?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Chitosan, a biopolymer known for its biocompatibility, biodegradability, and chemical adaptability, has attracted significant attention in scientific research. Chitosan-metal nanocomposites represent an emerging area of exploration. Among these, Chitosan-Fe nanocomposite has gained prominence due to its various applications, including photoremediation, bioimaging, and drug delivery systems. In this study, we delved into the synthesis and physicochemical properties of Chitosan-Fe nanocomposites. These nanocomposites exhibited a spherical structure with active binding sites that allowed for the functionalization of Fe3+ ions on their surface. Density functional theory simulations corroborated this alteration, demonstrating changes in surface charge properties resulting in increased mechanical strength. A key finding of this study is the enhanced antibacterial activity exhibited by the Cs-Fe nanocomposites against both Escherichia coli (62.5 µg/mL) and Staphylococcus aureus (125.25 µg/mL), surpassing that of bare Chitosan nanoparticles. Furthermore, our investigation confirms the therapeutic safety of the Chitosan-Fe nanocomposite. The cell viability calculated by MTT assay and accepted therapeutic dose concentration for CS-Fe NC was 125 ug/mL whereas for CSNP < 15.62 µg/mL. This underscores the nanocomposite’s potential in biomedical applications. In addition to its biomedical promise, the Chitosan-Fe nanocomposite also demonstrates remarkable potential in environmental remediation, particularly in the removal of hexavalent chromium.
1. Introduction
In recent years, the urgent need for effective solutions to combat environmental pollution and address biomedical challenges has spurred significant interest in the development of innovative materials and technologies. The overuse and miscues of antibiotics are leading to an increase in multidrug resistant bacteria strains. Conventional antibiotics follow the predictive mode of action as an antimicrobial mechanism. To overcome these limitations nanomaterials have been established as an innovative key aspect as an antimicrobial agent due to their high exposed surface, and peculiar mechanical, thermal, magnetic, electrical, biological, and optical properties. Chitosan is a cationic polysaccharide that is the second most abundant natural biopolymer, making it relatively inexpensive exhibits excellent biological properties which make it a promising candidate for numerous biomedical applications. Metal nanomaterials have emerged as one of the most exciting tools due to the increased surface-to-volume ratio, and tuneable physicochemical and optoelectronic properties. [Citation1]. However, these metallic nanoparticles possess nanotoxicity, enhanced permittivity, and cell retention and may get deposited in human vital organs [Citation2]. In this context, polymeric nanomaterials will be promising agents for biomedical applications because of the increased surface-to-volume ratio offering association for macromolecule binding and improved drug/metal/aptamer incorporation [Citation3]. Considering the advantages of polymeric and metal nanomaterials, numerous methods have been developed for preparing nanocomposites [Citation4,Citation5]. Chitosan possesses unique properties such as antimicrobial activity, and wound healing properties, making it an attractive candidate for drug delivery systems, tissue engineering scaffolds, and wound dressings. However, the intrinsic limitations of chitosan, such as poor mechanical properties and limited control over drug release, have spurred research into enhancing its properties through composite materials. Generally metal precursor is added to the polymeric solution for the formation of a new metal-polymer nanocomposite. Due to the increased biocompatibility cationic characteristics of chitosan makes favourable conditions for the absorption/adsorption of drug/metal/aptamer [Citation6–8]. The amalgamation of chitosan with metal nanoparticles has yielded a class of materials known as chitosan-metal nanocomposites, which have shown great promise for a wide range of biomedical applications. These promising features, leads to higher biocompatibility, bioavailability, and reduced toxicity of metals.
Researchers have explored the area of Chitosan-metal and metal oxide nanocomposites like CS-Silver nanocomposites are mainly utilized as an antimicrobial, antifungal agent [Citation9] as well in biofilm reduction, environmental remediation, and for increased bioavailability to cells [Citation10,Citation11]. CS-Gold, CS-Iron oxide, CS-Copper oxide, CS-Zinc oxide [Citation12] for the enhanced antibacterial, antifungal activities, drug delivery, wound healing, sensing applications, as well as an adsorbent for the heavy metals [Citation13–15]. However, both Silver and Copper belong to the heavy metal ions; they will affect the health of human easily by accumulating into human vital organs. Whereas metal oxide nanomaterials are less stable and more susceptible to dissolution, can be cytotoxic and have adverse effects on the environment and human health [Citation16]. Different chitosan nanocomposites have been functionalized the antibiotics to achieve increased bioavailability, antibacterial effect and decreased dosage values against the multidrug resistant bacteria [Citation17,Citation18].
Iron is an essential micronutrient which regulates carbohydrate, protein, and fat metabolism but researchers have mainly explored the area of iron oxide nanoparticles in the field of biomedical and environmental applications due to its increased magnetic properties, but it produces less colloidal stability, due to the calcination processes used in the synthesis of nanomaterials along with that it produces the nanotoxicity. To reduce the toxicity and increase the bioavailability, colloidal stability of iron mainly in Fe3+ and Fe2+ forms we explored the area of metal-polymer nanocomposites for advanced biomedical, environmental applications [Citation19,Citation20].
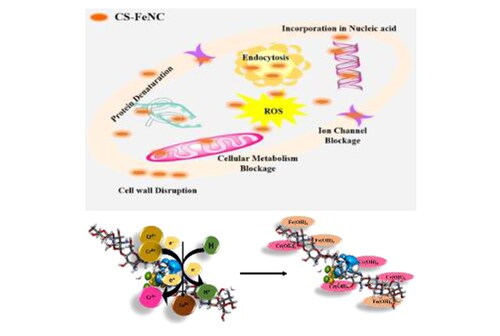
To overcome the limitations of the metal oxides and to enhance the biomedical activity of composites without antibiotics functionalization we aim to provide an insightful exploration of the physicochemical properties, synthesis methodology, and diverse applications of CS-Fe nanocomposite, emphasizing its pivotal role in the mitigation of environmental pollutants and its potential contributions to the realm of biomedicine. In this study, we report the one step preparation of chitosan-metal nanocomposite. The chitosan- iron (CS-Fe NC) nanocomposites were prepared by in-situ ionic gelation method. In addition to this, the optical, structural, and morphological properties of these nanocomposites were also investigated. Our study suggests that the iron functionalization in CS, modulates physiochemical properties of CS. Further, we have endeavoured surface charge distribution around CS-Fe NC using COMSOL Multiphysics simulation and first principles DFT to understand and corroborate the experimental observations. The developed useful insights on morphological and physicochemical changes in CSNPs due to the Fe3+ functionalization may pave the new way towards the tuneable nanocomposite synthesis, structural functionalization as well as increased antibacterial activity against broad spectrum of bacteria with the less dosage values. The synthesized CS-Fe Nanocomposite serves as the micronutrient to the cells and promote the cell growth which was confirmed by the cell cytotoxicity studies. The synthesized composite not only has increased biomedical applications but also it serves as a good adsorbent for the conversion of hexavalent chromium to trivalent chromium which makes it a promising agent in environmental remediation applications with reduced time and enhanced adsorption capabilities [Citation21].
