Abstract
Background
Vibrio cholerae is a highly motile Gram-negative bacterium which is responsible for 3 million cases of diarrhoeal illness and up to 100,000 deaths per year, with an increasing burden documented over the past decade. Current WHO guidelines for the treatment of paediatric cholera infection (tetracycline 12.5 mg/kg four times daily for 3 days) are based on data which are over a decade old. In an era of increasing antimicrobial resistance, updated review of the appropriate empirical therapy for cholera infection in children (taking account of susceptibility patterns, cost and the risk of adverse events) is necessary.
Methods
A systematic review of the current published literature on the treatment of cholera infection in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was undertaken. International clinical guidelines and studies pertaining to adverse effects associated with treatments available for cholera infection were also reviewed.
Results
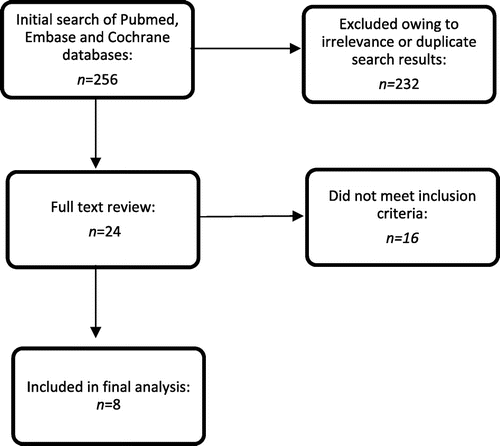
The initial search produced 256 results, of which eight studies met the inclusion criteria. Quality assessment of the studies was performed as per the Grading of Recommendations Assessment, Development and Evaluation guidelines.
Conclusions
In view of the changing non-susceptibility rates worldwide, empirical therapy for cholera infection in paediatric patients should be changed to single-dose azithromycin (20 mg/kg), a safe and effective medication with ease of administration. Erythromycin (12.5 mg/kg four times daily for 3 days) exhibits similar bacteriological and clinical success and should be listed as a second-line therapy. Fluid resuscitation remains the cornerstone of management of paediatric cholera infection, and prevention of infection by promoting access to clean water and sanitation is paramount.
Introduction
Vibrio cholerae is a highly motile, halophilic Gram-negative, comma-shaped bacterium. The main reservoirs of V. cholerae are people and aquatic sources such as brackish water and estuaries [Citation1]. V. cholerae is serologically classified on the basis of variations in the 0-antigen lipopolysaccharide structure, and, while over 200 serogroups have been identified, only two (V. cholerae 01 and 0139) cause cholera epidemics [Citation2].
V. cholerae 01 predominates as the cause of cholera globally. This species is further divided into two main serotypes — Inaba and Ogawa serogroups — and two biotypes (El Tor and classical) on the basis of biochemical differences and susceptibility to specific bacteriophages, with the latter now appearing to be extinct [Citation3]. Most environmental V. cholerae are not toxigenic. However, the pathogenic strains of V. cholerae 01 and 0139 may harbour genes within a filamentous bacteriophage, known as CTXφ that encode for ‘cholera toxin’ (CT) which acts by entering the surface of epithelial cells and increasing cyclic adenosine monophosphate activity, leading to chloride secretion at the apical surface. This results in significant water and sodium losses, leading to the massive fluid and electrolyte efflux that is the hallmark of clinical cholera infection [Citation4].
Cholera is endemic in approximately 50 countries — placing 1.4 billion people at risk — and the vast majority of the clinical burden is borne in resource-limited settings owing to restricted access to clean water sources. Each year, cholera is estimated to cause 3 million cases of diarrhoeal illness worldwide, and up to 100,000 deaths [Citation5]. During epidemics, the case fatality rate is 1–4%, higher in rural areas [Citation2]. Importantly, the burden of cholera has been increasing in the past decade [Citation6]. Patterns of transmission and infection differ between endemic areas (where seasonal distribution occurs after rainy seasons, and the incidence is highest in young children owing to a lack of protective immunity) in contrast with regions which experience cholera epidemics (where attack rates are similar in adults and children) [Citation7]. Superimposed epidemics may also occur in endemic regions in response to fluctuations in population-based immunity and climate [Citation8]. Since the early 1800s, there have been seven cholera pandemics, with the current pandemic (of V. cholerae 01 El Tor) commencing in 1961 and continuing in three successive waves — from South Asia to other regions of Asia, the Oceania and Africa [Citation9].
The infectious dose of V. cholerae required to cause infection is relatively high (over 108 V. cholerae), although human-shed organisms are more infectious and require a lower inoculum [Citation10]. Once infected, V. cholerae causes a spectrum of illness — from asymptomatic disease to life-threatening dehydration — depending on bacterial load, degree of background immunity and presence or absence of malnutrition [Citation11]. The incubation period varies between hosts and inoculum size, from 1 to 5 days. Mild cases may be indistinguishable from other causes of diarrhoeal illness, while profound infection causes rapid loss of fluid and electrolytes in ‘rice water’ stool (containing large amounts of sodium, potassium and bicarbonate) at rates of 10–20 ml/kg/h [Citation3]. Severe hypovolaemia may occur within hours of symptom onset, resulting in hypovolaemic shock, hypokalaemia, lactic acidosis (owing to bicarbonate loss), acute renal failure and hypoglycaemic coma. The mortality of untreated cholera is 50–70%, and children have a 10 times greater risk of death than adults [Citation5].
