Abstract
Objective: The objective of the study was to determine the prevalence and degree of anaemia in low-birthweight neonates exposed to intrauterine zidovudine (AZT) at a regional hospital neonatal unit in Durban.
Design: This was a descriptive, cross-sectional study.
Background: Perinatal exposure to AZT has been shown to cause anaemia in full-term babies. The effects of AZT in this group need to be established as there is a low-birthweight rate of 12.8% of anaemia in South Africa.
Outcome measures: Outcomes measures of the study were the neonatal haemoglobin (Hb) levels, gestational age and duration of AZT exposure.
Results: The total prevalence of anaemia was 47% in the 95 neonatal birth records analysed. The prevalence of anaemia was 16.7% and a mean Hb of 17 g/dl in the 26- to 28-week gestational age category; a prevalence of anaemia of 37% with a mean Hb of 16 g/dl in the 29- to 31-week category; and a prevalence of 54.8% of anaemia with a mean Hb of 17 g/dl in the > 31-week category. The mean Hb in the neonates exposed to more than 28 days of AZT was lower than that in neonates exposed to less than 28 days in the 29- to 31-week and > 31-week gestational age categories.
Conclusion: The haematological side-effects of neonatal anaemia resulting from intrauterine AZT exposure were mild and clinically insignificant in keeping with existing international and continental studies. The severity of anaemia in low-birthweight neonates appears to be the same as that in neonates with a normal birthweight. Current recommendations for the routine use of AZT for the prevention of mother-to-child transmission (PMTCT) of HIV are adequate, but further research involving larger numbers is needed.
Keywords:
Introduction
More than one million babies are born each year in South Africa, of whom up to 300 000 are human immunodeficiency virus (HIV) exposed. Of the estimated 22 000 neonatal deaths in South Africa in 2008, 43% were attributed to HIV infection.Citation1 Without the prevention of mother-to-child transmission of HIV (PMTCT), up to 40% of HIV-exposed infants develop HIV infection during pregnancy, birth or in the postnatal period.Citation2 In 1998, antepartum and intrapartum maternal zidovudine (AZT) and a six-week period of neonatal AZT prophylaxis became the benchmark of the PMTCT in most First World countries following the Paediatric Aids Clinical Trial Group-076 (PACTG-076) trial.Citation3 In 2005, a large clinical trial in Thailand demonstrated a 2% transmission rate of HIV from mother to child when the mother was given AZT from 28 weeks gestation and single-dose nevirapine (NVP) at the onset of labour. The baby was also given single-dose NVP at delivery and a daily dose of AZT for one week.Citation4
Figure depicts the evolution of the PMTCT of HIV programme in South Africa dating back to 1999. The PMTCT of HIV programme in South Africa dates back to 1999 in Khayelitsha where the PMTCT utilising AZT from 34 weeks gestation and at birth was initiated. In 2004, the Western Cape commenced AZT for all HIV-positive pregnant women from 28 weeks of gestation, as well as single-dose NVP at the onset of labour.Citation5 In 2004, the National Department of Health initiated a national PMTCT policy comprising single-dose NVP given at the onset of labour. The landmark study in Thailand in 2005 led to the introduction of dual therapy (AZT and NVP) in South Africa in 2008 for all HIV-positive pregnant women, as well as AZT prophylaxis for their babies.Citation4
Figure 1: Timeline of the prevention of mother-to-child transmission of human immunodeficiency virus programme in South Africa
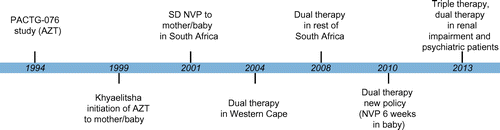
In April 2010, a revised PMTCT programme was launched in South Africa advocating AZT from 14 weeks gestation in all women with a cluster of differentiation 4 (CD4) count of > 350 cells/mm3.Citation6 In addition to the AZT, the mother received single-dose tenofavir (TDF) and emtracitabine (FTC) at delivery. The baby was also given six weeks of NVP for the duration of breastfeeding and no AZT. Other key components of the new policy included the HIV testing of pregnant women, treating pregnant HIV-positive women with antiretroviral drugs if the CD4 was less than 350 cells/m3, and the follow-up of mothers and infants at six weeks postpartum. The guidelines were revised in April 2013, and replaced dual therapy with TDF, efavirenz and FTC/lamivudine. AZT is administered to pregnant women who have evidence of psychiatric illness or renal impairment.Citation7 Currently, mother-to-child transmission rates vary widely across the country, averaging 8% nationally,Citation7 highlighting the need for a constantly improving the PMTCT programme.