2. Materials and methods
2.1. Chemicals and reagents
All the chemicals used for the preparation of CSNPs and CS-Fe NC are of analytical grade and used as received without further purifications. All the experiments were performed with Milli-Q water. The low molecular weight chitosan (bio-extracted (C6H11NO4)n) with acetylation degree of 90%, Glacial Acetic acid (C6H6O3, AA), Polyethylene Glycol 400 (PEG-400), and Sodium tripolyphosphate (Na5P3O10, STPP) and Iron Chloride hexahydrate (FeCl3.6H2O) were used for the synthesis of CS-Fe NCs. Potassium dichromate (K2Cr2O7) was used as a chromium source. Vero cells procured from NCCS, Pune, DMEM, FBS, Antibiotics solution, TPVG 1X solution, MTT reagent procured form Himedia. Tissue culture flasks (T25 cm2), 96-well flat bottom plates, microtips (10 µL, 200 µL, and 1000 µL), centrifuge tube (15 mL and 50 mL) procured form Tarson.
2.2. Synthesis of CSNP and CS-Fe NC
The ionic gelation method was employed for the synthesis of CSNPs and chitosan nanocomposites with slight modification in the previously described method [Citation22]. The entire synthesis of CSNPs and nanocomposites was carried out at room temperature (300 K). In a typical synthesis process, 50 mg of chitosan powder was dissolved in dilute AA (1%), to this mixture PEG-400 of 500 µL volume was added and stirred until clear solution was obtained. The CSNPs were precipitated by the dropwise addition of the STPP solution of concentration of 1.5 mM in Milli-Q water under stirring condition. The solution was then kept under constant stirring condition at room temperature followed by aging for 12 h. For the synthesis of chitosan-Fe nanocomposite. Typically, we have added the ferric chloride (1 mM) dropwise to the previously prepared CSNP in the ratio of 1:5 (FeCl3: CSNP) under constant stirring and then solution was vigorously stirred for the next 3 h. CS-Fe NC solution was then centrifuged and washed using Milli-Q water to remove excess and unreacted Fe3+, acetic acid moieties and other residual byproducts. The pH of CSNPs solution turns to 7.0 as a result of washing. These samples were utilized for biomedical and environmental remediation studies [Citation23].
2.3. Physicochemical characterization of CSNP and CS-Fe NC
2.3.1. UV–Vis spectroscopy
The confirmation of the formation and assessment of optical properties properties (UV–Vis) of CSNPs and CS-Fe NC were determined using Jasco V-750 Spectrophotometer. The absorption spectra were acquired in the range 190–900 nm with a resolution 1 nm.
2.3.2. Fourier-transform infrared spectroscopy analysis
To determine the presence of functional groups of biomolecules present on the surface of CSNPs, and CS-Fe NC which may be responsible for reduction and stabilization during the synthesis. The room temperature Fourier-transform infrared (FTIR) spectroscopy was performed using Shimadzu IR Affinity in the range of 400–4000 cm−1 with diamond Attenuated Transmittance and Reflectance accessory.
2.3.3. Zeta potential
The surface charge distribution of synthesized nanoparticles and nanocomposites, were measured by Dynamic Light Scattering Anton Parr Litesizer.
2.3.4. X-ray diffraction
The crystalline structure of the CSNPs and CS-Fe NC were investigated by X-ray diffraction (XRD) analysis. Powdered samples were subjected to XRD analysis and data was recorded.
Rigaku Miniflex operating at 40 kV and 40 mA equipped with Cu Kα radiation source (λ = 1.54060 Å). The diffraction pattern was acquired in the range 2θ = 5 – 80°.
2.3.5. Brunauer–Emmett–Teller (analysis)
The Brunauer–Emmett–Teller (BET) specific surface area was determined by the adsorption–desorption data, in relative pressure (P/Po) range of 0.1 to 0.99. The analysis was carried out on the Quantachrome TouchWin v1 .2 2.
2.3.6. Transmission electron microscopy
To obtain morphology, mean particle size of nanoparticles transmission electron microscopy (TEM) analysis was performed. Drop coating of synthesized CS-Fe NC solution on carbon-coated copper TEM grids was done for TEM analysis. Further, the TEM analysis was performed on TECNAI G2 SPIRIT BIOTWIN. At an accelerating voltage of 200 kV at room temperature, the TEM equipment was operated. Selected Area Electron Diffraction and SAED analysis were investigated using the same grid.
2.4. Simulation tools
To evaluate the interaction between CS and Fe3+ ions, DFT simulation was performed using Discovery Studio suite 2017 (Accelrys) [Citation22]. All the calculations were performed by setting fine grid quality. First, using DMol3 routine and generalized gradient approximation (GGA) and Perdew–Burke–Ernzerhof (PBE) exchange-correlation function along with density functional theory semi-core pseudopotential (DSPP) core treatment and double numerical plus polarization (DNP+) as basis set used for the simulation. Using these calculation routines, the single chitosan molecule (PubChem CID: 71853) and single FeCl3.6H2O molecule (PubChem CID: 6093258) was geometry optimized to get the lowest energy conformation structure. Subsequently, energy calculations were performed using random sampling search method and universal force field to obtain HOMO-LUMO states and binding energies of interactions. COMSOL Multiphysics package Version 5.4 was used for surface charge density (SCD) simulation [Citation24].