Cholera is commonly diagnosed and treated presumptively on the basis of clinical features. It can be confirmed by isolation of V. cholerae from stool cultures performed on specific media (TCBS or TTGA agar), with rapid diagnostic tests also available (which tend to be highly sensitive but poorly specific, limiting their usefulness in endemic areas) [Citation5]. The 2013 World Health Organization (WHO) Pocketbook for Hospital Care defines cholera as ‘profuse watery diarrhoea with severe dehydration’ during a cholera outbreak or a positive stool culture for V. cholerae 01 or 0139 [Citation12].
Fluid resuscitation is the mainstay of treatment (reducing mortality to <0.5%) [Citation1] and, while antimicrobial therapy does not have an immediate effect on disease progression (as the toxin is already bound to intestinal cells), they decrease the duration of the disease by diminishing further production of the toxin by inhibiting bacterial protein synthesis or promoting bacterial cell death [Citation9]. Importantly in epidemics, antimicrobial therapy also diminishes pathogen excretion which reduces person-to-person transmission of infection, as well as limiting environmental contamination by cholera by diminishing the volume and duration of stools passed (by approximately 50%), shortening the period of faecal excretion of V. cholera [Citation13]. Clinical recovery is therefore expedited, while the volume of rehydration fluid required (and burden on medical care) is diminished, optimising use of resources during outbreaks and decreasing the rate of infectivity [Citation14–19].
Currently, WHO recommends antibiotics (as soon as vomiting stops, usually 4–6 h after commencing oral rehydration therapy) for children aged > 2 years with ‘severe dehydration’ (Table ). However, the current WHO recommendations for antimicrobial therapy (Table ) are based on evidence from 2005 [Citation12,20]. In view of increasing antimicrobial resistance worldwide and the changing efficacy and safety profiles, this review of the international literature was undertaken to update the evidence surrounding the recommendations for antibiotic treatment in paediatric cholera infection.
Table 1. WHO classification of dehydration in children with cholera [Citation2,3].
Table 2. Published WHO recommendations for antibiotic therapy for children >2 years presenting with suspected cholera.
Methods
Search terms
A systematic search of systematic reviews, meta-analyses, multi-centre studies and randomised-controlled trials for relevant papers was conducted using the MeSH Search terms ‘cholera, ‘antibiotics’ and ‘antimicrobials’. The databases EMBASE, Cochrane database of systematic reviews and PubMed were searched. Trials were limited to those in humans published in the past decade to ensure that accurate and up-to-date information on antimicrobial resistance patterns was documented. The reference lists of relevant publications were also reviewed. Inclusion and exclusion criteria are documented in Table .
Table 3. Inclusion and exclusion criteria for review of the evidence for antimicrobial treatment of cholera infection.
Results
The initial search produced 256 results (Figure ), 24 of which qualified for full text review. Ultimately, eight studies met the inclusion criteria (Table ) and were abstracted as detailed in Appendix 1. Quality assessment of the studies was performed as per the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines [Citation21].
The search was initially restricted to results investigating the paediatric population, but owing to limited research in this area, it was expanded to include research in all age ranges. International clinical practice guidelines were also reviewed, including the Infectious Diseases Society of America (IDSA), World Gastroenterology Guidelines, ICDDR,B, the United States Centre for Disease Control, BMJ Clinical Evidence, the American Academy of Paediatrics, and Therapeutic Guidelines (Australia) [Citation16,22–26].
Characteristics of the studies included
Three studies were systematic reviews and meta-analyses, two of which were conducted across an international setting while one was conducted in sub-Saharan Africa [Citation27]. One study was a systematic descriptive analysis (which included a systematic search) of information regarding the epidemiology of cholera outbreaks in Asia and included descriptive analyses regarding increasing antimicrobial resistance patterns [Citation28]. There were two randomised controlled trials, one open-labelled-controlled clinical trial and one multi-centre study conducted in four sites.
Only two papers analysed the paediatric population specifically (age 2–16 years) [Citation6,19] while the remaining systematic reviews covered all age ranges [Citation27–29] and three clinical trials included only adults [Citation29–31]. Most clinical trials were conducted in Asia — Bangladesh [Citation19,29,30] and India [Citation31]. The studies were analysed according to GRADE level of evidence criteria (see Appendix 1 for description of methodologies and relevant limitations) [Citation21]. No studies were assessed as high-quality evidence. Three were classified as being of moderate quality [Citation19,32,33], four as low quality [Citation6,27,29,31] and one as very low quality [Citation28].
Evidence for current WHO recommendations
Erythromycin
Macrolides (azithromycin, clarithromycin, erythromycin and roxithromycin) have a broad spectrum of activity against Gram-positive and Gram-negative cocci (as well as Gram-negative anaerobic bacteria), attaining high intracellular concentrations beneficial for the treatment of infections caused by intracellular pathogens. As inhibitors of the cytochrome P450 (CYP3A4) enzyme system, drug interactions and adverse effects can occur (discussed below). Oral formulations of erythromycin have variable absorption and are poorly tolerated owing to adverse gastrointestinal effects, and poor adherence is exacerbated by the four times daily dosing schedule [Citation26]. A 2014 systematic review of the evidence of two trials (involving 179 participants) showed that single-dose erythromycin was inferior to azithromycin which shortened the duration of diarrhoea by half a day compared with erythromycin [mean duration (MD) 12.05, 95% CI 22.02–2.08] [Citation32].
