Abstract
Background: Cervical adenocarcinoma in situ (AIS) is the known precursor condition of cervical adenocarcinoma. The aim of this study is to evaluate treatment choices for AIS in terms of residual disease, recurrence and progression to invasive cancer.
Methods: This is a retrospective cohort study conducted at the Mercy Hospital for Women in Melbourne, Australia. Women diagnosed with AIS on histology between 2000 and 2013 were included. Patient records were reviewed for notes on symptoms, cytology, colposcopy and biopsy reports, initial treatment, any subsequent treatment, outcome and follow-up.
Results: A total of 114 patients were included: 87 patients were treated with one or more fertility-sparing procedures; 25 patients received a subsequent hysterectomy, one a trachelectomy and one chemo- and radiotherapy. Residual disease was found in 31% (9/29) after a previous procedure with positive margins. In 13.3% (2/15) residual disease was diagnosed despite clear surgical margins. The mean follow-up time was 78.6 months (range 7.9–183). Two patients (2/87, 2.3%) who primarily received fertility-sparing treatment had recurrent AIS. None of the patients progressed to invasive cancer.
Conclusion: Both positive and negative margins after initial treatment must be carefully considered in each case. With negative margins, fertility-sparing surgery should be a treatment choice available for all women, not only for those who want to preserve fertility. Fertility-sparing surgery requires regular follow-up.
Keywords (MESH terms):
Introduction
Due to successful cervical screening programmes, the overall incidence of invasive cervical cancer decreased substantially in developed countries. This is mainly caused by a decrease in incidence of squamous cell carcinomas (SCC), whereas incidence rates of adenocarcinoma (AC) stayed the same or often even increased, especially in younger women.Citation1–5 In Australia the incidence rate of cervical cancer in the period 1990–2011 decreased from 18 to 9.4 cases for every 100 000 women in the screening population aged 20–69. The number of squamous cell carcinomas decreased from 15.1 new cases per 100 000 in 1982 to 6.4 in 2011. In contrast, the incidence of adenocarcinoma has remained around two new cases per 100 000 women since 1991.Citation6 The proportion of adenocarcinoma relative to all cervical cancer has grown from 5–10% in the 1960s and 1970s to 20–30% currently.Citation1,3,6 This suggests the current screening programme does not efficiently detect the known precursor of adenocarcinoma, adenocarcinoma in situ (AIS), and is therefore unable to reduce the incidence of glandular cancer. Besides difficulties in diagnosing, the management of AIS is both challenging and controversial. Conservative treatment to preserve fertility in young patients is widely accepted in the case of negative surgical margins and careful follow-up. Nevertheless a hysterectomy remains the advised treatment for women who have completed childbearing. This is advised because AIS frequently extends high into the endocervical canal and it can be multifocal, therefore negative margins do not necessarily mean the lesion is completely excised.Citation7–10 A more recent study, however, considers conservative treatment with strict follow-up as a reasonable treatment option for all patients, not only for those who want to preserve fertility.Citation11
The aim of this study is to investigate treatment choices for adenocarcinoma in situ and their outcome by analysing the rate of residual disease, recurrence and progression to invasive cervical cancer, after completing treatment.
Methods
A retrospective cohort study was performed from February until May 2016 at the Mercy Hospital for Women in Melbourne. The research project was approved by the institutional Human Research Ethics Committee. Medical records of all women diagnosed with AIS on histology between 2000 and 2013 were identified using the hospital digitalised database. Women with coexisting squamous lesions and coexisting invasive adenocarcinoma were also included. Medical records were reviewed for notes on symptoms, cytology, colposcopy and biopsy reports, initial treatment, any subsequent treatment, outcome and follow-up. Primary management includes all procedures performed without any follow-up in between. The follow-up period was defined as the time between the date of the last treatment belonging to primary management and the date of last contact up to the time of this study, April 2016. In case a patient was not followed up in the Mercy Hospital, the GP and/or the Victorian Cytology Register were contacted for the missing follow-up PAP smears and HPV testing. Residual disease was defined as persisting disease diagnosed at a subsequent procedure within 12 months after initial treatment. After 12 months it was considered a recurrence. A positive margin is defined as the lesion present at the margin.