2.5. Antibacterial activity studies
CSNP and CS-Fe NC were evaluated for antibacterial activity against gram-negative bacteria Escherichia coli (ATCC 8739) and gram-positive bacteria Staphylococcus aureus (ATCC 6538 P) using the Minimum Inhibition Concentration (MIC) by conventional broth-dilution method. The CSNP and CS-Fe NC concentrations evaluated for determining the Minimum Inhibitory Concentration (MIC) at which bacterial growth is inhibited were 250, 125, 62.5, 31.25, and 15.62 µg/mL. It was tested in a 96-well microtiter plate using the microbroth dilution method. A log-phase bacterial culture with a 0.1 Optical Density (OD) at 595 nm was prepared. Bacterial culture was mixed with varied concentrations of nanostructures and NPs in triplicate along with negative control (bacterial growth curve) and incubated at 37 °C under shaking conditions, and optical density was monitored spectrophotometrically every 30 min in a plate reader (BioTek) [Citation25] The data collected during the 20-h incubation period was calculated average OD to determine the MIC of CSNP and CS-Fe NC.
2.6. Cell viability study
Vero cells were used to assess the cell viability of CS-Fe NCs and CSNP. The Vero cells were grown in Dulbecco’s Modified Eagles Medium (DMEM) supplemented with 10% Foetal Bovine Serum (FBS) and 1% Antibiotics (100 U Penicillin and 100 mg/mL Streptomycin) at 37 °C in a humidified 5% CO2 atmosphere. The stock solution of CS-Fe NCs and CSNP was filtered through 0.2 µ syringe filters, and concentrations ranging from (500, 250, 125, 62.5, 31.25, 15.62 µg/mL) was prepared by diluting it into a maintenance medium (DMEM with 2% FBS and 1% antibiotics). The 3-(4,5-dimethylthiazol-2yl)-2,5Diphenyltetrazolium (MTT) experiment was carried out in 96-well flat-bottom plates [Citation23], 2 × 104 cells per well were grown in 100 µL of growth media and incubated in optimal conditions to generate a confluent monolayer. After incubation, the growth media was replaced with 100 µL of test CS-Fe NCs and CSNP (500, 250, 125, 62.5, 31.25, 15.62 µg/mL) samples and the plate was incubated for 24 h in a CO2 incubator. Following that, 10 µL of 5% MTT solution was added to each well, followed by 4 h of incubation in the dark, 100 µL DMSO was added to dissolve the formazan crystals. The optical density at 570 nm was measured using a microplate reader (BioTek). Cell viability of CS-FE NC and CSNP treated cells were evaluated OD means ± standard deviation (SD) and percent growth inhibition was calculated by the following formula [Citation26,Citation27]
2.7. Remediation of chromium
The chromium remediation was checked by CS-Fe NC and confirmed by the UV–Vis analysis. The CS-Fe NC solution was prepared with concentration of 1 mg/mL. The hexavalent chromium stock solution with a concentration of 1000 ppm was prepared as previously discussed and stored at RT. Further, the CS-Fe NC solution mixed with hexavalent chromium solution in the ratio of 1:10 at pH 8 and vortexed. The UV–Vis absorption spectrums within the range of 200 nm to 900 nm were collected for 0, 10, 20,30, and 60 min. The change in the absorption spectra was considered for the further studies.
2.8. Statistical analysis
Experiments were set up in a completely randomized design. In this study, all the experiments were performed in triplicates. All the experimental average values were expressed as mean ± SD. For cell viability study we have compared various concentrations of CS-Fe NC with CSNP using Student’s t-test (two-tailed, type 2), average percent cell viability was meditated by ± standard deviation, and differences were considered significant for p < .05*, p < .01**, p < .001***, and p < .0001****.
3. Results and discussion
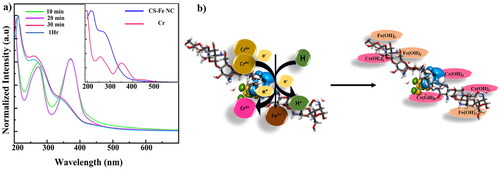
The synthesized CSNPs and CS-Fe nanocomposites were characterized using UV–Visible Spectroscopy. shows the absorption spectrum of CSNPs with the strong characteristics absorption peak at 210 nm, representing glucosamine functional group of CS (as shown in inset) [Citation22,Citation28]. Interestingly, after Fe3+ incorporation in CS, the spectra shows prominent absorption peak positioned at 278 nm, confirming successful functionalization of Fe3+ in CSNPs matrix as shown in and the peak corresponding to glucosamine functional group of CS shows blue shift [Citation29]. The functionalization of Fe3+ is also correlated with the visual appearance of the solution of CSNPs (white) and CS-Fe NC (pale orange) as shown in inset of . The simulated structures of CSNP and CS-Fe NC are shown in the inset of .
Figure 1. Represents the physicochemical characteristics of CSNP & CS-FeNC (a, b) shows the absorption spectra of CSNPs and CS-Fe NC, respectively, insets of a and b shows the changes in the first derivative of the corresponding spectrum in the region where optical transitions are observed along with the molecular structures) (c) shows room temperature FTIR spectra obtained for CSNP (Blue line) and CS-Fe NC (Brown line). (d) BET nitrogen adsorption/desorption isotherm of CS-Fe NC represents the pore size distribution of CSNP and CS-Fe NC.