Although they are outside the inclusion time-frame for this review, it is worth mentioning two studies completed in 2002 [Citation14] and 2005 [Citation34] which evaluated the clinical efficacy of erythromycin in childhood cholera. A double-blind randomised-controlled trial in a tertiary centre in Bangladesh of 128 children aged 1–15 years with severe dehydration treated with single-dose azithromycin (20 mg/kg) vs four times daily erythromycin (12.5 mg/kg) for 3 days found no significant difference in clinical success between the two groups (76% of patients receiving azithromycin vs 65% in the erythromycin group (95% CI 5–7, p = 0.24) and no significant difference in bacteriological success (71% of azithromycin group vs 82% of the erythromycin group, 95% CI 5–25, p = 0.26) [Citation14]. Furthermore, this RCT found that patients treated with azithromycin had a significantly shorter duration of diarrhoea [median 24 h vs 42 h, difference 12, 95% CI {surrounding difference} 0–18 h, p = 0.019) and fewer episodes of vomiting (1 vs 4, difference = 1, 95% CI surrounding difference 0–3, p = 0.023) [Citation14]. A second randomised, open-label-controlled clinical trial published in 2005 compared single-dose ciprofloxacin (20 mg/kg) with erythromycin (12.5 mg/kg four times daily for 3 days) in 180 children aged 2–15 years with V. cholerae infection (confirmed by stool microscopy) and found no significant difference in clinical success between children treated with erythromycin vs ciprofloxacin (difference 5%, 95% CI 10–21) [Citation34]. However, children treated with ciprofloxacin had less vomiting (58 vs 74%, difference 16%, 95% CI 2–30%), fewer stools [15 vs 21%, difference 6% (95% CI 0–9%)] and less stool volume [152 vs 196 ml/kg, difference 43 ml/kg (95% CI 13–87)] than those treated with erythromycin, yet bacteriological failure was significantly more common in ciprofloxacin-treated patients [58 vs 30%, difference 28% (95% CI 13–43)] [Citation34].
Ciprofloxacin
Historically, fluoroquinolones have been viewed as attractive agents for treating cholera because of their very good activity in vitro, high concentrations in the gut lumen, high therapeutic ratio and relatively long half-life [Citation33]. These characteristics have led to their widespread use as single dose therapy, or as a daily dose therapy (for 3 days). However, the evidence of this review demonstrates that resistance to this class of drugs for treating cholera is increasing.
A 2014 systematic review [Citation32] found no statistically significant difference in ciprofloxacin compared with tetracyclines in reducing the duration of diarrhoea or stool volume (three trials, 259 participants, moderate-quality evidence). A further systematic review in 2016 which assessed fluoroquinolone resistance in sub-Saharan Africa found high levels of resistance to nalidixic acid, with reduced susceptibility to ciprofloxacin observed in recent outbreaks (in the Democratic Republic of Congo, Kenya, Nigeria and Cameroon) [Citation27]. Data from four clinical trials in adults (n = 275) in Bangladesh [Citation30] also found a poor clinical response to a single, 1-g dose of ciprofloxacin (a standard treatment for adults with cholera in South-East Asia). Clinical success (defined as cessation of diarrhoea within 48 h) was achieved in only 18% of patients with nalidixic acid-resistant V. cholerae infection; the majority of isolates were found to be resistant [this clinical success improved to 67% in those treated with a 3-day course (difference 0.49, 95% CI −0.68 to −0.22, p < 0.001). This research emphasised the highly apparent increase in non-susceptibility to fluoroquinolones in the region during the study period, with a dramatically increasing MIC35 for ciprofloxacin, from 0.002 μg/ml in 1994 to 0.250 μg/ml in 2003, a 125-fold increase. Concurrently, all isolates became resistant to nalidixic acid [Citation30].
In the 2005 randomised, open-label-controlled trial discussed above which found that single-dose ciprofloxacin had similar clinical efficacy to a 3-day course of erythromyin (60 vs 55%, 95% CI 10–21), bacteriological failure was more common with ciprofloxacin (58 vs 30%, 95% CI 13–43%) [Citation34]. In a 2010 RCT of paediatric patients in Bangladesh, ciprofloxacin was also found to be clinically inferior to azithromycin and bacteriological success was, again, significantly less [Citation19].
A number of authors have noted that current thresholds of antimicrobial susceptibility to ciprofloxacin are inappropriately low, with poorer clinical outcomes in isolates defined by the Clinical Laboratory Standards Institute as susceptible in vitro [Citation29,30,35,36]. Owing to cross resistance with nalidixic acid (secondary to a single mutation in the gyrA gene coding the DNA gyrase) and the high worldwide resistance patterns to nalidixic acid [Citation30], ciprofloxacin is not recommended for use in cholera.
Tetracyclines
Tetracyclines have a broad spectrum of activity that includes Gram-positive and Gram-negative bacteria. Common adverse effects (discussed below) include oesophagitis, photosensitivity and enamel dysplasia which often precludes their use in children <8 years, although the risk appears to be minimal if single short courses are used [Citation25].
A recent systematic review assessed 39 trials in 4632 participants, and found that there was no difference in clinical outcomes between patients treated with tetracycline and those treated with doxycycline (three trials, 230 participants, very low quality evidence), or in patients treated with tetracycline compared with ciprofloxacin or norfloxacin (three trials, 259 participants, moderate quality evidence) [Citation32]. However, in indirect comparisons with substantially more trials, tetracycline exhibited benefits over doxycycline, norfloxacin and trimethoprim–sulphamethoxazole (TMP-SMX) for the primary review outcomes (reducing stool volume, vibrio excretion and the amount of rehydration fluids required) [Citation32]. Another systematic review [Citation6] identified one study which compared the efficacy of tetracycline in children aged 1–5 years in Bangladesh, revealing that, compared with tetracycline, the mean total times to recovery were prolonged by 66% with placebo (p < 0.001), 25% with ampicillin (p < 0.017) and 9% with erythromycin (p = 0.37), yet these data were collated in 1998 and so are unlikely to represent current susceptibility patterns.