Anaemia is a well known side-effect of AZTCitation8 in adults, and a number of studies have shown that perinatal exposure to AZT results in neonatal anaemia which is clinically insignificant and reversible.Citation3,9−12 A Tanzanian study on 82 HIV-positive pregnant women in 2008 demonstrated that AZT exposure in utero can cause mild and transient haematological alterations in infants.Citation13 The Promoting Maternal and Infant Survival Everywhere (PROMISE) International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT)Citation14 are currently being conducted in many resource-limited countries, including South Africa. A secondary objective is to document any side-effects of the PMTCT medication in the mother and baby.
Anaemia in neonates can result from a number of causes, including a decrease in red blood cell production, for example, anaemia of prematurity, an increase in red blood cell destruction in the case of infection, and blood loss during birth.Citation15,16 Most studies have excluded low-birthweight neonates because it is difficult to determine whether anaemia is due to prematurity or to AZT exposure in preterm babies, and infection is often present. 12.8% of babies born in South Africa in 2010/2011 had a low birthweight,Citation17 hence the need to evaluate the effect of AZT exposure in low-birthweight neonates. Low-birthweight babies are neonates with a birthweight of less than 2 500 g, irrespective of gestational age. The aim of this study was to evaluate the prevalence and clinical significance of anaemia in low-birthweight neonates exposed to intrauterine AZT at a regional hospital in Durban.
Method
This was a retrospective cross-sectional study, conducted at the neonatal unit at a regional hospital in Durban. The study population consisted of low-birthweight neonates of mothers who received AZT during pregnancy, and who were admitted to the neonatal unit and followed-up at the clinic for HIV-exposed neonates. Data were collected from May 2008 to February 2010 as the new Department of Health PMTCT of HIV guidelines were implemented in April 2008 and revised in April 2010. Therefore, relevant data were not available after this date. Two hundred and fifty HIV-exposed, low-birthweight neonates were admitted to the neonatal unit between May 2008 and February 2010, and included in the study. Inclusion criteria were AZT exposure during pregnancy and a birthweight of less than 2 500 g. Exclusion criteria were acute maternal blood loss in the peripartum period which resulted in haemodynamic instability or low Hb in the mother, acute blood loss in the baby and evidence of neonatal sepsis (raised or low white blood cells, low platelets, elevated C-reactive protein and positive blood cultures). Data were collected on birthweight, gestational age, neonatal Hb levels at birth and the duration of AZT intake by the mother during pregnancy. Data were captured and analysed using the SPSS® software package. Independent t-tests were used to demonstrate the relationship between the duration of AZT and the degree of anaemia. The numerical data were summarised by measures of central tendency, i.e. mean and median, and by measures of variability, i.e. range.
Anaemia was defined as a Hb or haematocrit concentration of more than two standard deviations below the mean for age.Citation16 The average Hb values on the first postnatal day were 15.1 g/dl (13.5–16.7 g/dl) for the gestational age of 26–28 weeks, 16.2 g/dl (14.5–17.9 g/dl for the gestational age of 28–31 weeks and 19.3 g/dl (17.1–21.5 g/dl) for the gestational age of > 31 weeks.Citation16 A baby was considered to be anaemic if the Hb was less than 13.5 g/dl at 26–28 weeks’ gestation, less than 14.5 g/dl at 29–31 weeks’ gestation and less than 17.1 g/dl at a gestational age of > 31 weeks.