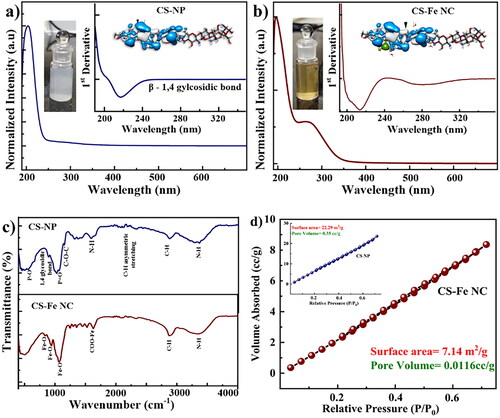
Further, we performed FTIR measurements of the samples to determine the interactions between CS-Fe NC and possible active sites for Fe3+ on CSNP matrix. shows the FTIR spectra of CSNP (Blue color) and CS-Fe NC (Brown color). The features observed in the FTIR for CSNPs are consistent with the reported work., The major peaks positioned at 500–750 cm−1 attributed P-O bonding, saccharine structure or beta 1, 4 glycosidic bond observed at 890–894 cm−1, 1064–1076 cm−1 corresponds P = O vibration coming from phosphate group of STPP [Citation22,Citation30] and C-O stretching of primary alcohol, asymmetric C-O-C stretching observed at 1129 and 1130 cm−1,1598–1602 cm−1 for N-H bending vibration of amine-I [Citation31] (Blue color spectra).Whereas in CS-Fe NC NCs the features positioned in the proximity to 1527 cm−1 and 1620 cm−1 correspond to the COO-Fe [Citation32], Fe-O vibrations observed at 941 and Fe-O 825 cm−1 [Citation33], Fe-O bending vibrations of hydroxyl group associated with Fe3+ are observed at 1234 cm−1 to 1365 cm−1, 2858–2864 cm−1 corresponds to C-H asymmetric stretching and 3443–3446 cm−1 are assigned for stretching vibrations of -OH and -NH [Citation33] (Brown color spectra). Furthermore, other FTIR vibrational features for CSNP and CS-Fe NC shows slight difference in symmetric, asymmetric, bending, and vibrational modes. Thus, alteration in dipole moments of CSNP and CS-Fe NC have been studied and concluded by FTIR studies. The BET Nitrogen adsorption and desorption isotherm was used to analyse surface area and pore diameter of the CS-Fe NC and CSNP shown in . The BET specific surface area was determined by the adsorption-desorption data, in the relative pressure (P/Po) range of 0.1–0.99. The nanopore distribution of CSNPs was determined using desorption curve of the nitrogen isotherm. Further, the average pore diameter was determined from the peak position of the distribution curve and it was observed about 376.58 nm. The change in the surface volume and surface area of the CSNP and CS-Fe NC confirms the loading of Fe3+ in CSNP. The surface area of CSNPs was 22.58 m2/g decreases to 7.14 m2/g indicating that the nanopores are blocked by the Fe3+ ions.
The crystallographic investigations have been carried out using powder XRD. The XRD pattern shown in and inset clearly indicates the difference between CSNPs and CS-Fe NC. The weak broad peak in the proximity to 2θ = 5 to 7° represents the characteristic diffraction peak of CS. The broadening of the peak in the range of 2θ = 20 – 25° is supported to the nanosized formation of CS [Citation24]. The XRD pattern also suggests the amorphous nature of the prepared sample. Whereas CS-Fe NC shows sharp peaks positioned at 2θ = 32°, 45° contributing to the cubic structure of iron in metallic form with the plane (112) and (111), respectively. Whereas weak peak at 2θo = 56, represent presence of α Fe-OOH with plane (221) [Citation34]. The understanding of the overall electronic interactions between CSNP and CS-Fe NC we have estimated the HOMO-LUMO gaps for both samples and found to be 7.6 and 6.2 eV, respectively (). The electron donation ability and electron acceptance ability are governed by HOMO and LUMO, respectively. The calculations showed that CSNPs shows highest value of HOMO suggesting strong tendency towards electron donation. However, while functionalization with Fe3+ LUMO of the CSNP slightly shifted accelerating the stabilization of CSNP. The decreased gap is directly attributed to the incorporation/functionalization of Fe in CSNP matrix. Specific functional groups present in CS-Fe NC contribute to both HOMO and LUMO. The functionalization affects the position of LUMO decreasing ΔE [Citation35].
Figure 2. (a) Shows XRD pattern of CS-FeNC the inset shows the XRD of CSNP. The functionalization with Fe3+ on CSNP matrix was confirmed by the appearance of new crystallographic planes/peaks. (b) Represents calculated HOMO-LUMO molecular orbital levels of CSNPs and CS-Fe NC by using DFT simulation studies. (c) Shows the TEM micrographs of polydisperse CS-Fe NC (highlighted in yellow), which are spherical in nature with an average particle size of ≈63 nm. Inset shows the particle size distribution & SAED pattern. (d) SCD simulation represents the ZP distribution and surface charge behaviour of CS-Fe NC.
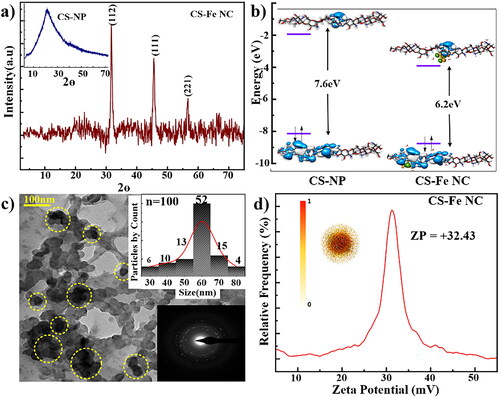
The depicts the TEM analysis of the synthesized CS-Fe NC which was done to decode the size and shape of the synthesized CS-Fe NC. From the , it is crystal clear that the synthesized CS-Fe NC are having moderate dispersity with a spherical shape. These nanocomposites confirming the functionalization Fe3+ shows appearance spherical structures on the surface of the CSNPs matrix. This is in good agreement with the XRD analysis. The nanocomposites are in the range of ≈63 ± 9.12 nm which is calculated by particle size distribution (inset of ) from the . Selected area electron diffraction (SAED) () pattern outlines the crystalline nature of the nanocomposites confirming the functionalization of Fe3+ on chitosan matrix. The charge distribution on the surface of the CS-Fe NC was further studied using Zeta potential (ZP) as shown in . CS-Fe NC exhibit high ZP = + 38 mV, indicating stable dispersion [Citation24]. The positive charge is due the presence of surfactant ions of STPP and Fe3+. The stability of CS-Fe NCs is also evident from FTIR studies, which shows association of -NH2 group present CS with Fe3+. The ZP behaviour was also simulated by surface charge density distribution around the CS-Fe NC, and electrophoretic mobility parameters obtained from ZP. Using Navier-Stokes equations the simulated electrostatic charge distribution shows strong charge distribution near the core of the composite as compared to its surface. The SCD behaviour of CS-Fe NC have a good correlation with experimental results.