Cotrimoxazole
Whilst outside the time frame for this review, two trials conducted more than 20 years ago evaluated the efficacy of cotrimoxazole. Both showed no difference from other antimicrobials but were statistically inadequately powered [Citation37,38].
Evidence for alternative antibiotics
Doxycyline
As outlined above, tetracyclines exhibit clinical benefit over doxycycline [Citation32]. Trials dated prior to this review period have found doxycycline to be inferior to alternative antibiotics (including ciprofloxacin) for treating cholera [Citation39]. Furthermore, there is evidence that in vitro doxycycline susceptibilities are not a useful indicator of the in vivo efficacy of the drug [Citation39], and concerns regarding their adverse effects limit its use in older children and adults.
Azithromycin
Four publications examined the efficacy of azithromycin in treating cholera [Citation19,29,31,32]. A recent systematic review [Citation32] found single-dose azithromycin to be superior to ciprofloxacin and erythromycin in shortening the duration of diarrhoea (vs ciprofloxaxcin, MD 32.43 h, 95% CI 62.90 to −1.95, two trials, 375 participants, moderate-quality evidence; and vs erythromycin MD 12.05 h, 95% CI −22.02 to −2.08, two trials, 179 participants, moderate-quality evidence). It was not compared with tetracycline.
In a 2010 clinical trial of 180 paediatric patients with cholera in Bangladesh [Citation19], single-dose azithromycin (20 mg/kg) was compared with single-dose ciprofloxacin (20 mg/kg), and azithromycin achieved greater clinical success (defined as resolution of diarrhoea within 24 h — earlier than the usual timeline of 48 h) than ciprofloxacin (95 vs 70.6%, RR 1.33, 95% CI 0.65–0.86). Similar outcomes were observed for bacteriological success (defined as eradication of V. cholerae in the stool sample from day 3: 100% for azithromycin vs 96% for ciprofloxacin, RR 1.04, 95% CI 0.91–0.99, p = 0.06).
A 2014 RCT in 120 adult males in Kolkata compared single-dose azithromyin (1 g) with norfloxacin (400 mg) twice daily for 3 days, and found no statistically significant difference in clinical outcome (stool volume and urine output, duration of diarrhoea, total fluid requirement); the authors concluded that azithromycin is not more effective than norfloxacin [Citation31]. However, they noted that azithromycin remained clinically superior in the paediatric age range owing to the ease of single-dosing and the availability of a syrup (norfloxacin is available only in tablets).
Further superiority of single-dose azithromycin (compared with single-dose ciprofloxacin) was also found in a 2006 double-blind RCT in 195 male adults in Bangladesh with 73% of patients achieving clinical success compared with 27% of those treated with ciprofloxacin [Citation29]. The authors concluded that, in adults and children, single-dose azithromycin is an effective (and inexpensive) drug for the treatment of cholera caused by susceptible strains of V. cholerae.
Finally, in a 2002 double-blind RCT in paediatric patients slightly earlier than this search period (detailed above) in which single-dose azithromycin (20 mg/kg) was compared with 12.5 mg/kg erythromycin four times daily for 3 days, there was no significant difference in clinical or bacteriological success between the two patient groups, although patients treated with azithromycin had significantly less vomiting and a shorter duration of diarrhoea [Citation19]. This is further evidence of the clinical efficacy of single-dose azithromycin.
There is, therefore, substantial evidence supporting the use of azithromycin for paediatric cholera. Azithromycin’s primarily trans-intestinal and biliary route of elimination results in high concentrations in the stool, and its ease of administration with a single-dose regimen and prolonged half-life (48–72 h) enhance its clinical efficacy [Citation31,40].
Synopsis of evidence from international guidelines
A summary of the international guidelines reviewed is presented in Table . While most guidelines recommend doxycycline as first-line therapy for cholera in adults, guidelines updated in the last decade cite single-dose azithromycin as the preferred first-line therapy for children [Citation9,24,26]. Recent consensus is that, owing to diminishing susceptibility of tetracyclines, this class of antibiotic should be reserved only for epidemics in which susceptibility has been documented. While ciprofloxacin is listed as a second-line therapy in several international guidelines, in view of recent evidence reviewed above regarding increasing worldwide ciprofloxacin resistance, it is not recommended as a routine treatment of cholera in children.
Table 4. Summary of international guidelines on the treatment of cholera.
Clinical dehydration and the indication for antibiotic treatment
Current WHO guidelines recommend antibiotics only for patients with severe dehydration. However, as outlined in Table , a number of international guidelines extend this to include patients with both moderate and severe dehydration [Citation9,16,23,25] with some even indicating antibiotic therapy for patients with mild dehydration [Citation26] or ‘clinically diagnosed cholera’, not limited by severity [Citation23,24].
The guidelines for expanded therapy regardless of fluid status are largely based on the results of the systematic reviews discussed above which document significant clinical and bacteriological success in patients with both severe and moderate dehydration treated with antibiotics [Citation6,32]. A 2014 systematic review of 39 trials in 4632 participants found that antibiotic therapy shortened the mean duration of diarrhoea by approximately 1.5 days compared with placebo or no treatment (MD 36.76 h, 95% CI −43.51 to −30.03, data from 19 trials in 1103 participants, moderate-quality evidence), reduced total stool volume by 50% (ratio of means 0.5, 95% CI 0.45–0.56, 11 trials, 1201 participants, moderate-quality evidence) and reduced mean duration of faecal excretion of vibrios by almost 3 days (MD 2.74, 95% CI −3.07 to −2.40, 740 participants, moderate-quality evidence) [Citation32].