Ethical consideration
Permission to conduct this study was obtained from the University of KwaZulu-Natal Research Ethics Committee (BE 174/010), King Edward VIII Hospital and the KwaZulu-Natal Department of Health.
Results
Of the 250 records on the paediatric database, 36% (90 patient files) could not be retrieved from the medical records department. From the 160 files located, 65 patient records did not meet the inclusion criteria for the following reasons: 21% (34/160) of the HIV-positive pregnant women did not receive AZT during gestation (seven of whom were unbooked), 7.5% (12/160) had no record of their full blood count results, 6.8% (11/160) of the mothers experienced antepartum haemorrhage, and 6.2% (10/160) had confirmed neonatal sepsis. Three women (1.9%) were found to be on highly active antiretroviral therapy and 1 neonate (0.6%) had a diagnosis of acute haemorrhage and was also excluded. Three mothers who were diagnosed with antepartum haemorrhage did not receive the PMTCT AZT, and three babies who were diagnosed with neonatal sepsis were born to mothers who did not receive AZT, therefore appearing twice in the above figures. Of the 250 admissions, 95 charts were analysed.
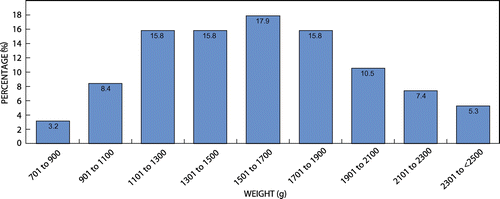
The majority of the neonates in the study population weighed between 1 501 g and 1 700 g (17.9% of n = 95, Figure ). 15.8% of the total number of neonates fell in the 1 101-1 300g category, 15.8% of the total number of neonates fell into the 1 301-1 500g category, and 15.8% of the total number of neonates fell into the 1 701-1 900g category (see Figure ). The lowest number of neonates weighed between 701 g and 900 g (3.2%)
The neonatal gestational age was categorised into three groups: 26–28 weeks, 29–31 weeks and > 31 weeks of age. Of these, the majority (62/95, 66%) of neonates fell into the > 31-week gestational age category, followed by the 29- to 31-week (27/95, 28%) and 26- to 28-week gestational age categories (6/95, 6%).
The mean Hb was 17 g/dl (13.4–19 g/dl) in the 26- to 28-week category, 16 g/dl (11.1–23.9 g/dl) in the 29- to 31-week and 17 g/dl (11.1–24 g/dl) in the > 31-week categories. One of six neonates, (16.7%) in the gestational age category of 26–28 weeks and 10 of 27 neonates (37%) in the gestational age category of 29–31 weeks had anaemia. The highest prevalence of anaemia (54.8%) was found in the gestational age category > 31 weeks, in which 34 of 62 neonates were affected. The overall prevalence of neonatal anaemia in low-birthweight, HIV-exposed babies whose mothers had been receiving AZT was 47% across all the gestational age groups.
There were six neonates in the 26- to 28-week gestational age category. Five were in the group that was exposed to AZT for less than 28 days, and only one neonate was exposed to AZT for more than 28 days. The mean Hb in neonates who were exposed to AZT for less than 28 days was 16.78 g/dl, and 18.1 g/dl for those exposed for longer than 28 days. The study found that the difference in Hb was statistically insignificant when the duration of AZT exposure was compared in this age group (p = 0.609).
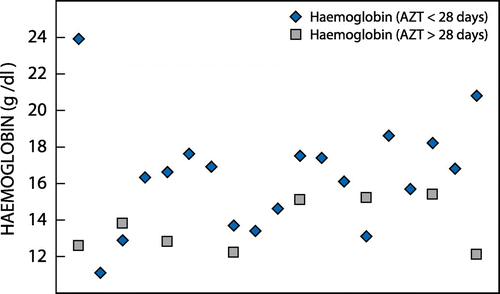
Figure illustrates the distribution of Hb in neonates who were exposed to AZT for less than 28 days in utero compared to those who had been exposed to it for more than 28 days in the gestational age category of 29–31 weeks. A total of 27 neonates were in the 29- to 31-week gestational age group, of whom 19 were exposed to AZT for less than 28 days and eight were exposed to it for more than 28 days. The mean neonatal Hb was lower in neonates exposed to a longer duration of AZT (13.63 g/dl vs. 16.37 g/dl), and there was a statistically significant correlation between the neonatal Hb and duration of AZT in this age category (p = 0.02).
