ABSTRACT
Introduction
Human papillomavirus (HPV) is identified as a culprit in a subset of head and neck squamous cell carcinomas (HNSCCs). The clinicopathologic profile displayed by this subset diverges from that of HPV-negative HNSCCs. Despite a variety of available tests, there is no consensus on which technique is the best for detection of HPV in HNSCCs. Although this field has received substantial interest within different continents, African and Egyptian populations are not yet well studied within the literature.
Methods
This cross-sectional study was carried out to detect HPV prevalence in HNSSC and to correlate the viral prevalence with different clinicopathologic parameters as well as with the patients’ outcome. For 51 patients with HNSCC, HPV-16 DNA was determined via PCR, while E6/E7 mRNA was detected employing real-time PCR. Immunohistochemistry (IHC) was performed to assess p16 status.
Results
P16 was overexpressed in 49% of cases, while HPV-16 DNA was detected in 52.9% of cases, and likewise, E6/E7 mRNA was found in 52.9% of cases. There was a very good agreement between HPV16 DNA and RNA results (κ = 0.843, P-value <0.001). Meanwhile, a good agreement was revealed between HPV16 DNA and p16 IHC results (κ = 0.608, P-value <0.001). Similarly, there was a good agreement between HPV RNA results and p16 IHC results (κ = 0.608, P-value <0.001). By the end of the study period, 13.7% of the enrolled patients died, with the overall survival of the studied patients being 17.29 months. Of note, there was no statistically significant correlation between the overall survival and HPV status.
Conclusion
The present study highlights the significant role played by HPV in HNSCC. Furthermore, it reveals that although p16 has been a marker of HPV existence in HNSCC, it should not be the sole determinant of HPV role in tumorigenesis.
1. Introduction
Around 600,000 people worldwide become afflicted with a head and neck carcinoma (HNC) per year [Citation1], with squamous cell carcinoma (HNSCC) constituting over 90% of these cases [Citation2]. HNSCC has been characteristically linked to risk determinants, e.g., alcohol intake and smoking. Nonetheless, over the last decade, the global incidence drifts have witnessed a sharp upsurge in a subset of HNSCC that are etiologically allied with high-risk HPV rather than the traditional risk factors [Citation3].
On a global scale, HPV16 has significantly been the most prevailing genotype (82% of positive cases); then HPV18 comes second, and sporadic genotypes come third [Citation4]. Notably, HPV-driven HNSCC depicts a clinically distinct disease group compared to other HNSCC, with the affected patients tending to be younger males who are typically nonsmokers [Citation5].
Despite more than 30 years of research on HPV and cancer and while it is now recognized that almost all cervical carcinomas are caused by HPV, there is often a debate on whether or not and to what degree other cancer types are caused by HPV [Citation6]. One reason for this is that HPV prevalence is assessed via different modalities employing strategies that vary in both design and targets. Examples of the targets have been viral DNA, viral RNA, HPV-specific antibodies, as well as viral oncoproteins [Citation7]. Meanwhile, in attempting to detect active HPV, some researchers have advocated utilizing immunohistochemistry (IHC) of p16 as a marker. This protein is encoded by CDKN2A which is a tumor-suppressor gene. Characteristically, HPV-associated cancers exhibit aberrant p16 over-expression that results from E7 viral oncogene transcription [Citation8].
Remarkably, HPV-positive HNSCCs have been significantly escalating in the USA since 1970, chiefly affecting the oropharynx of white males at middle age. And throughout 15 years, the incidence witnessed a 225% uprise [Citation9].
The pervasion of HPV-associated HNSCCs reported in literature is versatile depending on the geographic location and the tumor subsites. This work aimed at shedding light on HPV prevalence among HNSCC patients within the Egyptian population and its relation to patients’ outcome.
2. Materials and methods
2.1. Ethical consideration
Before commencing this study at the Faculty of Medicine, Cairo University, it has been accepted by the Research Ethics Committee of the Institutional Review Board. From every participant, an informed consent was procured.
2.2. Patients and samples
The current cross-sectional analytical study involved 51 participants having HNSCCs admitted at the Department of Otorhinolaryngology, Faculty of Medicine, Cairo University, during the period from November 2017 throughout August 2019. All cases clinically diagnosed with HNSCC and pathologically confirmed at the Pathology Department, Faculty of Medicine, Cairo University were enrolled.
