Abstract
The blood-brain barrier (BBB) is a multicellular neurovascular unit that serves as a highly impermeable cellular barrier to regulate brain homeostasis, protect the central nervous system, and respond to different physiological and pathological states. Recently, new drugs have been discovered to treat neuropathology. However, because BBB blocks the delivery of drugs to the central nervous system (CNS), it is still difficult to translate the treatment of CNS diseases into clinical results. Therefore, overcoming the BBB and realizing efficient delivery of therapeutic drugs is of great significance for the diagnosis and treatment of various neurological diseases such as Alzheimer’s disease, Parkinson’s disease and glioblastoma. Nano drug delivery system (NDDS) has the characteristics of high biocompatibility, high drug load, improving pharmacokinetic behavior of drugs in vivo, achieving targeted drug delivery, controlled drug release, etc. Therefore, in the field of trans-BBB drug delivery, It has broad application prospect for the diagnosis and treatment of various neurological diseases. This paper reviews the latest research on NDDS crossing BBB at home and abroad, providing new ideas for the diagnosis and treatment of CNS diseases.
1. BBB
The blood-brain barrier (BBB) is a multicellular neurovascular unit that acts as a highly impermeable cellular barrier which tightly regulates brain homeostasis. It is mainly composed of vascular endothelial cells, and physically connected with astrocyte end-feet and pericytes embedded in the vascular basement membrane to form continuous tight junctions (TJs) [Citation1]. Explicitly illustrated in , the end-feet of astrocytes represent nearly 99% of the luminal surface area of brain capillaries and extend into the vessel wall. Pericyte contributes to maintain and stabilize the monolayer of endothelial cells in the brain, and is essential for constituting the TJs. The interaction of these three types of cells makes the BBB a dynamic structure to regulate brain homeostasis, protect the central nerve, and respond to different physiological and pathological states, limiting the simple diffusion of blood-borne pathogens to the CNS. Also, the BBB relies on other biological macromolecules such as microglia and enzymes, to form physical, metabolic and immune barriers, strictly restricting the entry of drugs into the brain. About 98% of small molecules and almost all large molecules (for example: monoclonal antibodies and recombinant proteins), they cannot pass through the BBB. The movement of pharmacological agents from the blood circulation into the brain is influenced by the molecular weight, size, surface charge, hydrophobicity, hydrogen bond, and the affinity of the drug or drug carrier to the receptor expressed in the BBB. Obviously, the modification of the drug can reduce such effects, so as to achieve the effect of crossing the BBB.
Figure 1. Schematic diagram of the BBB [Citation2].
![Figure 1. Schematic diagram of the BBB [Citation2].](/cms/asset/87ca47bb-ac29-4e92-a28a-df07a0113d5d/ynan_a_2256466_f0001_c.jpg)
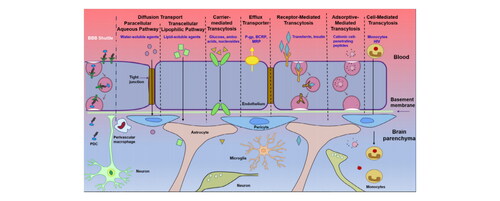
In the past decade, there are many explorations into trans-BBB delivery systems. The following are several solute or substance transport mechanisms through the BBB [Citation2]. First, some molecules of gases (such as O2 and CO2) and small lipophilic molecules (less than 400 Da) can be smoothly dispersed outside the endothelium. Water-soluble agents are transported through the paracellular pathway; lipid-soluble small agents, such as corticosteroids and alcohol, cross BBB by simple diffusion. Besides, adsorption-mediated endocytosis (AMT), receptor-mediated transport (RMT), the use of carrier, cell-mediated endocytosis and inhibition of efflux pumps are other trans-BBB mechanisms.
Now we need urgently innovative therapeutic strategies and drugs that can cross BBB and reach the focal location for enhancing the pharmacokinetic ability of the drugs and achieving higher drug concentrations in the brain. To solve this problem, so far, several targeting techniques have been developed, combined with nanoparticles (NP), to achieve highly efficient targeting across the BBB. There are several ways of BBB shuttling illustrated in [Citation2]. This paper reviews mainly RMT [Citation2], carrier-mediated exocytosis, and cell-mediated biomimetic membrane technology. It is no doubt that RMT has increasingly become a widely used method in BBB delivery research, while the research of cell membrane biomimetic technology (CMBT), such as exosomes (Ex), continues to rise, and gradually become a new delivery method.
Figure 2. Transport mechanism of the solute or substance BBB shuttle [Citation2].
![Figure 2. Transport mechanism of the solute or substance BBB shuttle [Citation2].](/cms/asset/b2bcc1c0-6b2f-4da5-8f95-2f1e197278dc/ynan_a_2256466_f0002_c.jpg)
The development of nanotechnology and its application in clinical drug preparation provide new ideas for the treatment of neurological diseases. NPs, ultrafine microparticles, generally refer to solid colloidal particles in the size range of 1 to 100 nm. The special properties of nanomaterials with large specific surface area give it special function in the nano and molecular level range. For example, modified hybrid nanoparticle drugs can make them easier through BBB and reach target sites such as brain tumors. So they are now used in the treatment of CNS disease. At the same time, Nano drug delivery system (NDDS) has the advantages of increasing drug solubility and prolonging the retention time of drugs in the body.
2. Pure trans-BBB nanomaterials
Recently, the promise of various nanomaterials in cancer treatment has been explored. Indeed, these agents have many advantages over conventional drugs, including lower systemic toxicity, specific targeting, and small volume, thus enabling them to target specific tumor cells through BBB. Nanoparticle (NPs) can be divided roughly into polymer nanoparticles, lipid nanoparticles, and inorganic nanoparticles. As shown in the , a wide variety of NPs were designed and used as carriers to provide drugs to the CNS, such as liposome [Citation3–5], polymeric NPs, lipid NPs [Citation3, Citation6] iron oxide NPs, gold NPs [Citation7], carbon group nanomaterials [Citation8], silica NPs [Citation9] and graphene quantum dot [Citation10–12].