Figure 3: The distribution of neonatal haemoglobin taken at birth in the 29- to 31-week gestational age category in relation to azidothymidine exposure of less than and more than 28 days
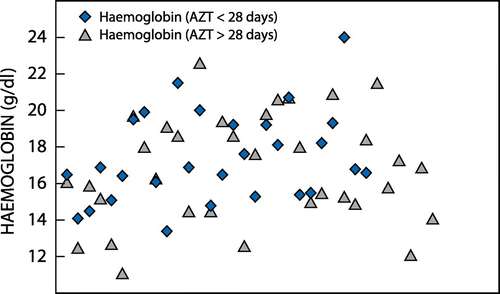
There were 62 neonates in the > 31-week gestational age category, of whom 28 (45%) fell into the group that was exposed to AZT for less than 28 days and 34 (55%) in the group that was exposed to it for more than 28 days. Figure illustrates the distribution of neonatal Hb in neonates with less than and more than 28 days of AZT exposure in this age category. The mean Hb was 17.42 g/dl in neonates with less than 28 days of AZT exposure, and 16.81 g/dl in those exposed to AZT for more than 28 days. There was no statistical significance in the difference in neonatal Hb between the two groups with exposure to AZT of differing duration (p 0.38).
Figure 4: The distribution of neonatal haemoglobin taken at birth in the > 31-week gestational age category in relation to azidothymidine exposure of less than and more than 28 days
Pearson’s product-moment correlation coefficient test for neonatal weight and Hb showed an insignificant correlation value of 0.041 and a p of 0.694. The correlation between neonatal Hb and gestational age was statistically significant, with a p of 0.01. There was no statistical significance between Hb and weight.
A t-test of gestational age and duration of AZT intake showed a statistically significant correlation of p −0.01.
Discussion
When conducting the analysis for this study, a significant number of charts could not be found and the blood results were misplaced. Poor recordkeeping and loss of data are significant weaknesses that are inherent to retrospective studies. The large number of records that could not be traced at this large regional teaching hospital was a cause for concern as an audit and reflection of best practice are dependent upon the review of patient management.Citation18
Of the neonates admitted who were HIV exposed, 21% (34/160) had not been commenced on a PMTCT programme. While this is better than the 27% found in an audit of the PMTCT programme in Khayelitsha,Citation19 it is still worrying. Factors contributing to the high rate of missed opportunity may include the fact that many clinics had not yet implemented the national guidelines which were only introduced in April 2008. There is often a significant lag time between policy dissemination and implementation. This is often a challenge when health strategies are being changed.Citation20 Despite the PMTCT programme becoming national policy in 2005, only one third of HIV-positive pregnant women received the PMTCT drug regimen in 2007.Citation20 A further reason for the PMTCT programme not being taken up was that a number of pregnant women presented for the first time in labour and were unbooked at the antenatal clinic. HIV testing was only performed intrapartum, and often postpartum, for these women, so at the very most a stat dose of nevirapine was administered to the pregnant women intrapartum, resulting in limited protection for the baby. Unbooked mothers represent a challenge with respect to the PMTCT programme. Other centres have reported a 29% rate of unbooked mothers presenting for delivery.Citation21 Home-based teams, as part of the re-engineering of primary health care, may represent an opportunity that could be used to address this problem. Missed opportunities possibly represent new, but potentially avoidable, paediatric HIV infections. Vigilance, regular audits, close cooperation between those providing antenatal care and perinatal care, as well as ongoing training are essential aspects of the PMTCT programme if the perinatal transmission of HIV is to be reduced to 2%.Citation22 In order to address this issue, the 2013 PMTCT guidelines stipulate that pregnant women who test positive for HIV at the initial antenatal visit should be commenced on the PMTCT programme at the same visit.Citation7
In this study, the mean neonatal Hb in neonates exposed to AZT was lower than the normal value for neonatal Hb at birth. As anaemia is said to be clinically significant and severe when Hb is less than 10 g/dl,Citation23 none of the neonates included in this study had severe anaemia at birth. These findings are similar to those of large, international, randomised, double-blinded placebo-controlled studies by Connor et al.Citation3 and Sperling et al.Citation8, who demonstrated mild and transient anaemia at birth in AZT-exposed, full-term babies, as well as those in the African study by Ziske et al,Citation13 which also showed similar mild haematological side-effects in the full-term newborn.