The assessment of antibacterial activity is crucial in the field of nanomedicine, as it provides valuable insights into the potential therapeutic applications of nanoparticles. In this study, we investigated the antibacterial properties of CS-Fe NC and CSNP against two standard bacterial strains, E. coli (gram-negative) and S. aureus (gram-positive). The minimum inhibition concentration (MIC) was estimated by conventional broth dilution method as shown in . The results shed light on these nanomaterials’ potential for combating bacterial infections. The MIC is an essential parameter for evaluating the efficacy of antimicrobial agents, as it represents the lowest concentration at which bacterial growth is inhibited. The study revealed that MIC of CSNPs was 62.5 µg/mL against E. coli as depicted in and 125 µg/mL against the S. aureus as shown in whereas, CS-Fe NC exhibited MIC values of 32.25 µg/mL against both E. coli and S. aureus the results are shown in . This suggests that these CS-Fe NC possess antibacterial activity one and twofold hinger than CSNP against E. coli and S. aureus, respectively, which suggests that NPs can be effective against a broad spectrum of bacteria, including both gram-negative and gram-positive strains [Citation23,Citation36]. The fact that CS-Fe NC were more effective than the CSNP according to the MIC values, which are similar as reported earlier by Divya et al. 2017 [Citation37]. In fact, CS-Fe NCs could potentially have additional benefits, such as enhanced stability or targeted delivery, due to the presence of iron. Chitosan nanoparticles loaded with Fe3+ showed significantly higher antimicrobial activity than chitosan and related metal ions.
Figure 3. Represents the antibacterial activity of CSNP and CS-FeNC (a, b) shows the antibacterial activity of CSNPs against E. coli and S. aureus, (c, d) shows the antibacterial activity of CS-FeNC against E. coli and S. aureus (e) schematic representation of the mechanism of antibacterial activity.
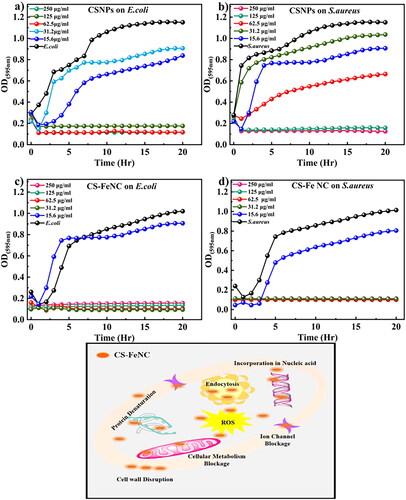
Chitosan is known to disrupt the bacterial cell membrane, leading to increased permeability and leakage of cellular contents as depicted schematic in . Additionally, the positively charged chitosan nanoparticles may interact with the negatively charged bacterial cell surface, disrupting its integrity and inhibiting bacterial growth. We can consider the basic function of Fe3+ chelated chitosan nanoparticles load destroys the bacterial cell wall. Because of the large surface area of the CS-Fe NC, nanoparticles could be highly adsorbed onto the surface of bacteria so as to disrupt the membrane, which leads to the leakage of intracellular components, thus, killing and inhibiting the growth of bacteria. Moreover, the outer membrane of Gram-negative bacteria such as E. coli consists of lipopolysaccharides containing phosphate and pyrophosphate groups which render the cell surface negatively charged. As the Fe3+ chelated chitosan nanoparticle is a cationic in nature (ZP + 32.43 mV), it can attach to the E. coli cell wall by electrostatic interaction. Gram-negative bacteria are more sensitive (MIC 62.5 µg/mL) than Gram-positive bacteria that’s why the Fe3+ chitosan nanoparticles exhibit higher antibacterial activity against Gram-negative bacteria than Gram-positive bacteria (S. aureus MIC 125.25 µg/mL).To sum up, metal chelated chitosan nanocomposites possess high antibactericidal performance as comparing to the chitosan nanoparticles.
To determine a therapeutically safe dose, the CS-Fe NC and CSNP were tested for its cytotoxic effect on Vero cells. The MTT assay was carried out at the following concentrations: (500, 250, 125, 62.5, 31.25, 15.62, µg/mL) as shown in . The statistical analysis was carried out to check the significance of CS-Fe NC, CSNP independently and in compared with respective concentrations. From the statistical analysis, it was confirmed that there was a significant reduction in cell viability at 500 and 250 µg/mL for CS-Fe NC. Whereas the CS-Fe NC concentrations below 125 µg/mL c retained the cell viability as compared to control without causing the cytotoxic effect. Whereas in case of CSNP there was a significant reduction in cell viability for all the tested concentrations. The study concluded that CSNP’s are cytotoxic in nature as compared to the CS-Fe NC which was analysed and confirmed by the statistical analysis. The better cell viability in CS-Fe NC may be because of the slow release of Fe3+ from CS-Fe NC, where Fe3+ acting as a micronutrient promoting cell growth [Citation27]. These findings clearly show that CS-Fe NC has less harmful effects than CSNP, implying that CS-Fe NC may be a useful nanomaterial for biological applications that need to be investigated.
Figure 4. Represents the comparative analysis of the cytotoxic effect on Vero cells when treated with CSNP and CS-Fe NC, respectively.
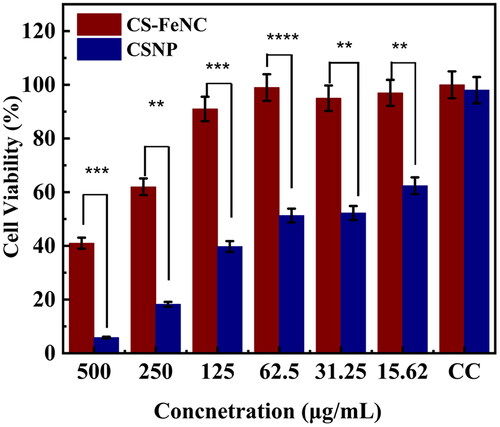
The cell viability assessment is critical in developing nanoparticles for biomedical applications. The results obtained in this study suggest that CS-Fe NCs have the potential to be cytotoxic at higher concentrations >250 ug/mL [Citation27]. Therefore, careful consideration should be given to the dosage and delivery methods when using these nanoparticles for therapeutic purposes. Additionally, investigations into the cellular uptake mechanisms and intracellular fate of CS-Fe NCs would provide valuable insights into their interactions with cells.