These clinical and public health (through diminished transmission) benefits were seen in trials recruiting only patients with severe dehydration and in those with less severe dehydration [Citation32], leading the authors to conclude that, in treating cholera, similar clinical and microbiological benefits are observed in both severely and non-severely ill patients. This was substantiated by a systematic review which also concluded that antibiotics have a clinical benefit in moderately dehydrated patients with cholera, and no adverse effects of their use were identified [Citation41].
In view of the evidence of these large systematic reviews and the international consensus in recently updated international guidelines, antibiotic therapy in cholera outbreaks should be extended, if resources allow, to all paediatric patients presenting with signs of dehydration (i.e. those requiring hospital-based oral or intravenous rehydration, defined as those with ‘some’ or ‘severe’ dehydration as per the WHO 2005 guidelines; Table ) [Citation13].
Evidence regarding the duration of antibiotic therapy
The duration of antimicrobial therapy depends on the choice of antibiotic (Table and ). Erythromycin and tetracycline require 3-day courses for bacteriological success, and, while doxycycline may be a useful single dose therapy in susceptible epidemics, resistance is increasing and its use should be limited to older children [Citation32]. As outlined above, the most promising evidence in the paediatric age range for single-dose therapy is for azithromycin because increasing minimal inhibitory concentrations (MICs) for ciprofloxacin now mean that it is not effective as a single-dose [Citation29,32]. Single-dose therapy has significant advantages: compliance is assured (and the development of resistance is, therefore, diminished), treatment is more affordable and logistics are improved, an important point when considering treatment strategies in rapidly spreading cholera epidemics [Citation23,33].
Table 5. Recommended duration and dosage of evidence-based antibiotics to treat cholera in children.
Table 6. Common adverse reactions to antibiotics currently indicated to treat cholera in children.
Reviews of harms and toxicity — summary of the evidence on safety
Common adverse effects
Common adverse effects of the currently recommended therapies for treating cholera and those which may be relevant when updating guidelines are detailed in Table .
Prolongation of the QT interval
Published case reports suggest that fluoroquinolones and macrolides are associated with prolongation of the QT interval [Citation42,43]. Independently, mild delays in ventricular repolarisation are clinically unnoticeable, though these antimicrobials may serve to amplify the risk of ‘torsades de pointes (TdP)’, a potentially fatal polymorphic ventricular tachyarrhythmia which may present as sudden death (owing to ventricular tachycardia), syncope, palpitations, seizures, or asymptomatically if the duration is short and terminates spontaneously [Citation45]. Of note, the current literature identifies this risk as requiring the presence of other risk factors, as highlighted in Table .
Table 6. Risk factors for the development of torsades de pointes.
The predominant risk factor for the development of TdP is co-administration of other medications which are substrates and/or inhibitors of cytochrome P450 (CYP) enzymes, and the associated ‘metabolic liability’ resulting from co-administration of medications synergistically interacting with this enzyme. This risk is enhanced by individual allelic variations in CYP3A4, the most important enzyme in human drug metabolism. CYP3A4 is responsible for the biotransformation of approximately 60% of all oxidised drugs [Citation44] and allelic variations can result in patients being poor metabolisers of CYP3A4-inducing medications [Citation45], resulting in reduced clearance of drug substrates and increasing exposure to toxicity effects. Overall, the individual risk of cardiac arrhythmias secondary to these antimicrobials is minimal; yet, if combined with a genetic propensity to poor metabolism of CYP3A4-inducing medications and co-administration with other CYP potentiators, the risk may be magnified, although the clinical impact of this is unknown.
Prolonged QT syndrome and azithromycin
Azithromycin has been identified as being distinguishable from macrolides as a group in terms of its cardiac toxicity, as it minimally inhibits CYP3A4, resulting in a lack of appreciable interaction with other CYP3A4 substrates. It is therefore classified as one of the safer macrolide antibiotics from a cardiac perspective [Citation45,47]. In recent years, however, increasing attention has been paid to azithromycin’s risks following a documented increased risk of cardiac death in a cohort of 347,795 patients aged 30–74 years taking azithromycin. The study found that patients taking 5 days of azithromycin compared with taking no antibiotics had a statistically significant increased risk of cardiac death [hazard ratio (HR) 2.88, 95% CI 1.25–2.75, p < 0.0001] as well as death from any cause (HR 1.85, 95% CI 1.25–2.75, p = 0.002). However, the risk was found to be most pronounced in patients with a high baseline risk of cardiovascular disease, and there was evidence of confounding by factors associated with both azithromycin use and risk of cardiovascular disease — namely a history of smoking, high body mass index, poor diet, and low physical activity [Citation46]. At present, published case reports of increased risk of sudden cardiac deaths in patients taking azithromycin are limited to adults, and whether these findings apply to the paediatric population cannot be concluded [Citation47].
A considerable risk in severe cholera is that of hypovolaemia-induced hypokalaemia owing to potassium loss in the stool, which in itself is a risk factor for arrhythmias (specifically, a prolonged PR interval and flattened T-waves) [Citation25]. As such, adequate fluid replacement with potassium-containing oral and intravenous solutions should remain of paramount importance in treating patients with cholera to minimise the possibility of this risk factor contributing to the risk of TdP.
Prolonged QT syndrome and fluoroquinolones
As with macrolides, there is interclass variability in the QT prolongation effect of fluoroquinolones. Ciprofloxacin’s inhibition of CYP1A2 has been described as ‘relatively inconsequential’ [Citation45], and the US Food and Drug Administration (FDA)’s Adverse Event Reporting System (AERS) supports the notion of multifactorial causes of fluoroquinolone-associated TdP, usually occurring in the context of co-administration with another QT-prolonging drug, underlying cardiac disease, renal impairment and electrolyte anomaly. However, combined with the increasing resistance of cholera to ciprofloxacin and the longer course that is required to overcome increasing MICs, ciprofloxacin should not be recommended as a first-line therapy for treating paediatric cholera.