Figure 3. A wide variety of NPs [Citation12].
![Figure 3. A wide variety of NPs [Citation12].](/cms/asset/a6c33399-d568-4979-95b5-cdd37e89bbd0/ynan_a_2256466_f0003_c.jpg)
In the latest progress of nanotechnology, the new strategy of crossing BBB is based on ultra-small NPs encapsulating drugs. Passive diffusion is the main mechanism of payload accumulation in the brain. As a drug carrier [Citation13, Citation14], NPs has ligands, antibodies, pegylation (PEG) or other carriers that enable actively transport the drug to the brain by targeting the receptor in the BBB, which is a two-step mechanism, namely binding to the endothelial membrane and heterogenetic exposure of receptors acting across cells. This should be an integral feature of the nanoconjugation-transcellular pathway of action. Ultra-small drug-free NPs and quantum dots (QDs) [Citation10, Citation11], can be designed according to composition, size, shape, and surface ligands; these structures can respond to external stimuli such as temperature, light (e.g. near-infrared light), magnetic/electric fields, or ultrasonic energy. For example, these drug-free NPs in phototherapy show considerable therapeutic effects on brain tumors.
Carbon group nanomaterials are composed of different carbon-based structures, including zero-dimensional fullerenes, carbon points, one-dimensional carbon nanotubes [Citation15] (single-walled and multi-walled), two-dimensional graphene and nanodiamonds. Different structures have different properties and are used as “vehicles” for drug delivery into the brain. Recently, graphene nanostructures [Citation8] showed great potential to span the BBB with special properties, such as high electron mobility, ease of synthesis and functionalization, and control of size, shape, and drug release profiles. Graphene nanostructures, present alone or in other materials, such as functionalized parts, ligands, proteins, receptors, and drugs, have been explored for treating brain diseases due to their enhanced ability to across BBB. In fact, some drugs are used for schizophrenia. For example, aripiprazole and paliperidone palmitate are mainly composed of nanocrystals, while the main ingredients of buprenorphine is poly(lactic-glycolic)NPs [Citation16].
Shiravalilou et al. synthesized a magnetic nanoparticle (MNPs) [Citation17] loaded with 5-iodine-2-deoxyuridine (IUDR) coated with PLA-hydroxyl acetate copolymer (PLGA) for targeted delivery of IUDR to glioblastoma multiforme (GBM). The results of in vivo experiments showed that compared with the control group, the tumor volume of GBM model rats in the IUDR/MNPS group was reduced significantly, and the survival time increased by 6 times. Similar results have been reported in other studies using similar radiation intensity. Kargar et al. developed a new targeted drug delivery systems (DDSs) using PLGA functionalized nano graphene oxide as carrier [Citation18] and IUDR as radiosensitizer. The application of IUDR-PLGA-NGO, an attractive treatment for GBM with synergistic effects, makes the combination of photothermal therapy and radiosensitizers. In addition to the applications of drug delivery [Citation18–20], they can be used as a CNS therapeutic agent [Citation21], and even some carbon nanostructures have neuroregenerative activity, while their effects on neuronal growth and anti-amyloid effects deserve attention.
Based on the key role of microglia in the regulation of AD microenvironment, the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, described in article [Citation22] the development of Ruth Blue/Polyamide Dendrimer/Vasculitis-2 (PPA) -NPs. The NPs can regulate the mitophagy of microglia as a potential treatment for AD. PPA-NPs exhibit superior BBB permeability and exert a synergistic effect in scavenging reactive oxygen free radicals and restoring mitochondrial function in microglia. Meanwhile, PPA-NPs reduced effectively the neurotoxic Aβ aggregates, thus rescuing the cognitive function in the mouse model.
3. Nanocomposite
We adopts a relatively novel classification method, which is generally divided into two categories through the difference or different forms of nanomaterials, as summarized in . One is simple nanoparticles as drugs. As its small molecular diameter and other characteristics, it can cross the BBB through passive diffusion, and accumulate in the brain, so as to play the role of diagnosis and treatment. Another category is the combination of nanomaterials and related transmembrane modes. To elucidate, this nanocomposites mode include receptor-mediated, carrier-mediated except for nanocarriers (such as peptide mediated, virus mediated), membrane-coated with bionic system modification.
Table 1. There is a relatively novel classification method, which is generally divided into two categories through the difference or different forms of nanomaterials.
3.1. Receptor-mediated trans-BBB nanomaterials
The RMT [Citation23, Citation44] is the most important pathway for the transport of biological macromolecules through the BBB. This is because most of these biological macromolecules, such as immunoglobulins and growth factors, are unable to cross the BBB alone, and the RMT systems used to recognize the ligands are often highly specialized. Therefore, only some molecules can be transported between the blood and the brain through this system, such as transferrin (Tf), folate, insulin, and leptin. They can be transported through their receptors, namely transferrin receptor (TfR), folate receptor (FR), insulin receptor (IR), and leptin receptor. There are also lipoprotein receptors on the BBB surface (such as low-density lipoprotein receptor-associated proteins (LRP)), which mediates the transport of lipoproteins through the BBB. Meanwhile, antibodies against the above receptors can also provide transport to the BBB via the RMT system.
3.1.1. Transferrin receptor (TfR)
Transport rates of biological macromolecules through the BBB are slower than those for nutrients. The demand for iron in highly proliferating cells (such as cancer cells) increases dramatically, which leads to an increase in the amount of TfR expression. This finding makes the TfR on these cells excellent candidates for tumor-targeted therapy. TfR is one of the most commonly used receptors for targeted delivery to the brain, primarily because of the high expression of this receptor on endothelial cells.