For statistical analysis Stata 13.1® was used (StataCorp LP, 2013, College Station, TX, USA).
Role of the funding source: not applicable. The corresponding author hereby confirms having the final responsibility for the decision to submit for publication.
Results
A total of 117 cases were identified. Two cases were excluded because of missing treatment details and one case was excluded because of a previous history of AIS treated elsewhere. Therefore 114 cases were included for analysis There was no documentation on HIV status. The baseline characteristics are summarised in Table .
Table 1: Baseline characteristics
Treatment
All 114 patients initially received fertility-sparing treatment (Figure ). A cold knife cone biopsy (CKC) was performed in 99 patients (86.8%), a large loop excision of the transformation zone (LLETZ) in 14 (12.2%) and laser treatment in one patient (0.9%). Histological reports were present for all patients except for the one with laser treatment. In one case no abnormalities were found and in another case only CIN II was diagnosed. Both cases had had AIS on a punch biopsy before. In 51 patients (51/113, 45.1%) solely AIS was diagnosed and in 60 patients (60/113, 53.1%) a mixed lesion was present, of whom 38 were diagnosed with AIS and coexisting CIN III.
Figure 1: Treatment flowchart of initial, secondary and tertiary treatment. There was no follow-up in between these treatments. All are part of primary management. Histology result of prior treatment is given below type of secondary and third treatment. CKC = cold knife cone; LLETZ = large loop excision of the transformation zone.
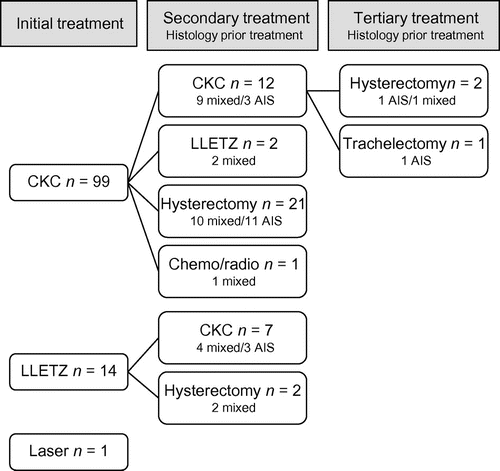
In nine mixed lesions (9/113, 8%) actual adenocarcinoma was found, combined with AIS in three cases and combined with AIS and CIN III in five cases. In one case adenocarcinoma was found with coexisting CIN III. The mean age of the patients diagnosed with adenocarcinoma was 43.7 years (range: 31–57 years). Five of these nine mixed cases with adenocarcinoma had previously had a biopsy, which showed AIS in four cases and possible high-grade glandular lesion in one. In two cases (2/113, 1.8%) early invasive adenocarcinoma combined with AIS and CIN was diagnosed. These patients were aged 26 and 27 years.
In 77 cases (77/113, 68.1%) the margins were clear and in 30 cases (30/113, 26.5%) the margins were involved, of whom 27 had at least AIS and three of them had solely CIN III present at the margin. In six cases (5.3%) the margin status was uncertain (Table ).
Table 2: Margin status of initial large loop excision of the transformation zone (LLETZ) vs. the margin status of initial cold knife cone biopsy (CKC)
Among the 113 histology reports, 15 multi-foci lesions were noted (13.2%).