Gastrointestinal side effects of macrolide administration
Previous clinical trials have documented significantly less vomiting in patients treated with azithromycin compared with erythromycin (1 vs 4, difference one episode, 95% CI 0–3 episodes, p = 0.023) [Citation19]. While vomiting is also a manifestation of cholera, the difference in the number of episodes of vomiting suggests that prolonged vomiting in patients treated with erythromycin may be attributed to an adverse effect rather than to the disease process itself. Azithromycin is therefore considered clinically superior to erythromycin because of its short-course requirement and subsequently diminished risk of gastro-intestinal side effects.
Antibiotic resistance and chemoprophylaxis regimens
Increasing the administration of antibiotics to children with less severe dehydration needs to be weighed against the effect it may have on antibiotic resistance in cholera. Alongside the clinical efficacy data discussed above, laboratory-based studies in Asia have found high levels of multi-drug resistance in strains of V. cholerae 01 in the past decade. A laboratory analysis of 302 strains associated with endemic cholera in Thailand found that 71% were resistant to erythromycin, 54% TMP-SMX, 23% to tetracycline and 31% to ampicillin, with 23% of the strains exhibiting multi-drug resistance [Citation48]. A 2012 study of 100 isolates in Vietnam (collected between 2007 and 2010) found all isolates were completely resistant to TMP-SMX and nalidixic acid, 29% were resistant to tetracycline and 85% exhibited multi-drug resistance (to nalidixic acid, TMP-SMX and tetracyclines), yet there was 95% susceptibility to azithromycin [Citation49]. Similarly, high levels of erythromycin and tetracycline resistance have been documented in laboratories in Dhaka [Citation50], while a laboratory analysis of 77 rectal swabs from patients presenting during cholera epidemics in Mozambique found high incidences of resistance to chloramphenicol (58%), TMP-SMX (97%) and tetracycline (97%) (yet quinolone resistance remained low at 4.2%) [Citation51].
These increasing resistance patterns must be taken into account when considering the appropriate first-line therapy for paediatric cholera and other interventions, such as the administration of chemoprophylaxis for contacts of patients with cholera. A systematic review and meta-analysis in 2011 found that chemoprophylaxis reduced infectivity rates (RR 0.39, 95% CI 0.29–0.51) and hospitalisation of contacts (RR 0.54, 95% CI 0.4–0.74) [Citation52], yet mass prophylaxis may lead to rising resistance rates in isolates, causing subsequently resistant clinical cases [Citation9,10]. Although families of patients with cholera are at high risk of contracting cholera themselves, they should receive targeted education about safe water and sanitation, plus appropriate administration of oral rehydration solution, rather than prophylactic antibiotic therapy.
Discussion
Cholera is an important cause of diarrhoeal illness, and the burden it imposes has increased over the past decade [Citation6]. It is responsible for 3 million cases and 100,000 deaths worldwide each year [Citation10], and 1.4 billion people live in places where cholera is endemic [Citation9]. Prevention of infection through adequate sanitation and access to clean water is paramount, and the cornerstone of treatment remains access to aggressive fluid rehydration which reduces mortality to <0.5% [Citation16].
Antimicrobial therapy decreases further production of the cholera toxin, and the current international literature supports antibiotic treatment of children with dehydration who require hospital admission during epidemics, when resources allow [Citation32]. The evidence demonstrates that antibiotic therapy reduces the volume of stool passed which diminishes the volume of rehydration required, minimises the burden on medical care in resource-constrained settings and reduces the transmission of infection.
The 2005 WHO guidelines listed tetracycline (12.5 mg/kg qid for 3 days) as the treatment of choice for children >2 years with severe dehydration, with an expanded list of antimicrobial choices published in the 2013 Pocketbook of Hospital Care for Sick Children (including doxycycline, TMP-SMX, erythromycin and chloramphenicol alongside zinc supplementation once vomiting has stopped) [Citation12]. However, this review has found increasing evidence of resistance to tetracyclines and ciprofloxacin for cholera infection in adults and children.
Alongside their patterns of increasing resistance, tetracycline antimicrobials are contraindicated in young children in higher income settings owing to their adverse effects.
The macrolide azithromycin has been shown to be clinically superior to tetracyclines in treating cholera infection in children, and the benefits of instituting this as first-line therapy in treating cholera outweigh the limited evidence to suggest macrolides are associated with cardiac arrhythmias by prolonging the QT interval. Moreover, in its class of antibiotics, azithromycin has been distinguished as one of the safest macrolides in terms of its cardiac side-effects. While single-dose erythromycin is inferior to azithromycin, when administered four times daily, it has been shown to exhibit similar clinical efficacy and bacteriological success in treating children with cholera [Citation46,47], although the regular and prolonged (3-day) course required makes adherence challenging. However, erythromycin is clinically superior to ciprofloxacin as an alternative therapy for cholera, and because of its lower cost and improved bacteriological clearance rates [Citation22,47] it is an appropriate second-line therapy for cholera in children, although the increasing resistance needs to be closely monitored.
Microscopy and susceptibility testing conducted in laboratories with external quality assurance should continue to be of paramount importance prior to commencing therapy, and if this is not locally available susceptibility testing from neighbouring regions should be used. Future research should continue to monitor the resistance profiles of antimicrobials used to treat cholera infection to diminish the spread of further antimicrobial resistance in V. cholerae infection, and monitor adverse effects of antimicrobials used to treat cholera infection in the paediatric population.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the World Health Organisation; The Nuffield Department of Medicine (The University of Oxford); General Sir John Monash Foundation; The Wellcome Trust [grant number MR/M007367/1]; and the Bill and Melinda Gates Foundation [grant number OPP1131320].