The clinicopathologic parameters (age, sex, date of diagnosis, TNM stage, stage group as per Head and Neck Cancer Study Group (HNCSG) [Citation10], history of smoking/alcohol intake, treatment modalities, and patients’ outcome data) were collected from the patients’ files. Finally, direct communication with the patients or their first-degree relatives was done to update the follow-up data on patients’ survival.
Surgical specimens were collected, then sent to the Pathology Department, Faculty of Medicine, Cairo University, fixed in 10% formalin, processed, and embedded within blocks of paraffin. A microtome was used to cut the paraffin-embedded (FFPE) blocks to obtain a thickness of 5 µm.
Sections were subjected to hematoxylin and eosin staining. Smears were screened to verify both the diagnosis and the grading of the tumor, and further validation was made by two pathologists. Additional sections were cut on positively charged slides for immunohistochemical staining against p16INK4a. Five micrometers of tissue sections were added into Eppendorf tubes for the extraction of DNA and RNA, and subjected to PCR and RT-PCR for the evaluation of HPV status, at the Department of Medical Biochemistry and Molecular Biology, Faculty of Medicine, Cairo University.
2.3. P16 immunohistochemistry
In the current study, p16 IHC was carried out via Dako REAL™ EnVision™ Detection System (Dako, Agilent, USA) in addition to the autostainer immunostaining instrument on the FFPE tissues. Antigen retrieval was done using a p16 rabbit polyclonal antibody (CDKN2A/p16-INK4a antibody, 1:400–800 dilution; Chongqing Biopsies Co., Ltd, Chongqing, China). The substrate + chromogen used was 3–3′-diaminobenzidine (DAB), which on staining results in a brown-colored precipitate at the antigen site. Hematoxylin was used as a counterstain. Samples were classified by qualitative measurement as being positive (cells depicting staining in the cytoplasm and/or nucleus) or being negative [Citation11].
2.4. HPV16 DNA detection
Genomic DNA extraction from FFPE tissues
The DNA Purification Kit (Gene JET) from Thermo scientific, United States (K0721) was operated to extract the DNA as per the manufacturer’s standard protocol [Citation12].
PCR amplification
Primers were synthesized by Bio Basic Inc., Canada. The amplification was performed in Biometra T personal thermal cycler. The sequence of the sense primer was 5′-CAAAGC CGTCGCCTTGGGCA-3′, while the sequence of the antisense primer was 5′-GGTGT GGCAGGGGTTTCCGG-3′[Citation13].
2.5. HPV 16 E6/E7 mRNA detection
Extraction of RNA and synthesis of complementary DNA
The RNeasy mini kit from Qiagen (cat no. 74,104), United States was operated to isolate RNA as per the manufacturer’s instructions [Citation14]. The total RNA obtained was utilized for cDNA synthesis via cDNA reverse transcription kit from Fermentas, United States (K1621).
Real-time PCR employing SYBR Green I:
To determine the expression of E6 and E7 mRNA, the primers depicted in were employed. These primers have been synthesized and bought from Bio Basic Inc., Canada.
Table 1. The primer sequences used for HPV16 E6/7 mRNA detection [Citation15].
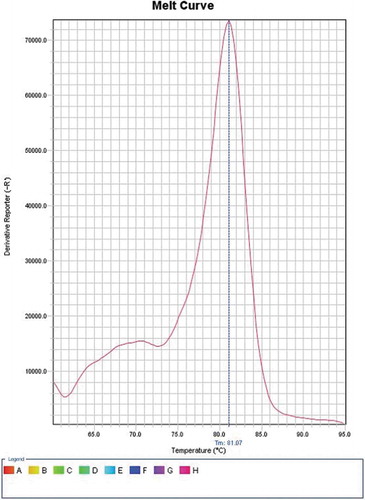
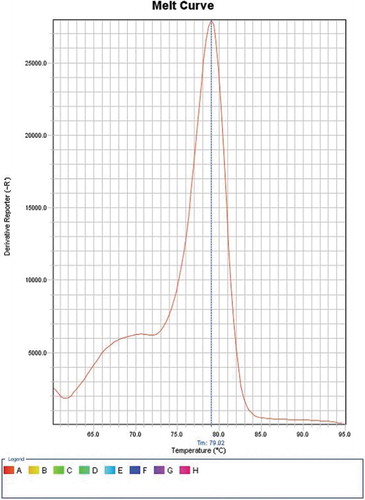
SYBR Green I (Qiagen, USA) was employed to perform real-time PCR as per its manufacturer’s instructions. Reagents without cDNA were used as a negative control. For amplification and analysis, StepOne Thermal Cycler from Applied Biosystems, United States, was utilized to process the reaction mixtures. Cycling was under these conditions: a denaturation step for 5 min at 95°C, ensued by forty cycles for 1 min, also at 95°C, then 1 min at 60°C, and 1 min at 72°C. This was ensued by extension for 5 min at 72°C. Melting curve analysis (with temperature 81.07⁰C for E6 and 79.02⁰C for E7) was performed ( and )
The PCR data sheet included Ct values of E6, E7, and the housekeeping gene (β-actin gene), which is the gene continuously, and normally expressed in human cells, as an internal control.