TfR has been used extensively in studies targeting CNS delivery of drugs. In the context of the delivery of therapeutic NPs to the brain, studies have utilized a small group of rare lysosomal storage disorders with abnormal lipid metabolism caused by acid sphingomyelinase deficiency, called Niemann-Pick disease (NPD) [Citation45]. In the disease model, investigators found that cell surface levels of TfR, clathrin heavy chain expression, cellular transmigration of anti-TfR and brain accumulation in mice would be reduced. LI et al. linked the tau aptamer to the TfR aptamer to form a nanostructure [Citation23], and this group of suitable ligands would facilitate trafficking of the construct through TfR-mediated transcytosis at the BBB cell membrane. In both in vitro and in vivo studies, modified tau-TfR bispecific aptamers still had strong binding capacity with tau protein, showing the ability to enhance plasma stability and improve BBB permeability, and reducing effectively the levels of relevant biomarkers and the protective effect for impaired memory recovery in traumatic brain tissue induced by traumatic brain injury.
There are also many studies on the treatment of tumor brain metastasis. EGFR TKI target therapy has become the first-line treatment for patients with non-small-cell lung cancer (NSCLC). Programmed cell death ligand-1 (PD-L1) is an immune checkpoint that is overexpressed not only on tumor cells, but also on tumor-associated immune cells. Yongzhuo Huang research group of Shanghai Institute of Materia Medica, Chinese Academy of Sciences developed T12 peptide and anti-PD-L1 modified liposomes (T12/P-Lipo)NPs [Citation3] for simvastatin/gefitinib treatment of NSCLC brain metastasis (BMs). The T12 peptide is a TfR binding peptide that can mediate BBB penetration and targeting of brain tumors, with its binding site with TfR distinct from Tf, thus avoiding competition for binding with endogenous Tf. Therefore, it is expected that the dual-targeting strategy can overcome BBB, directly target BMs and tumor immune microenvironment (TIME), and improve the therapeutic efficacy.
3.1.2. Folate receptor (FR)
Folic acid (FA) is essential for CNS function. FA transport is mediated by 3 major pathways, namely reduced folate carriers, proton-coupled folate transporters, and FRα, which are known to be regulated by ligand-activated nuclear receptors. FRα occurs in various epithelial cells of the CNS (e.g., choroid plexus). Folate transport into and out of the CNS occurs at the blood-cerebrospinal fluid barrier (BCSFB), mediated by FRα and PCFT. Impairment of folate transport at the BCSFB results in cerebral folate deficiency in infants characterized by severe neurological deficiencies and seizures. Therefore, there is no doubt that folate receptors are highly expressed in the human BBB. The primitive and FRα-modified NPs showed good compatibility with primary human choroid plexus epithelial cells (CPEC) [Citation24]. The PEGb-PCL NPs surface modified with the FRα-FA complex had a better ability of the NPs to span the CPEC monolayer than their unmodified counterparts. It is clear that FR is promising in drug delivery to CNS diseases.
To date, TfR [Citation46–48], FR [Citation24, Citation49–51] have been widely used in targeted CNS delivery of therapeutic antibodies, enzymes and chemotherapeutic agents. The application of IR [Citation25] and LRP-1 [Citation26] in NDDS has also been studied and will not be discussed here.
3.2. Carrier-mediated nanomaterials across the BBB
In order to develop specific brain-targeted DDSs, it can be used that various types of solute carriers highly expressed on BBB. The use of substrate corresponding to the solute vectors to synthesize precursor drugs or to connect to the DDSs is solute carrier-mediated across BBB. At present, the commonly used mediated carriers include: nanocarrier, polypeptide carrier, viral vector and so on.
Molecular carriers–known as BBB shuttle carriers–hold great promise for safely overcoming this serious obstacle of BBB. In recent years, shuttle peptides have received increasing attention due to their lower cost, low immunogenicity, and higher chemical versatility compared to conventional Trojan antibodies and other proteins. Here we will use the word peptide to refer to small proteins (with or without structure) that contain up to 50 amino acid residues. Simply, we divided shuttle peptides into two categories. One type of BBB shuttle peptide can increase intake of brain drugs in a non-selective way, mainly belonging to the cell-penetrating peptides (CPP) family. CPPs consists of short amphiphilic and (or) cationic sequences, with the ability to cross the cell membrane (CM) without the receptor, which is believed generally to have the ability to cross CM by the AMT mechanism. For example, the CPP-modified PLGA-NPs [Citation52] achieved significant brain transfer efficiency via nasal route, and the non-CPP-modified brain transmission efficiency was only 1.36% as reported by Lu et al. Significantly, incorporation of CPP into the NLC surface increased NPs transport to 46%, and CPP-CS-NLC [Citation27] for nose-to-brain drug delivery represents a new promising brain shuttling. Albumin-binding proteins (e.g., SPARC and gp 60) are overexpressed in many tumors. Transport albumin acts as an amino acid to provide energy for rapidly growing cancer cells. BBB penetration, tumor infiltration, and cellular uptake of albumin nanoparticles were enhanced by the modification of CPP LMWP [Citation53].
Another type of BBB shuttle peptide needs to bind to transporter, and most of these peptides are screened from neurotropic biomolecules or phage. Natural polypeptides or proteins targeting the brain can be endogenous, such as hormones and apolipoproteins, or exogenous, such as certain viruses and neurotoxins. The chimeric peptide [Citation2], chimeric compounds as the name suggests, are not exclusively transported through the BBB and must be bound to a transport carrier in a covalently bound form usually, for efficient transport [Citation54]. These conjugation vectors can be endogenous polypeptides, modified proteins, monoclonal antibodies and peptidomimetic antibodies, etc. There are different transport pathways behind the construction of these peptides, such as the peptide-specific receptor DWSW (DSDYDPDGDWDSDW) peptide [Citation28], which effectively assists in crossing the BBB and shows measurable tumor targeting, providing an effective platform for targeted treatment of glioma.