Notes on contributors
Phoebe C. M. Williams, MBBS(Hons.), received her medical degree from the University of Sydney and a master’s in Global Health Science from the University of Oxford. She is a paediatric registrar and dual trainee in Infectious Diseases at Sydney Children’s Hospital, Australia. She is a DPhil candidate through the University of Oxford, with her research focusing on antimicrobial resistance in paediatric patients.
James A. Berkley FRCPCH, MD is a professor of Paediatric Infectious Diseases at the University of Oxford based at the KEMRI–Wellcome Trust Research Programme in Kilifi, Kenya. He is the principal investigator of the CHAIN network with a research focus on serious infection and survival in highly vulnerable groups of infants and children.
Acknowledgments
We would like to thank the WHO Department of Newborn, Child and Adolescent Health for their valuable input to the conclusions arising from this review.
References
- Lutz M, Noorian P, Sun S, et al. Environmental reservoirs and mechanisms of persistence of Vibrio cholerae. Front Microbiol. 2013;4:1–15. DOI:10.3389/fmicb.2013.00375
- Harris JB, LaRocque RC, Charles R, et al. Cholera’s western front. Lancet. 2010;376:1961–1965.
- Harris R, Qadri F, Ryan E, et al. Cholera. Lancet. 2012;379:466–476.
- Viswanathan VK, Hecht G, Hodges K. Enteric infection meets intestinal function: how bacterial pathogens cause diarrhoea. Nat Rev Microbiol. 2009;7:110–119.
- Ali A, You Y, Kim Y, et al. The global burden of cholera. Bull WHO. 2012;90(3):157–244.
- Das A, Salam R, Bhutta Z, et al. Antibiotics for the treatment of cholera, shigella and cryptosporidium in children. BMC Public Health. 2013;Suppl. 3:S3–S10.
- Deen JL, von Seidlein L, Sur D, et al. The high burden of cholera in children: Comparison of incidence from endemic areas in Asia and Africa. PLoS Negl Trop Dis. 2008;2:e173.
- Koelle K, Rodó X, Pascual M, et al. Refractory periods and climate forcing in cholera dynamics. Nature. 2005;436:696–700.
- American Academy of Pediatrics. Vibrio cholera. In: Kimberlin M, Jackson M, Long S, et al., editors. Red Book, 2015. Report of the committee on infectious diseases. 30th ed. Elk Grove Village (IL): American Academy of Pediatrics; 2015. P. 850–853.
- Merrell DS, Butler SM, Qadri F, et al. Host-induced epidemic spread of the cholera bacterium. Nature. 2002;417:642–645.
- Griffith DC, Miller MA, Kelly-Hope LA. Review of reported cholera outbreaks worldwide, 1995–2005. Am J Trop Med Hyg. 2006;75:973–977.
- World Health Organization. Pocket book of hospital care for children: guidelines for the management of common illnesses with limited. Resources. 2013. Available from: http://apps.who.int/iris/bitstream/10665/43206/1/9241546700.pdf
- World Health Organization. The treatment of diarrhoea: a manual for physicians and other senior health workers. 4th revision. Available from: http://apps.who.int/iris/bitstream/10665/43209/1/9241593180.pdf
- Khan WA, Saha D, Rahman A, et al. Comparison of single-dose azithromycin and 12-dose, 3-day erythromycin for childhood cholera: a randomised, double-blind trial. Lancet. 2002;360:1722–1727.
- Nelson EJ. Antibiotics for both moderate and severe cholera. N Engl J Med. 2010;364:5–7.
- Centers for Disease Control and Prevention. Recommendations for the use of antibiotics for the treatment of cholera. 2015. Available from: http://www.cdc.gov/cholera/treatment/antibiotic-treatment.html
- Greenough WB, Rosenberg IS, Gordon RS, et al. Tetracycline in the treatment of cholera. Lancet. 1964;283:355–357.
- Lindenbaum J, Greenhough WB, Islam MR. Antibiotic therapy of cholera. Bull WHO. 1967;36:871–883.
- Kaushik JS, Gupta P, Faridi MMA, et al. Single dose azithromycin versus ciprofloxacin for cholera in children: a randomized controlled trial. Indian Pediatr. 2010;47:309–315.
- World Health Organization. Pocket book of hospital care for children: guidelines for the management of common illnesses with limited. Resources. 2005. Available from: http://www.who.int/maternal_child_adolescent/documents/9241546700/en/
- Balshem H, Helfand M, Schünemann HJ, et al. Grade guidelines 3: rating the quality of the evidence – introduction. J Clin Epidemiol. 2011;64:401–406.
- Guerrant RL, Van Gilder T, Steiner TS, et al. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001;32:331–351.
- Siddique AK, Nasim SMA. Guidelines for operating makeshift treatment centres in cholera epidemics. ICDDR, B Cent Popul Heal Res. 1997. Available from: http://dspace.icddrb.org/jspui/bitstream/123456789/4021/1/ICDDRBSpecialPub-61.pdf
- World Gastroenterology Guidelines. Acute diarrhoea in adults and children. 2012. Available from: http://www.worldgastroenterology.org/guidelines/global-guidelines/acute-diarrhea/acute-diarrhea-english
- Green M, Sack D, Alam N, et al. Cholera. Br Med J Best Practice. 2017. Available from: http://bestpractice.bmj.com/best-practice/monograph/451.html
- Cholera. eTG complete [Internet]. Melbourne: Therapeutic Guidelines Limited; [revised November 2014], 2015.