In this study, the prevalence of anaemia was shown to increase with gestational age and with duration of AZT exposure, with a statistically significant correlation between gestational age and duration of AZT intake (p = 0.01). This is consistent with the finding of Lallemant et al,Citation24 in which it was demonstrated that mothers exposed to AZT for a lengthy period experienced more neonatal complications than those exposed for a short period. The findings of this study suggest that pregnant women may have taken AZT for a greater duration when continuing to a later gestation, which, in turn, caused a higher prevalence of anaemia. However, the numbers in this study were small and the results must be treated with caution as our findings were different to those of the PACTG-076 studyCitation3 which concluded that the risk of anaemia was not associated with the duration of maternal AZT intake. Further research is required to adequately assess the correlation between AZT duration and degree of neonatal anaemia at birth, as the latest PMTCT guidelines recommend that pregnant women with renal impairment and psychiatric illness receive AZT from the first antenatal visit, which may be at an early gestation. For now, it is believed that the benefits of PMTCT AZT medication commenced at an earlier gestation outweigh the overall risks.
The high prevalence of neonatal anaemia in this study was in keeping with this commonly known side-effect of AZT.Citation25 However, the overall rate of 47% was higher than 29% for grade 1 anaemia in full-term infants found in the Tanzanian study.Citation13 The high level of anaemia may relate to the fact that the normal values for mean neonatal Hb taken at birth in low-birthweight babies who are born preterm were taken from the recent literature, based on findings in a First World setting,Citation15 as there are no national normal reference ranges for preterm neonates in South Africa.
Because of missing records, the small sample size and use of only one centre, all limiting factors in the study, the results presented must be treated with caution.
Conclusion
The prevalence of neonatal anaemia in low-birthweight, HIV-exposed babies whose mothers had been receiving AZT at a regional hospital was in keeping with this commonly known side-effect of AZT. The haematological side-effects of anaemia in neonates resulting from AZT exposure in utero were found to be of a mild and clinically insignificant nature. Issues were not identified that warrant an amendment to the current recommendations for the use of AZT for the PMTCT. Further research is required to confirm the results of the study in view of the small sample size. The ongoing PROMISE IMPAACT clinical trialCitation14 will detail the side-effects of the PMTCT medication, provide more information on the significance of duration of exposure to AZT with regard to neonatal anaemia, and may influence current PMTCT guidelines.
Acknowledgements:
The authors wish to extend their gratitude to Prof M Adhikari and Dr N Nair for their assistance in completing this article.
References
- MacDonald M, Yadler R. Maternal child and women’s health summit by Dr Aaron Motsoaledi. 2009.
- Kak L Chitsike ILuo C Rollins N. Prevention of Mother-to-Child Transmission of HIV/AIDS Programmes, Opportunities for Africa’s Newborns. 2011;113–126
- Connor EM, Sperling RS, Gelber R, et al. Paediatric AIDS Clinical Trials Group Protocol 076 Study Group. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med. 1994;331: 1173–80.10.1056/NEJM199411033311801
- Lallemant M, Jourdain G, Le Coeur S, et al. Single-dose perinatal nevirapine plus standard nevirapine to prevent mother to child transmission of HIV-1 in Thailand. New Eng J Med. 2004;351(3): 217–28.10.1056/NEJMoa033500
- Draper B, Abdullah F. A review of the prevention-of-mother-to-child-transmission programme of the Western Cape provincial government, 2003-2004. S Afri Med J. 2008;98(6): 431–42.