Relative quantification was determined by obtaining the Ct for both E6 and E7 of the examined samples and comparing it with a predetermined HPV16 positive SSC with known Ct for both E6 and E7 (retrieved from the Pathology Department, Faculty of Medicine, Cairo University) which was used as a positive control.
2.6. Statistical analysis
Following the coding of data, they were entered into the statistical package for the Social Sciences version no. 25 (IBM Corp., USA). For all variables that are quantitative, the mean, the median, the standard deviation, the minimum, and maximum were utilized; and for variables that are categorical, frequencies, and percentages were employed. To compare different groups, the t-test (unpaired) was applied. To compare categorical data, Chi-square (χ2) test was employed. If the expected frequency was below 5, the exact test was used instead. Kappa (k) measure of agreement was employed to assess the agreement between variables that are categorical. P-values were regarded as statistically significant if they were below 0.05.
3. Results
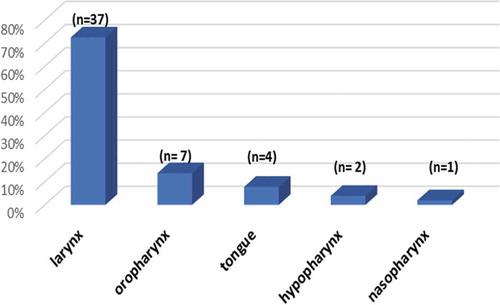
The current cross-sectional study included 51 cases of HNSCC, of which 43 cases (84.3%) were males. The age range of the enrolled patients was 29 to 92 years revealing a mean of 56.67 years and a standard deviation (SD) of ±13.34 years. The sites of SCC in the studied patients are displayed in .
Of the 51 patients, 36 patients (70.6%) were smokers. There were only two cases (3.9%) with history of alcohol intake.
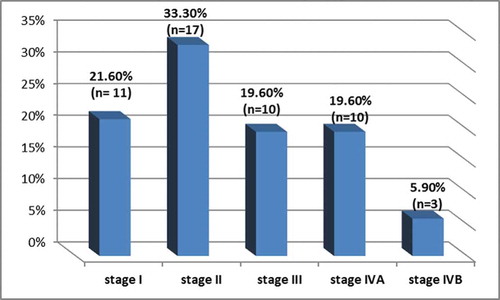
On the other hand, 87.4% of the patients had carcinoma of grade II histological differentiation and 21.6% had grade III. Out of the 51 patients, 17 cases (33.3%) had the carcinoma in T2 stage, 13 cases (25.5%) in T3 stage, 10 cases (19.6%) in T1 stage, 7 cases (13.7%) in T4a stage, 2 cases (3.9%) in T4b stage, one case (2%) in Tis stage, and one case (2%) in Tx stage. Meanwhile, 38 patients (74.5%) had the tumor in N0 stage, 6 patients (11.8%) in N1 stage, 6 patients (11.8%) in N2 stage, and only one patient (2%) in N3 stage. All patients were in M0 stage. According to UICC classification, the distribution of tumor stages in the studied patients is shown in .
Regarding treatment modalities, 24 patients (47.1%) underwent surgery and received radiotherapy, 18 patients (35.3%) underwent surgery alone, 7 patients (13.7%) received radiotherapy alone, and 2 cases (3.9%) underwent surgery and received both radiotherapy and chemotherapy.
3.1. HPV DNA state of the tumors
In this study, 27 patients (52.9%) were positive for HPV16 DNA. The mean age was 54.63 years (SD± 14.84 years) for HPV16 DNA-positive cases and 58.96 years (SD ±11.3 years) for HPV16 DNA-negative cases. We have not detected any significant correlation between HPV16 DNA result and any of the other variables ().