3.1. Chromium remediation studies
The UV–Vis based remediation studies of chromium are shown in . Chromium is a strong oxidizing agent and it readily undergoes reduction reactions in the presence of electron donors [Citation38]. Researchers have utilized the chitosan-silver hydrogel for the remediation of Chromium with 83% of removal efficacy [Citation39]. Chitosan-MnFe2O4 is used as a adsorbent for the remediation of chromium with removal capacity of 35.2 mg g−1 [Citation40]. Chitosan-Fe3O4 is widely used for the separation and removal of Cr [Citation41]. Iron ions particularly (Fe3+) ions play a pivotal role in remediation of chromium from wastewater [Citation42]. They facilitate the process by reducing toxic hexavalent chromium to trivalent chromium followed by the precipitation of Fe (III)/Cr(III) hydroxides. In brief in the presence of Fe3+ ions in CS-Fe NC, Cr undergoes reduction reaction. This chemical reaction results in the conversion of Cr(VI) to Cr(III) ions which are less soluble in water and less toxic. Fe3+ ions ac as an electron donor transferring electrons to the Cr. In the precipitation step once Cr(VI) reduced to Cr(III) both the Cr(III) and Fe(II) ions form insoluble hydroxides [Citation43]. These hydroxides precipitate out of the solution effectively removing them from the aqueous phase. The resulting chromium-iron hydroxide compounds are less stable and tend to settle as solid particles [Citation44]. The possible reaction mechanism is shown in the reaction given below and depicted in .
Figure 5. (a) UV–Vis absorption spectrum representing the formation of coordination complex between CS-Fe NC and Cr (VI) the inset shows the spectra of pristine CS-Fe NC & Cr standard (K2Cr2O7) (b) schematic representation of reaction mechanism between CS-Fe NC and Cr (VI).
Reduction of Cr (VI) to Cr (III)
Precipitation of Iron (III)/Chromium (III) hydroxide
The UV–Vis spectra analysis shows the reduction in the peak intensity for chromium when treated with the CS-Fe NC. The intensity of the absorption peak at 395 nm is reduced with time. The change and blue shift of the absorption peak from 395 nm to 360 nm confirms the formation of Cr-Fe hydroxides and also reduction and conversion of Cr (VI) to Cr (III).
4. Conclusion
In the summary, we report the in-situ ionic gelation method for the synthesis of CS-Fe NC. The morphological, surface charge, optical, and structural properties of CS-Fe NC were evaluated using FESEM, ZP, UV–Vis, FTIR, and XRD. The synthesized CSNPs are spherical in nature while in Fe3+ functionalized on CSNPs matrix were observed. FTIR studies show the involvement of different functional groups in the functionalization of Fe3+ with CSNPs matrix. The morphological TEM studies confirmed the spherical formation of CSNPs and the functionalization of Fe3+ in spherical form onto CSNPs matrix. Electrostatic charge distribution suggests the good stability of CS-Fe NC. All the results confirmed the formation CS-Fe NC are correlated with SCD and DFT studies. From the overall investigation, we conclude that this newly synthesized nanocomposite is high potential material with great surface modifications for active loading of drug molecules/aptamer/dyes for the applications in biomedical and environmental field. Our findings show that CS-Fe NC has improved and enhanced antibacterial activity against E. coli and S. aureus with a lower MIC than previously reported. Incorporating Fe3+ into chitosan nanoparticles, increases its bioavailability and antibacterial efficacy as compared to the bare chitosan nanoparticles. These findings open up exciting possibilities for developing chitosan-based nanomaterials as potential antimicrobial agents for treating bacterial infections without any external functionalization with antibiotics and other biomolecules. The cell viability studies on Vero cells demonstrated a dose-dependent increase with decrease in CS-Fe NC concentration from 500 to 15.62 µg/mL. Careful consideration of the concentration and exposure duration will be crucial in the therapeutic potential of CS-Fe NC while minimizing any adverse effects. The conversion of toxic Cr(VI) to Cr(III) by the help of CS-Fe NC also shows promising chromium remediation which can help in environmental pollution monitoring.
Authors’ contributions
Atul Kulkarni: Conceptualization, Supporting experiments, draft editing. Santosh Koratkar: Conceptualization, investigation, data curation, draft editing, Supporting experiments. Mandar M. Shirolkar: Simulation Studies & Supporting experiments. Rutuja S Gumathannavar: Experimentation, investigation, data curation, writing original draft and editing. Anil Thormothe & Pankhudi Bhutada: experimentation, Supporting experimentations, data curation, draft editing.
Disclosure statement
The authors declare that they have no known competing interests that could have appeared to influence the work.
Additional information
Funding
Notes on contributors
Rutuja Gumathannavar
Rutuja Gumathannavar is a Research Assistant with Symbiosis Centre for Nanoscience and Nanotechnology (SCNN), Symbiosis International (Deemed University). Her research interests are towards the utilization of novel nanofabrication techniques, to develop Functional Nanostructures for diverse biomedical diagnostic applications.
Anil Thormothe
Anil Thormothe, MSc (Microbiology) working as a Research Assistant at Symbiosis School of Biological Sciences (SSBS), Symbiosis International (Deemed University), Pune, Maharashtra, India. His research interest areas are Microbiology, Virology and Immunology.
Pankhudi Bhutada
Pankhudi Bhutada is a Junior Research Fellow at Symbiosis School of Biological Sciences (SSBS), Symbiosis International (Deemed University), Pune, Maharashtra, India. Her research interests are Clinical Microbiology, Bacteriology and Virology.
Mandar M. Shirolkar
Dr. Mandar M. Shirolkar is an assistant professor at Symbiosis Centre for Nanoscience and Nanotechnology (SCNN) of the Symbiosis International (Deemed University), India. His research focuses in Material sciences and simulation studies for applications in various fields.
Atul Kulkarni
Dr. Atul Kulkarni is currently associated with Symbiosis Centre for Nanoscience and Nanotechnology (SCNN) of the Symbiosis International (Deemed University), India, in the capacity of Research Professor and Director. His research focuses on multidisciplinary Nano biosensor development. He has published over 90 research papers in international journals and has 13 patents (Korean, US and PCT) to his credit.
Santosh Koratkar
Dr. Santosh Koratkar is currently working as an Assistant professor with Symbiosis School of Biological Sciences (SSBS), of the Symbiosis International (Deemed University), India. He is actively involved in areas of Pathogenesis and virus-host cell interactions, Immunology, vaccine development Ecology of bacteriophages and phage therapy etc.