Further treatment
Forty-five of all 114 (39.5%) patients received further elective treatment (see Figure ), in the majority of cases (66%) because of positive or uncertain margins found at previous treatment. Twenty-three patients received a hysterectomy as secondary treatment; among them were six of the nine patients with adenocarcinoma. Nineteen patients received a CKC as second treatment, among them were two out of nine patients with adenocarcinoma. One patient with adenocarcinoma received chemotherapy and radiotherapy after an initial cold knife cone biopsy with positive margins. Only three patients had a third procedure as part of their primary management, although only two of them had involved margins at secondary treatment.
In summary, 87 patients (87/114, 76.3%) were managed conservatively using one or more fertility-sparing procedures, while 25 (25/114, 21.9%) underwent a subsequent hysterectomy, one patient a trachelectomy and one patient chemotherapy and radiotherapy.
Noteworthy is that three patients with involved margins, one solely CIN III and three with uncertain margins, did not have any further treatment.
Residual disease
Nine patients (9/44, 20.5%) who had a secondary procedure were diagnosed with residual AIS; one of them had coexisting CIN II. Two patients (2/44, 4.5%) had residual adenocarcinoma, combined with AIS and CIN III in one case. Five patients had a purely squamous residual lesion, of whom two had only CIN I. See Table for residual disease in relation to margin status at prior procedure.
Table 3: Margin status of initial procedure in relation to residual disease found in the 44 secondary procedures performed
All three women who underwent a third procedure had residual AIS again and, besides this, one of them had also progressed to early invasive adenocarcinoma.
Three out of nine patients with adenocarcinoma had residual disease (33.3%), compared with 8/105 (7.6%) in the patients without adenocarcinoma. See Table for all pathology results of treatments belonging to primary management.
Table 4: Pathology results of all primary management treatments
Follow-up and recurrence
We did not find any follow-up for five patients; some had moved overseas. This left 109 patients for follow-up analysis. The mean follow-up time was 78.6 months (range 7.9–183). Eight patients were followed up for less than 24 months. Any further treatment given during the follow-up period is listed in Table . Two of eight patients who had a hysterectomy during follow-up were diagnosed with recurrent AIS at 3.5 and 6.4 years after initial treatment in which both had clear margins. One of these two patients was initially diagnosed with adenocarcinoma and received a second CKC before the hysterectomy during follow-up. The recurrence rate of the group with adenocarcinoma is 1/9 (11.1%) vs. 1/105 (1%) in the group without cancer. One patient had CIN III present in her hysterectomy specimen and one patient received a hysterectomy because of hematometra. Only one patient with uncertain margin status in primary management received a hysterectomy during the follow-up period. Three patients with positive margins and two more with uncertain margins did not have any further treatment in the follow-up period.
Table 5: Further treatment during follow-up period and initial treatment(s) patients had received before
To assess the completeness of follow-up we used the completeness index, C.Citation12 This is the ratio of the total observed follow-up time and the potential follow-up time and therefore it quantifies the effect of losses to follow-up. We calculated the median completeness of follow-up in our study as 85%.
None of the patients died of cervical cancer during the study period, had a recurrent carcinoma or progressed to cancer during the follow-up period.
Discussion
In Australia a modified Bethesda system (AMBS 2004) is used to describe cytology.
In the absence of consensus of what is considered residual disease and what is considered recurrent disease we defined these terms based on expert opinion.
Correlation cytology and histology
Most women do not have symptoms and they present with an abnormal Pap smear picked up by the national cervical screening programme. None of the nine cases with cancer or the two cases with early invasive adenocarcinoma were predicted on Pap smear or detected on punch biopsy. In a study conducted in Victoria (Australia) 16% of women with AIS on cytology were diagnosed with cancer on histology.Citation13 This is a high rate in comparison with women with squamous carcinoma in situ on cytology, among whom cancer is diagnosed in about 3–4%. For women with possible high-grade glandular or minor non-specific changes on Pap smear they found these rates to be 5% and < 1%, respectively.Citation13
In our study 42 women were referred with AIS on Pap smear and 4 (10%) were diagnosed with an adenocarcinoma in addition to AIS on histology. Thirty-three women had a possible high-grade glandular lesion on their referral Pap smear, 12 with a coexisting high-grade squamous lesion. Two (6%) of these 33 women were histologically confirmed as adenocarcinoma with AIS and CIN III.