Table 2. Correlation between HPV16 DNA and clinicopathologic variables of the studied patients.
3.2. E6/E7 mRNA
Concerning E6/E7 mRNA detection; the expression of transcripts of E6 or those of E7 was used to demarcate a carcinoma as positive for HPV16 RNA. Out of 51 patients, 27 cases (52.9%) were positive, with the mean age being 56.81 years (SD± 15.98 years) for HPV16 RNA-positive cases and 56.5 years (SD± 9.9 years) for HPV16 RNA-negative cases. A statistically significant association was noticed between E6/E7 mRNA expression and oropharyngeal subsite, where all patients with oropharyngeal carcinoma in this study (n = 7) were positive for E6/E7 mRNA (P-value = 0.007). Nonetheless, there was no notable correlation linking HPV16 RNA state to any other variable ().
Table 3. Correlation between E6/E7 mRNA and clinicopathologic variables of the studied patients.
3.3. P16 IHC
Out of 51 patients, 25 cases (49%) were p16 positive ( and ).
Figure 5. A: Photomicrograph showing moderately differentiated non-keratinized squamous cell carcinoma [H&E, magnification x200], B: Photomicrograph showing negative staining of p16 of the same case [p16-INK4a, original magnification x200].
![Figure 5. A: Photomicrograph showing moderately differentiated non-keratinized squamous cell carcinoma [H&E, magnification x200], B: Photomicrograph showing negative staining of p16 of the same case [p16-INK4a, original magnification x200].](/cms/asset/17e44841-b800-450a-8e3e-d7ab8896a840/tajm_a_1827944_f0005_oc.jpg)
Figure 6. A: Photomicrograph showing moderately differentiated keratinized squamous cell carcinoma [H&E, original magnification x400], B: Photomicrograph showing positive cytoplasmic p16 staining via IHC of the same case [p16-INK4a, magnification x400].
![Figure 6. A: Photomicrograph showing moderately differentiated keratinized squamous cell carcinoma [H&E, original magnification x400], B: Photomicrograph showing positive cytoplasmic p16 staining via IHC of the same case [p16-INK4a, magnification x400].](/cms/asset/d36ba2a5-6c4f-459e-97e6-fb95fd925e28/tajm_a_1827944_f0006_oc.jpg)
The mean age was 58.96 years (SD± 16.1 years) for p16-positive patients and 54.46 years (SD± 9.83 years) for p16-negative ones. The study revealed a notable correlation linking p16 results to the grade of histological differentiation; 23 cases (92%) of p16-positive cases had grade II carcinoma (moderately differentiated) and 2 cases (8%) had grade III (poorly differentiated), while in p16-negative cases, 17 cases (65.4%) had grade II carcinoma and 9 cases (34.6%) had grade III (P- value = 0.021). Meanwhile, no significant correlation was noticeable between p16 state of our studied patients and other variables ().
Table 4. Correlations between p16 and clinicopathologic variables of the studied patients.
3.4. Agreement between HPV16 DNA, E6/7 mRNA, and p16 IHC
Nineteen patients (37.3%) had simultaneously positive results for HPV16 DNA, E6/E7 mRNA, and p16 IHC, while 20 patients (39.2%) had simultaneously negative results for the three tests. Meanwhile, six patients (11.8%) had positive DNA and RNA results but were negative for p16. On the other hand, two cases (3.9%) had negative results for both DNA and RNA, but had positive p16 result. In addition, two patients (3.9%) had positive DNA and p16 results but negative RNA result, and 2 patients (3.9%) had positive RNA and p16 results but negative DNA result.
3.5. Comparing between HPV16 DNA and RNA results
A very good agreement was revealed between both tests (κ = 0.843, P-value <0.001). Out of 27 DNA-positive patients, 25 patients (92.6%) were also RNA-positive. However, two patients (7.4%) did not show HPV E6 or E7 oncogenes expression despite being DNA-positive. In the meantime, out of 24 DNA-negative patients, 2 patients (8.3%) were RNA-positive.
3.6. Comparing between HPV16 DNA and p16 IHC results
A good agreement was observed between the two tests (κ = 0.608, P-value <0.001); where out of 27 DNA-positive patients, 21 patients (77.8%) revealed p16 over-expression. Notably, six patients (22.2%) were p16-negative while being DNA-positive, and out of 24 DNA-negative patients, 4 patients (16.7%) revealed p16 overexpression.