Here, we present several successful studies of central drug delivery using different polypeptide carriers. For example, scientists at the Sizaki Institute of Biomedical Innovation covalently attached six peptide carriers to a 50 kDa poly (β-L-malate)-trileucine polymer to form the P/LLL carrier complex [Citation13]. The constructed vectors are vascular endothelin-2 (AP2), B6, Miniap-4 (M4) and D configuration peptide D1, D3 and ACI-89, with specificity for TfR, LRP-1, bee venom potassium ion channel and Aβ/LRP-1 associated transcellular complex, respectively. The experimental results showed that the BBB penetration efficiency is significantly improved (40%) compared with the control group P/LLL.
According to statistics, GBM is the most common malignant primary tumor in intracranial, accounting for 57% of all gliomas and 48% of CNS primary malignant tumors. As an important part of TIME, tumor associated macrophages (TAMs) can account for 30% to 50% of GBM mass, and mainly exhibit tumor supportive M2 TAMs rather than tumor killing M1 TAMs. Therefore, M2 TAMs have emerged as novel biomarkers for GBM immunotherapy and immunoimaging. After study, the M2PEP peptide with sequence YEQDPWGVKWWY has been shown to selectively and preferentially target M2 TAMs to treat a variety of tumor [Citation29–32]. In order to target M2 TAMs, Li Zhen and coworkers Team designed and prepared a novel M2 targeted imaging nanoprobe [Citation54] based on rare earth nanoparticles and M2 targeted polypeptides. Based on the NIR-IIB window (1500–1700nm), nanoprobes are efficiently delivered and accumulated in the tumor to form accurate imaging of M2 TAMS, which can be better used for M2 targeted imaging of GBM and help guide treatment options. It is clear that polypeptide carriers play an important role in the CNS drug delivery of nanomaterials.
First, the temporary destruction of BBB caused by ultrasound, mechanical action or heat production mediated by NPs can be used for reversible BBB opening. However, the destruction of the invasive BBB also allows the access of harmful substances to the CNS, possibly causing irreversible damage to the normal function of the CNS. Moreover, nanomedicines decorated with CPPs can pass through the AMT or RMT, with cross-BBB capability. However, the traversal efficiency of BBB still depends on the selectivity and affinity of CPPs. Accordingly, a selective and efficient way to open BBB has been developed, bionic membrane technology.
3.3. Cell-mediated biomimetic nanomaterials
Cell-mediated endocytosis. Cell membranes and composition of the blood-brain barrier both have a typical lipid bilayer structure. Specifically, cell membrane-coated NPs inherently mimic the surface properties of the source cells and thus acquire many unique characteristics, such as superior biocompatibility, decreased uptake by macrophage cells, prolonged circulation lifetimes, and enhanced BBB and tumor penetration. It is an excellent delivery system.
Based on endogenous immune cells (such as single cells, macrophages (MØ) and neutrophils) or stem cells, cell-mediated trans-BBB () can respond to immune factors or enzymes in the brain to achieve effective brain drug delivery. Although there are various modification ways of NPs, extracting CM and coating it on the surface of NPs to modify it is a unique method of functionalization of NPs, namely membrane biomimetic [Citation1, Citation3, Citation41, Citation55, Citation56]. below shows a simple diagram of membrane coating by extrusion or sonication [Citation40]. There are three possible results: uncoated, partially coated, and completely coated. In order to probe the integrity of the cell membrane coating, LIU L and coworkers developed a fluorescence quenching assay [Citation40]. They hypothesized that if the NPs were fully coated, then the fluorescence signal would remain after the addition of the DT quencher into the solution. In contrast, if the NPs were only partially coated or totally uncoated, the fluorescence intensity would progressively disappear due to the reduction of the NBD dye with DT. Therefore, by measuring the remaining fluorescence, the proportion of fully coated NPs would be calculated. Based on the homologous targeting mechanism, as shown in , the research team used ultrasonic fusion method to mix cancer cell membrane (CCMs) with paclitaxel (PTX) nanosuspension (NS), developed a bionic CCM- (PTX) NS [Citation28]. And the DWSW-CCM-(PTX)NS with trans-BBB and tumor targeting function was obtained by adding DWSW peptide modification on the membrane surface using liposome insertion method. CCM camouflages the NS effectively so that it is not cleared by the immune system and is able to cross the BBB and target tumor tissue selectively for action.
Figure 4. Simple simulation diagram of biomimetic nanomaterials [Citation40].
![Figure 4. Simple simulation diagram of biomimetic nanomaterials [Citation40].](/cms/asset/4b2a75bc-0c4b-4daa-be7f-acbac1407f0a/ynan_a_2256466_f0004_c.jpg)
Figure 5. Preparation of a biomimetic nanosuspension (NS) [Citation28].
![Figure 5. Preparation of a biomimetic nanosuspension (NS) [Citation28].](/cms/asset/f7603718-6a41-4fcf-b04b-7e314ae96133/ynan_a_2256466_f0005_c.jpg)
CM wraps are a novel drug delivery carrier and biomimetic membrane technology. Due to the cell adhesion molecules present in the source cells, biomimetic NPs still exhibit source cell-specific targeting capability [Citation40], while CMs can also reduce the risk of inflammation because of the lack of cell activity. Membrane coating provide the stealth capability for drug-loaded nanomaterials, avoiding the interception of the reticuloendothelial system, and prolong blood circulation, thereby increasing the drug concentration in the brain parenchyma [Citation55]. It has been reported that antiretroviral accumulation of drugs (such as, indinavir, ritonavir and efavirenz) in mononuclear phagocytes is higher than that of negatively charged nanocarrier alone [Citation57]. Therefore, in recent years, CM has received more and more attention in developing intelligent DDSs with high drug loading content, enhanced brain targeting, and multiple therapeutic modalities.
According to the recent studies on CM biomimetic material in BBB shuttle, a series of specific cells from the body, including blood cells, cancer cells [Citation28, Citation39], extracellular vesicles (EV), etc. have been used for nervous system delivery through cell-mediated transport.