In this study we found only one case with solely atypical glandular cells of undetermined significance (AGC-US) on cytology, which was diagnosed as AIS and CIN III on histology. Besides this one case, we found six cases of AGC-US combined with low- and high-grade squamous lesions. Two of these six cases were diagnosed with adenocarcinoma and the other four all had AIS, mostly with coexisting high-grade squamous disease. In a large recent population-based study in Sweden a high and persistent risk of invasive cancer was found, especially adenocarcinoma, for up to 15 years if atypical glandular cells were present on cytology compared with women who had normal cytology.Citation14 They also found the incidence rate of invasive cervical cancer after AGC to be significantly higher than for women with HSIL and LSIL for up to 6.5 years and similar to HSIL for up to 10.5 years. Despite this, they stated that only 54% of women with AGC underwent histology assessment, which is much less than after HSIL (86%).Citation14 It seems that glandular abnormalities on cytology can present a wide range of histology and, in comparison with squamous lesions, in a substantial number of cases even cancer.
Margins, residual disease and recurrence
In two (13%) cases with clear margins we found residual AIS at a subsequent procedure. In one case the distance to the closest endocervical margin was 1 mm and for the other case the distance to the closest margin was not described. In a review of 35 studies by Baalbergen et alCitation.15 the risk of residual disease after a cone biopsy with clear margins was 16.5% (range 0–57%). In another study, Krivak et alCitation.9 reviewed 14 studies and found residual disease in 26% (range 0–44%). In three more studies, by Costales et alCitation.16, Baalbergen et alCitation.11 and Costa et al.,Citation17 the rates of residual disease after negative margins were 13.5, 24 and 11.6%. Possible explanations for residual disease despite clear margins are the existence of multifocal ‘skip’ lesions or inadequate histopathologic examination of the cone specimen.
In nine (31%) cases with involved or uncertain margins we found residual disease at a subsequent procedure, two of whom had residual adenocarcinoma. In the literature this rate is more often described as around 50%.Citation9,11,15–17 We found no residual disease in two-thirds of the patients with positive margins. The elimination of residual disease can possibly be explained by the postsurgical inflammation and granulation tissue reaction.Citation11
Two of 87 patients (2%) who were treated conservatively with CKC, LLETZ or a combination were later diagnosed with recurrent AIS. There were no recurrences in the group that went on to have a hysterectomy or trachelectomy. Baalbergen et alCitation.11 found a 3.5% recurrence rate of AIS and Costales et alCitation.16 2%.
Conclusion
Both positive and negative margins after initial treatment must be carefully considered in each case. With negative margins, fertility-sparing surgery should be a treatment choice available for all women, not only for those who want to preserve fertility. Fertility-sparing surgery requires regular follow-up.
IRB status: approved.
Summary
Margin status, fertility-sparing surgery and long-term follow-up are important issues for management of cervical adenocarcinoma in situ (AIS), the known precursor condition of cervical adenocarcinoma.
List of abbreviations
AC = adenocarcinoma.
AGC-US = atypical glandular cells of undetermined significance.
AIS = adenocarcinoma in situ.
Ca = cancer.
CIN = cervical intraepithelial neoplasia.
CKC = cold knife cone biopsy.
HPV = human papillomavirus.
HSIL = high-grade squamous intraepithelial lesion.
LLETZ = large loop excision of transformation zone.
LSIL = low-grade squamous intraepithelial lesion.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
The authors would like to thank Dr Richard Hiscock for his contribution to the statistics of this research project. In addition they would like to thank Christine Smith for her assistance in obtaining the data.
References
- Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Women’s Health. 2012;21:1031–7. doi:10.1089/jwh.2011.3385. PMCID: PMC3521146.