- DOH Clinical guidelines. The South African antiretroviral treatment guidelines. National Department of Health SA: PMTCT guidelines revised; 2013.
- DOH Clinical guidelines: prevention of mother to child transmission. National Department of Health SA. 2010.
- Moyle G, Sawyer W, Law M, et al.. Changes in haematological parameters and efficacy of thymidine-analogue-based, highly active antiretroviral therapy: a meta-analysis of six prospective, randomised, comparative studies. Clin Ther. 2004;26(1): 92–7.10.1016/S0149-2918(04)90009-4
- Sperling RS, Shapiro DE, McSherry GD, et al. Safety of maternal-infant zidovudine utilized in the Paediatric AIDS Clinical Trial Group 076 Study .AIDS 1998;12(14):1805–09. (London, England) .10.1097/00002030-199814000-00012
- Lahoz R, Noguera A, Rovira Nu, et al. Antiretroviral-related haematological short-term toxicity in healthy infants - implications of the new neonatal 4 week zidovudine regimen. Paediatr Infect Dis J. 2010;29(4): 376–9.
- Taha TE, Kumwenda N, Gibbons A, et al. Effect of HIV-1 antiretroviral prophylaxis on hepatic and haematological parameters of African infants. . AIDS.2002;16(6):851–8. (London, England).10.1097/00002030-200204120-00004
- Taha TE, Kumwenda N, Kafulafula G, et al. Haematological changes in African children who received short-term prophylaxis with nevirapine and zidovudine at birth. Ann Trop Paediatr. 2004;24(4):301–9.10.1179/027249304225019127
- Ziske J, Kunz A, Sewangi J, et al. Haematological changes in women and infantsexposed to an AZT-containing regimen for prevention of mother to child transmission of HIV in Tanzania. PLoS One. 2013;8(2):e55633.10.1371/journal.pone.0055633
- PROMISE IMPAACT 1077BF ongoing randomized clinical trial. 2010.
- Fanaroff AA, Martin RJ, Walsh MC. Fanaroff and Martin’s Neonatal perinatal medicine, Diseases of the fetus and infant. 9th ed. JFletcher editor. St. Louis, MI: Elsevier Mosby. (vol 2) 2011. p. 1309 –10
- Newland AC, Evans TGJR. ABC of clinical haematology: haematological disorders at the extremes of life. Br Med J. 1997;314(7089):1262–65.10.1136/bmj.314.7089.1262
- DHIS data 2010/11. Extracted January 2012
- Zegers M, de Bruijne M, Spreeuwenberg P, Wagner C, Groenewegen P, van der Wal G. Quality of patient record keeping: an indicator of the quality of care? BMJ Qual Saf. 2011;20(4):314–8.10.1136/bmjqs.2009.038976
- Fitzgerald FC, Bekker LG, Kaplan R, et al.. Mother to child transmission in a community based antiretroviral clinic in South Africa. S Afr Med J. 2010;100:827–31.
- World Health Organization. Towards Universal Access: scaling up priority HIV/AIDS interventions in the health sector. Geneva: World Health Organization; 2008.
- Sibeko S, Moodley J. Health care attendance patterns by pregnant women in Durban, South Africa. SA Fam Pract. 2006;48(10):17–17e.
- Republic of South Africa. National Strategic Plan on HIV, STIs and TB: 2012–2016. SANAC:Pretoria; 2011.
- Division of AIDS Table for Grading the Severity of Adult and Paediatric Adverse Events. Version 1.0, December, 2004.
- Lallemant M, Jourdain G, Le Coeur S, et al. A trial of shortened zidovudine regimens to prevent mother-to-child transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;343(14):982–91.10.1056/NEJM200010053431401
- Volmink J, Siegfried NVan de Merwe LBrocklehurst P. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database of Systematic Reviews [Internet; Peer Reviewed; UK & Ireland]. 2007.