3.3.1. Blood cells
Studies found that MØ, neutrophils and platelets in blood cells can be used in biomimetic membrane technology. Among these, MØ is one of the largest number of cells in the tumor microenvironment. MØ interacts with the vascular cell adhesion molecule-1 of cancer cells through α4 and β1 integrins to promote the delivery of biomimetic material to tumor sites, thus achieving effective tumor targeting [Citation35, Citation36]. Therefore, the biomimetic MØ membrane coating strategy is expected to make drug-loaded nanomaterials with BBB penetration and tumor targeting as effective therapies. UZPM (an optical response system) is introduced into MØ by functional liposome fusion and modified with hydroxylamine groups on the cell surface. Aldehyde-modified cytotoxic T lympxhocyte-associated protein-4 is used as a chimeric antigen receptor (CAR) targeting group to target precisely the central M1 microglia via aldehyde-hydroxylamine condensation on the MØ surface to generate CAR-M-UZP [Citation34]. It has shown in vitro and in vivo experiments that CAR-M-UZPM DDSs can penetrate effectively BBB, targeting accurately central activated microglia, thus inhibiting M1 polarization of microglia, which reduces effectively the expression of central proinflammatory factors and produces continuous vaccine-like anti-inflammatory effect to treat the occurrence and development of inflammation-related depression [Citation34]. The study did not continue on whether it is applicable to other brain diseases treatment methods has not been further developed and needs to be explored in future studies.
Because of the short life span and limited action radius of reactive oxygen species, the efficacy of tumor photodynamic therapy is limited. If the photodynamic drugs are endowed with good ability of targeting cancer cell, it can shorten the distance between the photodynamic drugs and the tumor cells, thus improving the efficacy. Platelets can naturally target many tumor cells. By coating platelet membrane [Citation37] on the surface of photodynamic NPs, photodynamic nanodrugs with various advantages such as tumor cell targeting and long blood circulation are produced. The resulting platelet membrane-coated NPs are significantly harvested by tumor cells rather than normal fibroblasts; in contrast, the red blood cell membrane-coated NPs did not show this tumor cell selectivity. Therefore, at the skin-tolerated doses of sun-light, platelet membrane-coated nanoparticles showed stronger tumor cytotoxicity and better photodynamic therapeutic efficacy than red cell membrane-coated NPs both in vitro and in vivo teats.
3.3.2. Cancer cell membrane (CCM)
CM-coated NPs have unique functions of specific cells. For example, CCM-coated NPs that can specifically identify tumors for homologous targeted diagnosis and treatment. The CCM-coated lanthanide-doped nanoparticles (LnNP) in the near-infrared IIb window (NIR-IIb, 1500–1700nm), are designed for brain tumor imaging and surgical navigation. [Citation38] is a schematic diagram of this technique. The package of CCM conferred the ability of CC-LnNPs for immune escape, BBB shuttling, and cognate tumor targeting. Glioma tissue (size <3 mm, depth > 3 mm) guided by NIR-IIb fluorescence can be clearly visualized and completely removed. This study provides new ideas for the future design of nanoprobes for brain tumor imaging, accurate diagnosis and surgical navigation.
Figure 6. near-infrared IIb window, CC-LnNPs are used for accurate imaging and diagnosis and treatment technology in brain tumors [Citation38].
![Figure 6. near-infrared IIb window, CC-LnNPs are used for accurate imaging and diagnosis and treatment technology in brain tumors [Citation38].](/cms/asset/61e43a04-21e4-49b7-938e-910eef440fc0/ynan_a_2256466_f0006_c.jpg)
Currently, with its high sensitivity and fast signal acquisition, fluorescence imaging is the most common technique in GBM diagnosis. SU J team [Citation41] prepared the CM of mouse skin melanoma modified with TfR ligand as a shell, endowed the dual BBB targeting of TMPSM receptor mediated and CM biomimetic coating. Overall, TMPSM is for the first time based on self-assembly imaging triggered by transglutaminase 2 in a single cell and siRNA therapy, simultaneously achieving accurate diagnosis and effective treatment of GBM.
3.3.3. Vesicle system– Ex
EV is a bilayer carrier composed of multiple amphiphilic components. EV has different subgroups, including Ex, metastatic body and viron, and the most popular study is the Ex subgroup. Ex comes from intramembranous sprouting of polyvesicles. It is a nanoscale lipid inclusion structure with a diameter of 50–150nm, which is coated with protein, mRNA, microRNA and other substances, and is a key messenger of intercellular communication. Because of its small size, good biocompatibility, non-immunogenicity, and persistent blood circulatory capacity, it can serve as an excellent nanocarrier for drug and gene delivery [Citation4, Citation58]. That is the main advantage of Ex over other artificial materials. The Ex fraction isolated from brain endothelial cells acts as a regulator to exchange compounds inside and outside the BBB, maintaining cell-cell signals in the brain, and its surface proteins can communicate with primary astrocytes and cortical neurons. While the interaction between Ex and brain cells is attributed to the receptor on Ex, which can carry macromolecules across the BBB.
By patching doxorubicin-loaded heparin-based nanoparticles (DNs) onto the surface of natural grapefruit EVs, experimenters at the Institute of Oncology, School of Basic Medical Sciences, Southern Medical University have fabricate biomimetic EV-DNs, achieving efficient drug delivery and thus significantly enhancing antiglioma efficacy. Compared with traditional EVs, the capacity of drug loading of patched EV-DNs has increased by 4-fold [Citation5, Citation42]. The potential of Si X L et al. to use Ex to diagnose PD comes from the accumulation of α-syn, which is critical for transmission between neurons via Ex. Ex release is stimulated by inflammasome activation of three Pyrin-containing proteins of the NLR family (NLRP3) [Citation59] and subsequent release of pro-inflammatory cytokines in microglia, an important event associated with PD progression.