- Bulk S, Visser O, Rozendaal L, et al. Cervical cancer in the Netherlands 1989-1998: decrease of squamous cell carcinoma in older women, increase of adenocarcinoma in younger women. Int J Cancer. 2005;113:1005–9. doi:10.1002/ijc.20678. PMID: 15515017.
- Smith HO, Tiffany MF, Qualls CR, et al. The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States – a 24-year population-based study. Gynecol Oncol. 2000;78:97–105. doi:10.1006/gyno.2000.5826. PMID: 10926787.
- Vizcaino AP, Moreno V, Bosch FX, et al. International trends in the incidence of cervical cancer: I. adenocarcinoma and adenosquamous cell carcinomas. Int J Cancer. 1998;75:536–45. PMID: 9466653. 10.1002/(ISSN)1097-0215
- Sherman ME, Wang SS, Carreon J, et al. Mortality trends for cervical squamous and adenocarcinoma in the United States. Relation to incidence and survival. Cancer. 2005;103:1258–64. doi:10.1002/cncr.20877. PMID: 15693030.
- Australian Institute of Health and Welfare. Cervical screening in Australia 2012-2013. Cancer series no. 93. Cat. No. CAN 91. Canberra: AIHW.
- Soutter WP, Haidopoulos D, Gornall RJ, et al. Is conservative treatment for adenocarcinoma in situ of the cervix safe? BJOG. 2001;108:1184–9. PMID: 11762660.
- Massad LS, Einstein MH, Huh WK, et al. 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Lower Gen Tract Dis. 2013;17:1–27. doi:10.1097/AOG.0b013e3182883a34. PMID: 23635684.
- Krivak TC, Rose GS, McBroom JW, et al. Cervical adenocarcinoma in situ: a systematic review of therapeutic options and predictors of persistent or recurrent disease. Obstet Gynaecol Surv. 2001;56:567–75. PMID: 11524622. 10.1097/00006254-200109000-00023
- Australian Government, National Health and Medical Research Counsil. Screening to prevent cervical cancer: guidelines for the management of asymptomatic women with screen-detected abnormalities. 2005. ISBN: 0 642 82714 1.
- Baalbergen A, Molijn AC, Quint WGV, et al. Conservative treatment seems the best choice in adenocarcinoma in situ of the cervix uteri. J Lower Gen Tract Dis. 2015;19:239–43. doi:10.1097/LGT.0000000000000114. PMID: 25943864.
- Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359:1309–1310. PMID: 11965278.10.1016/S0140-6736(02)08272-7
- Mitchell HS. Outcome after a cytological prediction of glandular abnormality. Aust N Z J Obstet Gynaecol. 2004;44:436–440. doi:10.1111/j.1479-828X.2004.00288.x. PMID: 15387866.
- Wang J, Andrae B, Sundström K, et al. Risk of invasive cervical cancer after atypical glandular cells in cervical screening: nationwide cohort study. BMJ. 2016;352:i276. doi:10.1136/bmj.i276. PMID: 26869597.
- Baalbergen A, Helmerhorst TJM. Adenocarcinoma in situ of the uterine cervix – A systematic review. Int J Gynecol Cancer. 2014;24:1543–8. doi:10.1097/IGC.0000000000000260. PMID: 25238167.
- Costales AB, Milbourne AM, Rhodes HE, et al. Risk of residual disease and invasive carcinoma in women treated for adenocarcinoma in situ of the cervix. Gynecol Oncol. 2013;129:513–6. doi:10.1016/j.ygyno.2013.03.015. PMID: 23541795.
- Costa S, Venturoli S, Negri G, et al. Factors predicting the outcome of conservatively treated adenocarcinoma in situ of the uterine cervix: An analysis of 166 cases. Gynecol Oncol. 2011;124:490–5. doi:10.1016/j.ygyno.2011.11.039. PMID: 22188786.
