV/AIDS Projection: AIDS Epidemic Model-Projection for HIV/AIDS in Thailand. 2010–2030. Summary Report. Bangkok: Ministry of Public Health, Thailand; 2012. Available from http://www.aidsdatahub.org/aids-epidemic-model-projection-hivaids-thailand-2010-2030-summary-report-thailand-working-group
- The Asian Epidemic Model (AEM) projections for HIV/AIDS in Thailand: 2005–2025. Family Health International and Ministry of Public Health, Thailand. Available from: http://www.aidsdatahub.org/sites/default/files/documents/The_Asian_Epidemic_Model_Projections_for_HIVAIDS_in_Thailand_2005_2025.pdf
- Nepomuceno T. The ‘anatomy’ of Thailand’s successful family planning program. ICMH Newsl. 1991;18:1.
- SEARO. Thailand and Family Planning: An Overview. New Delhi, India: World Health Organization South-East Asia Regional Office; 2016. Available from: http://www.searo.who.int/entity/maternal_reproductive_health/documents/tha-fp.pdf?ua=1
- Liulak W, Hemungkorn M, Thisyakorn U. Children and youth away from AIDS: a Pediatric Society of Thailand Initiative Program. Asian Oceanian J Pediatr Child Health. 2010;7:1–5.
- Phoolcharoen W. HIV/AIDS prevention in Thailand: success and challenges. Science. 1998;280:1873–1874.10.1126/science.280.5371.1873
- Kongsri S, Limwattananon S, Sirilak S, et al. Equity of access to and utilization of reproductive health services in Thailand: national reproductive health survey data, 2006 and 2009. Reprod Health Matters. 2011;19:86–97.10.1016/S0968-8080(11)37569-6
- World Health Organization. Medical eligibility criteria for contraceptive use: a WHO family planning cornerstone. 4th ed. Geneva: WHO; 2010. p. 15–82.
- Munsakul W, Lolekha R, Kowadisaiburana B, et al. Dual contraceptive method use and pregnancy intention among people living with HIV receiving HIV care at six hospitals in Thailand. Reprod Health. 2016;13:8.
- World Health Organization. Towards the elimination of mother-to-child transmission of HIV: report of a technical consultation, 2010 Nov 9–11; Geneva: WHO.
- Ford N, Wilson D, Costa Chaves G, et al. Sustaining access to antiretroviral therapy in the less-developed world: lessons from Brazil and Thailand. AIDS. 2007;21(Suppl 4):S21–29.10.1097/01.aids.0000279703.78685.a6
- UNGASS. Thailand AIDS Response Progress Report, 2015. Available from: http://www.unaids.org/sites/default/files/country/documents/THA_narrative_report_2015.pdf
- Patcharanarumol W, Thammatacharee N, Kittidilokkul S, et al. Thailand’s HIV/AIDS program after weaning-off the global fund’s support. BMC Public Health. 2013;13:1008.10.1186/1471-2458-13-1008