Recently, Tingting Wu and her research team [Citation43] used an engineered MØ-Ex modified biodegradable nanodrug carrier to achieve precise sonodynamic therapy (SDT) for GBM. After loading ICG (a three-carbon cyanine dye with near-infrared characteristic absorption peaks), CAT@SiO2-ICG (CSI) was transfected into a mono-core MØ-Ex with AS1411 aptamer modification (Ex-A). Ex-A modification endows CSI@Ex-A with efficient BBB penetration and cancer cell targeting. Thus, the obtained CSI@Ex-A achieves targeted imaging and enhanced SDT of GBM, demonstrating good biomedical applications and potential for further clinical applications.
4. Conclusion
In the nutshell, BBB plays a crucial role in maintaining brain homeostasis and drug transport to the brain. Brain disease remain a significant health, social and economic burden, and translating treatment into the clinic is a challenge that needs to be overcome in current research. NDDS represents a promising area for technological investment in improving drug bioavailability and for delivery to the brain. Among them, bio-nanomaterials are of increasing interest. There are various ways across BBB, and various combination strategies are adopted in the research process. Especially, in order to overcome the safety and toxicity problems of using nanocarriers, the utilization of CMBT can play an important role. Meanwhile, the combination of nanotechnology with multiple trans-BBB mode improves the shuttle efficiency clearly. For example, there are polypeptide carrier combined with RMT, polypeptide carrier combined with nano-carrier, CM or liposome shell and RMT, and peptide carrier and CMBT. At present, a small part of the preliminary studies have been used in clinical practice, and most of them are still in the experimental stage, so more mature methods of drug delivery are needed to improve cure rates of brain diseases.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Jinyu Wang
Jinyu Wang, graduate student, Department of Neurology, Shanxi Medical University, affiliated to the Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital.
Yaqin Yu
Yaqin Yu, Doctor of Neurology, Shanxi Medical University. She is affiliated to the Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital.
Chongqing Zhang
Chongqing Zhang, Ph.D. student at Harbin Medical University. He specializes in modeling across the blood-brain barrier (BBB), and is developing cancer cell membrane-coated NPs across the BBB.
Junling Song
Junling Song, master of Neurology, Department of Neurology, Shanxi Medical University. He is affiliated to the Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital.
Ziliang Zheng
Ziliang Zheng, Lecturer, Master Tutor, Shanxi Medical University. He received his PhD in Chemical Engineering and Technology (double first-class) from Taiyuan University of Technology and joined Shanxi Medical University in 2017. He is a member of the nano-oncology Professional Committee of Shanxi Anti-Cancer Association and deputy director of Shanxi Engineering Research Center of “Nano-biomedicine”.
Weihong Yan
Weihong Yan, M.D. deputy director of the Department of Neurology of Shanxi University Hospital, member of the Youth Council of China Stroke Society, chairman of the Professional Committee of Movement Disorders in Shanxi Province, member of the Neurology Branch of Shanxi Medical Association, and member of Shanxi Doctors Association.
References
- Furtado D, Björnmalm M, Ayton S, et al. Overcoming the Blood-Brain barrier: the role of nanomaterials in treating neurological diseases. Adv Mater. 2018;30(46):e1801362. doi: 10.1002/adma.201801362.
- Zhou X, Smith QR, Liu X. Brain penetrating peptides and peptide-drug conjugates to overcome the blood-brain barrier and target CNS diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13(4):e1695. doi: 10.1002/wnan.1695.
- Yin W, Zhao Y, Kang X, et al. BBB-penetrating codelivery liposomes treat brain metastasis of non-small cell lung cancer with EGFR(T790M) mutation. Theranostics. 2020;10(14):6122–6135. doi: 10.7150/thno.42234.
- Poustforoosh A, Nematollahi MH, Hashemipour H, et al. Recent advances in bio-conjugated nanocarriers for crossing the blood-brain barrier in (pre-) clinical studies with an emphasis on vesicles. J Control Release. 2022;343:777–797. doi: 10.1016/j.jconrel.2022.02.015.
- Zhang Y, Yang D, Nie J, et al. Transcranial non-genetic neuromodulation via bioinspired vesicle-enabled precise NIR-II optical-stimulation. Adv Mater. 2022;2022:e2208601.
- Xie Y, Ye L, Zhang X, et al. Transport of nerve growth factor encapsulated into liposomes across the blood-brain barrier: in vitro and in vivo studies. J Control Release. 2005;105(1–2):106–119. doi: 10.1016/j.jconrel.2005.03.005.
- Sztandera K, Gorzkiewicz M, Klajnert-Maculewicz B. Gold nanoparticles in cancer treatment. Mol Pharm. 2019;16(1):1–23. doi: 10.1021/acs.molpharmaceut.8b00810.
- Llenas M, Sandoval S, Costa PM, et al. Microwave-assisted synthesis of SPION-reduced graphene oxide hybrids for magnetic resonance imaging (MRI). Nanomaterials. 2019;9(10):1364. doi: 10.3390/nano9101364.
- Kaur S, Silveira Fiates AL, Rezwan K, et al. Monometallic and bimetallic SiC(O) ceramic with Ni, Co and/or Fe nanoparticles for catalytic applications. Nanocomposites. 2022;8(1):194–203. doi: 10.1080/20550324.2022.2106396.
- Li S, Peng Z, Dallman J, et al. Crossing the blood-brain-barrier with transferrin conjugated carbon dots: a zebrafish model study. Colloids Surf B Biointerfaces. 2016;145:251–256. doi: 10.1016/j.colsurfb.2016.05.007.
- Huang N, Cheng S, Zhang X, et al. Efficacy of NGR peptide-modified PEGylated quantum dots for crossing the blood-brain barrier and targeted fluorescence imaging of glioma and tumor vasculature. Nanomedicine. 2017;13(1):83–93. doi: 10.1016/j.nano.2016.08.029.
- Barani M, Mukhtar M, Rahdar A, et al. Progress in the application of nanoparticles and graphene as drug carriers and on the diagnosis of brain infections. Molecules. 2021;26(1):186. doi: 10.3390/molecules26010186.