References
- Taylor-Pashow KM, Della Rocca J, Huxford RC, et al. Hybrid nanomaterials for biomedical applications. Chem Commun (Camb). 2010;46(32):5832–5849. doi: 10.1039/c002073g.
- Sarkar A, Ghosh M, Sil PC. Nanotoxicity: oxidative stress mediated toxicity of metal and metal oxide nanoparticles. J Nanosci Nanotechnol. 2014;14(1):730–743. doi: 10.1166/jnn.2014.8752.
- Sharma S, Sudhakara P, Omran AAB, et al. Recent trends and developments in conducting polymer nanocomposites for multifunctional applications. Polymers (Basel). 2021;13(17):2898. doi: 10.3390/polym13172898.
- Huang H, Yuan Q, Yang X. Preparation and characterization of metal-chitosan nanocomposites. Colloids Surf B Biointerfaces. 2004;39(1–2):31–37. doi: 10.1016/j.colsurfb.2004.08.014.
- Wang P, Yao L, Pan Z, et al. Ultrahigh energy storage performance of layered polymer nanocomposites over a broad temperature range. Adv Mater. 2021;33(42):e2103338.
- Zhu C, Liu F, Zhang Y, et al. Nitrogen-doped chitosan-Fe(III) composite as a dual-functional material for synergistically enhanced co-removal of Cu(II) and Cr(VI) based on adsorption and redox. Chem Eng J. 2016;306:579–587. doi: 10.1016/j.cej.2016.07.096.
- Karki N, Tiwari H, Tewari C, et al. Functionalized graphene oxide as a vehicle for targeted drug delivery and bioimaging applications. J Mater Chem B. 2020;8(36):8116–8148. doi: 10.1039/d0tb01149e.
- Bhoopathy S, Inbakandan D, Thirugnanasambandam R, et al. A comparative study on chitosan nanoparticle synthesis methodologies for application in aquaculture through toxicity studies. IET Nanobiotechnol. 2021;15(4):418–426. doi: 10.1049/nbt2.12047.
- Joughi NFG, Farahpour MR, Mohammadi M, et al. Investigation on the antibacterial properties and rapid infected wound healing activity of silver/laterite/chitosan nanocomposites. J Ind Eng Chem. 2022;111:64–75. doi: 10.1016/j.jiec.2022.03.034.
- Namasivayam SKR, Pattukumar V, Samrat K, et al. Evaluation of methyl orange adsorption potential of green synthesized chitosan-silver nanocomposite (CS–AgNC) and its notable biocompatibility on freshwater tilapia (Oreochromis nitoticus). Chemosphere. 2022;308(Pt 2):135950. doi: 10.1016/j.chemosphere.2022.135950.
- Ramachandran G, Alharbi NS, Chackaravarthy G, et al. Chitosan/silver nanocomposites enhanced the biofilm eradication in biofilm forming gram positive S. aureus. J King Saud Univ Sci. 2023;35(4):102597. doi: 10.1016/j.jksus.2023.102597.
- Agnes J, Ajith P, Sappani Muthu M, et al. Preparation and characterization studies of chitosan encapsulated ZnO nanoparticles modified with folic acid and their antibacterial activity against selected bacterial species. Part. Sci. Technol. 2022;41:774–784. doi: 10.1080/02726351.2022.2145587.
- Sathiyavimal S, Vasantharaj S, Kaliannan T, et al. Bio-functionalized copper oxide/chitosan nanocomposite using Sida cordifolia and their efficient properties of antibacterial, anticancer activity against on breast and lung cancer cell lines. Environ Res. 2023;218:114986. doi: 10.1016/j.envres.2022.114986.
- El-Sayyad GS, Abdel Maksoud M, Fahim RA, et al. Gamma radiation induced synthesis of novel chitosan/gold/bioactive glass nanocomposite for promising antimicrobial, and antibiofilm activities. J Clust Sci. 2023;34(4):1877–1891. doi: 10.1007/s10876-022-02357-9.
- Iqbal H, Khan BA, Khan ZU, et al. Fabrication, physical characterizations and in vitro antibacterial activity of cefadroxil-loaded chitosan/poly (vinyl alcohol) nanofibers against Staphylococcus aureus clinical isolates. Int J Biol Macromol. 2020;144:921–931. doi: 10.1016/j.ijbiomac.2019.09.169.
- Mujahid MH, Upadhyay TK, Khan F, et al. Metallic and metal oxide-derived nanohybrid as a tool for biomedical applications. Biomed Pharmacother. 2022;155:113791. doi: 10.1016/j.biopha.2022.113791.
- Zafar N, Uzair B, Menaa F, et al. Moringa concanensis-mediated synthesis and characterizations of ciprofloxacin encapsulated into Ag/TiO2/Fe2O3/CS nanocomposite: a therapeutic solution against multidrug resistant E. coli strains of livestock infectious diseases. Pharmaceutics. 2022;14(8):1719. doi: 10.3390/pharmaceutics14081719.
- Zafar N, Uzair B, Niazi MBK, et al. Green synthesis of ciprofloxacin-loaded cerium oxide/chitosan nanocarrier and its activity against MRSA-induced mastitis. J Pharm Sci. 2021;110(10):3471–3483. doi: 10.1016/j.xphs.2021.06.017.
- Zheng L, Wang C, Shu Y, et al. Utilization of diatomite/chitosan–Fe (III) composite for the removal of anionic azo dyes from wastewater: equilibrium, kinetics and thermodynamics. Colloids Surf A. 2015;468:129–139. doi: 10.1016/j.colsurfa.2014.12.015.
- Chaudhary M, Rawat S, Jain N, et al. Chitosan-Fe-Al-Mn metal oxyhydroxides composite as highly efficient fluoride scavenger for aqueous medium. Carbohydr Polym. 2019;216:140–148. doi: 10.1016/j.carbpol.2019.04.028.
- Aslam MMA, Kuo H-W, Den W, et al. Recent trends of carbon nanotubes and chitosan composites for hexavalent chromium removal from aqueous samples. Sep Sci Technol. 2022;15:177–207.