- Chattaway A, Fashae K, Okoro C, et al. Fluoroquinolone-resistant enteric bacteria in sub-Saharan Africa: clones, implications and research needs. J Front Microbiol. 2016;7:1–20. DOI:10.3389/fmicb.2016.00558.
- Mahapatra T, Mahapatra S, Babu GR, et al. Cholera outbreaks in South and South-East Asia: descriptive analysis, 2003–2012. Jpn J Infect Dis. 2014;67:145–156.
- Saha D, Karim M, Khan W, et al. Single-Dose azithromycin for the treatment of cholera in adults. N Engl J Med. 2006;354:2452–2462.
- Khan W, Saha D, Ahmed S, et al. Efficacy of ciprofloxacin for treatment of cholera associated with diminished susceptibility of ciprofloxacin to Vibrio cholerae 01. PLoS One. 2015;10:e0134921.
- Bhattaharya S, Ramamurthy T, Rajendran K, et al. Comparison between single-dose azithromycin and six dose, 3-day norfloxacin for treatment of cholera in adults. Int J Biomed Sci. 2014;10:248–251.
- Leibovici-Weissman Y, Neuberger A, Bitterman R, et al. Antimicrobial drugs for treating cholera. Cochrane Database Syst Rev. 2014; 6:1–182. CD0086.
- Wasif A, Khan AR, Salam MA, et al. Comparison of single-dose azithromycin and 12-dose, 3-day erythromycin for childhood cholera: a randomised, double-blind trial. Lancet. 2002;360:1722–1727.
- Saha D, Karim MM, Chowdhury HR, et al. Single-dose ciprofloxacin versus 12-dose erythromycin for childhood cholera: a randomised controlled trial. Lancet. 2005;366:1985–1993.
- National Committee for Clinical Laboratory Standards. Approved standards M2-A6 performance standards for antimicrobial disk susceptibility tests. 6th ed.; 1997. Available from: https://clsi.org/media/1631/m02a12_sample.pdf
- Gitanjali B. Essential medicines for children: should we focus on a priority list of medicines for the present? J Pharmacol Pharmacother. 2011;2:1–2.
- Kabir W, Haider R, Mitra A, et al. Erythromycin and trimethoprim-sulphamethoxazole in the treatment of cholera in children. J Diarrhoeal Dis Res. 1996;14:243–247.
- Burans J, Podgore J, Mansour MM, et al. Comparative trial of erythromycin and sulphatrimethoprim in the treatment of tetracycline-resistant Vibrio cholerae 01. Trans R Soc Trop Med Hyg. 1989;83:836–838.
- Khan WA, Bennish ML, Seas C, et al. Randomised controlled comparison of single-dose ciprofloxacin and doxycycline for cholera caused by Vibrio cholerae 01 or 0139. Lancet. 1996;348:296–300.
- Shentag JJ. Tissue directed pharmacokinetics. Am J Med. 1991;91:40–45.
- Das SK, Klontz EH, Azmi IJ, et al. Characteristics of multidrug resistant shigella and Vibrio cholerae 01 infections in patients treated at an urban and a rural hospital in Bangladesh. ISRN Microbiol. 2013; 2013:8. Article ID 213915. DOI:10.1155/2013/213915
- Hancox M, Vieweg V, Crouse E, et al. Azithromycin, cardiovascular risks, QTc interval prolongation, torsade de pointes, and regulatory issues: a narrative review based on the study of case reports. Ther Adv Infec Dis. 2013;1:155–165.
- Owens RC, Nolin TD. Antimicrobial-associated QT interval prolongation: pointes of interest. Clin Infect Dis. 2006;43:1603–1611.
- Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000;38:41–57.
- Samarendra P, Kumari S, Evans SJ, et al. QT prolongation associated with azithromycin/amiodarone combination. Clin Electrophysiol. 2001;24:1572–1574.
- Ray W, Murray K, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890.
- Howard P. Azithromycin-induced proarrhythmia and cardiovascular death. Ann Pharmacol. 2013;47:1547–1551.
- Chomvarin C, Johura FT, Mannan SB, et al. Drug response and genetic properties of Vibrio cholerae assocated with endemic cholera in north-east Thailand, 2003–2011. J Med Microbiol. 2013;62:599–609.
- Tran HD, Alam M, Trung N, et al. Multi-drug resistant Vibrio cholerae 01 variant El Tor isolated in northern Vietnam between 2007 and 2010. J Med Microbiol. 2012;61:431–437.
- Rashed S, Mannan S, Joshura F, et al. Genetic characteristics of drug-resistant Vibrio cholerae 01 causing endemic cholera in Dhaka, 2006–2011. J Med Microbiol. 2012;44:4211–4213.
- Mandomando I, Espasa M, Vallès X, et al. Antimicrobial resistance of Vibrio cholerae 01 serotype Ogawa isolated in Manhiça District Hospital, southern Mozambique. J Antimicrob Chemother. 2007;60:662–664.
- Reveiz L, Chapman E, Ramon-Pardo P, et al. Chemoprophylaxis in contacts of patients with cholera: systematic review and meta-analysis. PLoS ONE. 2011;6:e27060.
- Christopher P, David K, John S, et al. Antibiotic therapy for Shigella dysentery. Cochrane Database Syst Rev. 2010;8. DOI:10.1002/14651858.CD006784.pub4
- British National Formulary for Children. BMJ Publishing Group; 2016. Available from: https://www.medicinescomplete.com.acs.hcn.com.au/about/publications.htm