- Israel LL, Galstyan A, Cox A, et al. Signature effects of vector-guided systemic nano bioconjugate delivery across blood-brain barrier of normal, Alzheimer’s, and tumor mouse models. ACS Nano. 2022;16(8):11815–11832. doi: 10.1021/acsnano.1c10034.
- DAS SS, Bharadwaj P, Bilal M, et al. Stimuli-responsive polymeric nanocarriers for drug delivery, imaging, and theragnosis. Polymers. 2020;12(6):1397. doi: 10.3390/polym12061397.
- Sireesha M, Jagadeesh Babu V, Kranthi Kiran AS, et al. A review on carbon nanotubes in biosensor devices and their applications in medicine. Nanocomposites. 2018;4(2):36–57. doi: 10.1080/20550324.2018.1478765.
- Joseph A, Nance E. Nanotherapeutics and the brain. Annu Rev Chem Biomol Eng. 2022;13(1):325–346. doi: 10.1146/annurev-chembioeng-092220-030853.
- Shirvalilou S, Khoei S, Khoee S, et al. Enhancement radiation-induced apoptosis in C6 glioma tumor-bearing rats via pH-responsive magnetic graphene oxide nanocarrier. J Photochem Photobiol B. 2020;205:111827. doi: 10.1016/j.jphotobiol.2020.111827.
- Kargar S, Khoei S, Khoee S, et al. Evaluation of the combined effect of NIR laser and ionizing radiation on cellular damages induced by IUdR-loaded PLGA-coated nano-graphene oxide. Photodiagnosis Photodyn Ther. 2018;21:91–97. doi: 10.1016/j.pdpdt.2017.11.007.
- Abdel Hady M, Sayed OM, Akl MA. Brain uptake and accumulation of new levofloxacin-doxycycline combination through the use of solid lipid nanoparticles: formulation; optimization and in-vivo evaluation. Colloids Surf B Biointerfaces. 2020;193:111076. doi: 10.1016/j.colsurfb.2020.111076.
- Rinaldi F, Oliva A, Sabatino M, et al. Antimicrobial essential oil formulation: chitosan coated nanoemulsions for nose to brain delivery. Pharmaceutics. 2020;12(7):678. doi: 10.3390/pharmaceutics12070678.
- MENDONçA MC, Soares ES, DE Jesus MB, et al. PEGylation of reduced graphene oxide induces toxicity in cells of the blood-brain barrier: an in vitro and in vivo study. Mol Pharm. 2016;13(11):3913–3924. doi: 10.1021/acs.molpharmaceut.6b00696.
- Zhong G, Long H, Zhou T, et al. Blood-brain barrier permeable nanoparticles for Alzheimer’s disease treatment by selective mitophagy of microglia. Biomaterials. 2022;288:121690. doi: 10.1016/j.biomaterials.2022.121690.
- Li X, Yang Y, Zhao H, et al. Enhanced in vivo blood-brain barrier penetration by circular Tau-transferrin receptor bifunctional aptamer for Tauopathy therapy. J Am Chem Soc. 2020;142(8):3862–3872. doi: 10.1021/jacs.9b11490.
- Kuplennik N, Lang K, Steinfeld R, et al. Folate receptor α-modified nanoparticles for targeting of the central nervous system. ACS Appl Mater Interfaces. 2019;11(43):39633–39647. doi: 10.1021/acsami.9b14659.
- Garcia-Caceres C, Quarta C, Varela L, et al. Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell. 2016;166(4):867–880. doi: 10.1016/j.cell.2016.07.028.
- Storck SE, Meister S, Nahrath J, et al. Endothelial LRP1 transports amyloid-β(1-42) across the blood-brain barrier. J Clin Invest. 2016;126(1):123–136. doi: 10.1172/JCI81108.
- Gartziandia O, Egusquiaguirre SP, Bianco J, et al. Nanoparticle transport across in vitro olfactory cell monolayers. Int J Pharm. 2016;499(1–2):81–89. doi: 10.1016/j.ijpharm.2015.12.046.
- Fan Y, Cui Y, Hao W, et al. Carrier-free highly drug-loaded biomimetic nanosuspensions encapsulated by cancer cell membrane based on homology and active targeting for the treatment of glioma. Bioact Mater. 2021;6(12):4402–4414. doi: 10.1016/j.bioactmat.2021.04.027.
- Qian Y, Qiao S, Dai Y, et al. Molecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumor-associated macrophages. ACS Nano. 2017;11(9):9536–9549. doi: 10.1021/acsnano.7b05465.
- Xiao H, Guo Y, Li B, et al. M2-like tumor-associated macrophage-targeted codelivery of STAT6 inhibitor and IKKbeta siRNA induces M2-to-M1 repolarization for cancer immunotherapy with low immune side effects. ACS Cent Sci. 2020;6(7):1208–1222. doi: 10.1021/acscentsci.9b01235.
- Cieslewicz M, Tang J, Yu JL, et al. Targeted delivery of proapoptotic peptides to tumor-associated macrophages improves survival. Proc Natl Acad Sci USA. 2013;110(40):15919–15924. doi: 10.1073/pnas.1312197110.
- Ngambenjawong C, Cieslewicz M, Schellinger JG, et al. Synthesis and evaluation of multivalent M2pep peptides for targeting alternatively activated M2 macrophages. J Control Release. 2016;224:103–111. doi: 10.1016/j.jconrel.2015.12.057.
- Yao Y, Wang J, Liu Y, et al. Variants of the adeno-associated virus serotype 9 with enhanced penetration of the blood-brain barrier in rodents and primates. Nat Biomed Eng. 2022;6(11):1257–1271. doi: 10.1038/s41551-022-00938-7.
- Liu Y, Hu P, Zheng Z, et al. Photoresponsive vaccine-like CAR-M system with high-efficiency central immune regulation for inflammation-related depression. Adv Mater. 2022;34(11):e2108525. doi: 10.1002/adma.202108525.