- Shirolkar MM, Athavale R, Ravindran S, et al. Antibiotics functionalization intervened morphological, chemical and electronic modifications in chitosan nanoparticles. Nano-Struct Nano-Objects. 2021;25:100657. doi: 10.1016/j.nanoso.2020.100657.
- Qian J, Pan C, Liang C. Antimicrobial activity of Fe-loaded chitosan nanoparticles. Eng Life Sci. 2017;17(6):629–634. doi: 10.1002/elsc.201600172.
- Athavale R, Sapre N, Rale V, et al. Tuning the surface charge properties of chitosan nanoparticles. Mater Lett. 2022;308:131114. doi: 10.1016/j.matlet.2021.131114.
- Sreelatha S, Kumar N, Yin TS, et al. Evaluating the antibacterial activity and mode of action of thymol-loaded chitosan nanoparticles against plant bacterial pathogen Xanthomonas campestris pv. campestris. Front Microbiol. 2021;12:792737. doi: 10.3389/fmicb.2021.792737.
- Nimbalkar UD, Seijas JA, Borkute R, et al. Ultrasound assisted synthesis of 4-(benzyloxy)-N-(3-chloro-2-(substitutedphenyl)-4-oxoazetidin-1-yl) benzamide as challenging anti-tubercular scaffold. Molecules. 2018;23(8):1945. doi: 10.3390/molecules23081945.
- Shukla S, Jadaun A, Arora V, et al. In vitro toxicity assessment of chitosan oligosaccharide coated iron oxide nanoparticles. Toxicol Rep. 2015;2:27–39. doi: 10.1016/j.toxrep.2014.11.002.
- Tan SC, Khor E, Tan TK, et al. The degree of deacetylation of chitosan: advocating the first derivative UV-spectrophotometry method of determination. Talanta. 1998;45(4):713–719. doi: 10.1016/s0039-9140(97)00288-9.
- Abbott AP, Al-Bassam AZM, Goddard A, et al. Dissolution of pyrite and other Fe–S–as minerals using deep eutectic solvents. Green Chem. 2017;19(9):2225–2233. doi: 10.1039/C7GC00334J.
- Mohammadpour Dounighi N, Eskandari R, Avadi MR, et al. Preparation and in vitro characterization of chitosan nanoparticles containing Mesobuthus eupeus scorpion venom as an antigen delivery system. J Venom Anim Toxins Incl Trop Dis. 2012;18(1):44–52. doi: 10.1590/S1678-91992012000100006.
- Mansour SF, El-Dek SI, Dorozhkin SV, et al. Physico-mechanical properties of Mg and Ag doped hydroxyapatite/chitosan biocomposites. New J Chem. 2017;41(22):13773–13783. doi: 10.1039/C7NJ01777D.
- Zhang J, Chen N, Tang Z, et al. A study of the mechanism of fluoride adsorption from aqueous solutions onto Fe-impregnated chitosan. Phys Chem Chem Phys. 2015;17(18):12041–12050. doi: 10.1039/c5cp00817d.
- Zhang B, Chen N, Feng C, et al. Adsorption for phosphate by crosslinked/non-crosslinked-chitosan-Fe(III) complex sorbents: characteristic and mechanism. Chem Eng J. 2018;353:361–372. doi: 10.1016/j.cej.2018.07.092.
- Wang J, Li L, Wong CL, et al. Controlled synthesis of α-FeOOH nanorods and their transformation to mesoporous α-Fe2O3, Fe3O4@C nanorods as anodes for lithium ion batteries. RSC Adv. 2013;3(35):15316. doi: 10.1039/c3ra41886c.
- Abdel-Bary AS, Tolan DA, Nassar MY, et al. Chitosan, magnetite, silicon dioxide, and graphene oxide nanocomposites: synthesis, characterization, efficiency as cisplatin drug delivery, and DFT calculations. Int J Biol Macromol. 2020;154:621–633. doi: 10.1016/j.ijbiomac.2020.03.106.
- Tamara FR, Lin C, Mi FL, et al. Antibacterial effects of chitosan/cationic peptide nanoparticles. Nanomaterials (Basel). 2018;8(2):88. doi: 10.3390/nano8020088.
- Divya K, Vijayan S, George TK, et al. Antimicrobial properties of chitosan nanoparticles: mode of action and factors affecting activity. Fibers Polym. 2017;18(2):221–230. doi: 10.1007/s12221-017-6690-1.
- Wankar S, Alset U, Gumathannavar R, et al. Assessment of nano-functionalized cellulosic paper for selective estimation of Cr (VI) using diffuse reflectance spectroscopy. Environ Pollut Bioavailab. 2023;35(1):2215944. doi: 10.1080/26395940.2023.2215944.
- Wankar S, Sapre N, Gumathannavar R, et al. Silver-chitosan (Ag-CH) nanocomposite hydrogel for remediation of aqueous medium. Mater Today: Proc. 2022. doi: 10.1016/j.matpr.2022.12.023.
- Xiao Y, Liang H, Wang Z. MnFe2O4/chitosan nanocomposites as a recyclable adsorbent for the removal of hexavalent chromium. Mater Res Bull. 2013;48(10):3910–3915. doi: 10.1016/j.materresbull.2013.05.099.
- Rimu SH, Rahman MM. Insight of chitosan-based nanocomposite for removal of hexavalent chromium from wastewater-a review. Int J Environ Anal Chem. 2022;102(18):6801–6818. doi: 10.1080/03067319.2020.1817426.
- Ulatowska J, Stala Ł, Polowczyk I. Comparison of Cr (VI) adsorption using synthetic schwertmannite obtained by Fe3+ hydrolysis and Fe2+ oxidation: kinetics, isotherms and adsorption mechanism. Int J Mol Sci. 2021;22(15):8175. doi: 10.3390/ijms22158175.
- Muedi KL, Masindi V, Maree JP, et al. Rapid removal of Cr (VI) from aqueous solution using polycationic/di-metallic adsorbent synthesized using Fe3+/Al3+ recovered from real acid mine drainage. Minerals. 2022;12(10):1318. doi: 10.3390/min12101318.
- Yang Y, Zhang Y, Wang G, et al. Adsorption and reduction of Cr (VI) by a novel nanoscale FeS/chitosan/biochar composite from aqueous solution. J Environ Chem Eng. 2021;9(4):105407. doi: 10.1016/j.jece.2021.105407.