- Cao H, Dan Z, He X, et al. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano. 2016;10(8):7738–7748. doi: 10.1021/acsnano.6b03148.
- Chen Q, Zhang XH, Massagué J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20(4):538–549. doi: 10.1016/j.ccr.2011.08.025.
- Bahmani B, Gong H, Luk BT, et al. Intratumoral immunotherapy using platelet-cloaked nanoparticles enhances antitumor immunity in solid tumors. Nat Commun. 2021;12(1):1999. doi: 10.1038/s41467-021-22311-z.
- Wang Z, Zhang M, Chi S, et al. Brain tumor cell membrane-coated lanthanide-doped nanoparticles for NIR-IIb luminescence imaging and surgical navigation of glioma. Adv Healthc Mater. 2022;11(16):e2200521. doi: 10.1002/adhm.202200521.
- Mo J, Chen X, Li M, et al. Upconversion nanoparticle-based cell membrane-coated cRGD peptide bioorthogonally labeled nanoplatform for glioblastoma treatment. ACS Appl Mater Interfaces. 2022;14(44):49454–49470. doi: 10.1021/acsami.2c11284.
- Liu L, Bai X, Martikainen MV, et al. Cell membrane coating integrity affects the internalization mechanism of biomimetic nanoparticles. Nat Commun. 2021;12(1):5726. doi: 10.1038/s41467-021-26052-x.
- Su J, Yao ZP, Chen ZX, et al. TfR aptamer enhanced blood-brain barrier penetration of biomimetic nanocomplexes for intracellular transglutaminase 2 imaging and silencing in glioma. Small. 2022;18(40):e2203448. doi: 10.1002/smll.202203448.
- Niu W, Xiao Q, Wang X, et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021;21(3):1484–1492. doi: 10.1021/acs.nanolett.0c04753.
- Wu T, Liu Y, Cao Y, et al. Engineering macrophage exosome disguised biodegradable nanoplatform for enhanced sonodynamic therapy of glioblastoma. Adv Mater. 2022;34(15):e2110364. doi: 10.1002/adma.202110364.
- Sharma G, Modgil A, Sun C, et al. Grafting of cell-penetrating peptide to receptor-targeted liposomes improves their transfection efficiency and transport across blood-brain barrier model. J Pharm Sci. 2012;101(7):2468–2478. doi: 10.1002/jps.23152.
- Solomon M, Loeck M, Silva-Abreu M, et al. Altered blood-brain barrier transport of nanotherapeutics in lysosomal storage diseases. J Control Release. 2022;349:1031–1044. doi: 10.1016/j.jconrel.2022.07.022.
- Sun P, Xiao Y, DI Q, et al. Transferrin receptor-targeted PEG-PLA polymeric micelles for chemotherapy against glioblastoma multiforme. Int J Nanomedicine. 2020;15:6673–6688. doi: 10.2147/IJN.S257459.
- Wiley DT, Webster P, Gale A, et al. Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc Natl Acad Sci USA. 2013;110(21):8662–8667. doi: 10.1073/pnas.1307152110.
- Clark AJ, Davis ME. Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proc Natl Acad Sci USA. 2015;112(40):12486–12491. doi: 10.1073/pnas.1517048112.
- Alam C, Aufreiter S, Georgiou CJ, et al. Upregulation of reduced folate carrier by vitamin D enhances brain folate uptake in mice lacking folate receptor alpha. Proc Natl Acad Sci USA. 2019;116(35):17531–17540. doi: 10.1073/pnas.1907077116.
- Lu L, Shen X, Tao B, et al. The nanoparticle-facilitated autophagy inhibition of cancer stem cells for improved chemotherapeutic effects on glioblastomas. J Mater Chem B. 2019;7(12):2054–2062. doi: 10.1039/c8tb03165g.
- Luiz MT, Viegas JSR, Abriata JP, et al. Docetaxel-loaded folate-modified TPGS-transfersomes for glioblastoma multiforme treatment. Mater Sci Eng C Mater Biol Appl. 2021;124:112033. doi: 10.1016/j.msec.2021.112033.
- Oller-Salvia B, Sánchez-Navarro M, Giralt E, et al. Blood-brain barrier shuttle peptides: an emerging paradigm for brain delivery. Chem Soc Rev. 2016;45(17):4690–4707. doi: 10.1039/c6cs00076b.
- Lin T, Zhao P, Jiang Y, et al. Blood-brain-barrier-penetrating albumin nanoparticles for biomimetic drug delivery via albumin-binding protein pathways for antiglioma therapy. ACS Nano. 2016;10(11):9999–10012. doi: 10.1021/acsnano.6b04268.
- Zhu H, Ren F, Wang T, et al. Targeted immunoimaging of tumor-associated macrophages in orthotopic glioblastoma by the NIR-IIb nanoprobes. Small. 2022;18(30):e2202201. doi: 10.1002/smll.202202201.
- Xie J, Shen Z, Anraku Y, et al. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials. 2019;224:119491. doi: 10.1016/j.biomaterials.2019.119491.
- He W, Li X, Morsch M, et al. Brain-targeted codelivery of Bcl-2/Bcl-xl and Mcl-1 inhibitors by biomimetic nanoparticles for orthotopic glioblastoma therapy. ACS Nano. 2022;16(4):6293–6308. doi: 10.1021/acsnano.2c00320.
- Batrakova EV, Gendelman HE, Kabanov AV. Cell-mediated drug delivery. Expert Opin Drug Deliv. 2011;8(4):415–433. doi: 10.1517/17425247.2011.559457.
- Xu M, Feng T, Liu B, et al. Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics. 2021;11(18):8926–8944. doi: 10.7150/thno.62330.
- SI X L, FANG Y J, LI L F, et al. From inflammasome to Parkinson’s disease: Does the NLRP3 inflammasome facilitate exosome secretion and exosomal alpha-synuclein transmission in Parkinson’s disease?.Exp Neurol, 2021;336:113525. doi: 10.1016/j.expneurol.2020.113525.