3.7. Comparing E6/E7 mRNA detection and p16 IHC results
There was a good agreement between both tests (κ = 0.608, P-value <0.001). However, six patients (22.2%) were p16 negative while being RNA-positive. Meanwhile, out of 24 RNA-negative patients, 4 patients (16.7%) revealed p16 overexpression.
3.8. Patients’ outcome
The mean follow-up length (defined as the date of diagnosis till the date of the patient’s final follow up, or till the end of this study period, or till the date of the patient’s demise) was 17.29 months (SD ±3.06 months). In the end of this study, seven participants (13.7%) passed away, 3 (42.9%) of them had disease-related mortalities. Concerning these three mortalities; two of them had stage IVA laryngeal SCC with negative DNA, RNA and p16 results. The third case had stage II oropharyngeal SCC with positive DNA, RNA and p16 results.
4. Discussion
HPV prevalence in carcinoma of the oropharynx has been recognized, and the interrelationship between HPV and the patients’ survival was evident among various reports [Citation16,Citation17]. Nonetheless, the implication of HPV in HNSCC originating from sites outside the oropharynx is still obscure. The crude incidence rates of HNSCC are strongly related to gender and age; being the double in males, and occurring at an average age of 65 years [Citation18]. In this study, male predominance (84.3%) and the involvement of old patients (mean age was 56.67 years, SD = 13.34 years) were observed. These findings were concordant with previous studies [Citation19,Citation20]. The predominance of male patients and senile age may be clarified by the commoner subjection to some risk determinants e.g. alcohol and tobacco, though occupational and environmental factors may also contribute [Citation21].
In the present study, cancers of the larynx constituted the majority of cases (72.54%), while oropharyngeal SCC constituted 13.7% of the cases. These results were somewhat divergent from the worldwide distribution where most of the tumors in the upper respiratory system arise in the oral cavity and oropharynx (43% and 31%, respectively), and are followed by laryngeal tumors (26%) [Citation12,Citation22]. The primary tumor site distribution is likely a reflection of smoking and alcohol consumption habits of the participants. While smoking is firmly linked to laryngeal cancers, alcohol intake tends to be linked to carcinomas of the oropharynx [Citation23]. However, some reports highlight the increasing percentage of patients having HNSCC but lacking history of alcohol intake and smoking at diagnosis. This trend is especially common in those having HPV-positive HNSCC [Citation24,Citation25].
In this research, 70.6% of the patients gave history of smoking, while only 3.9% had a history of alcohol consumption. The link between smoking and HNCs has been prominent for over three decades, and cigarette smoke is a notable ample source of carcinogenic elements [Citation26]. While smokers have been commoner than nonsmokers in this research, there was an absent significant association linking HPV state to smoking history. Our observations agreed with those of Lindel et al. [Citation27] concerning the absence of a significant association linking smoking to HPV16. Nonetheless, most studies found a significant inversely proportion relation between smoking and HPV state; HPV positive-patients were mainly nonsmokers [Citation28,Citation29]. However, some studies suggested a synergism between smoking and HPV16 state [Citation28,Citation30]. These versatile results are chiefly accredited to: geographical distribution, site of the investigated tissue, technical considerations, or the HPV-16 molecular test operated.
The prevalence of HPV-DNA in HNSCCs can differ widely according to the cancer site, method of detection, and the geographical area [Citation31,Citation32]; being high among HNSCC cases in Sweden (93%) [Citation33], the United States (71%) [Citation34] and Eastern Denmark (62%) [Citation35]. In the current study, HPV16 DNA prevalence was 52.9%, which was in concordance with former studies where HPV16 DNA prevalence was 51.7% [Citation36] and 50% [Citation37]. In contrast, previous research demonstrated either absent HPV DNA in HNSCC, e.g., in China and Mozambique [Citation38,Citation39], or a low (15.5%) or intermediate (34.4%) prevalence in Brazil and Germany [Citation40].
Some authors do not judge an HNSCC to be positive for HPV unless biological examination is accompanied by positive result for E6/E7 mRNA or p16 INK4a [Citation41]. In a study by Gheit et al. [Citation42], the examination of further FFPE blocks from five participants classified as possessing HPV DNA-positive and HPV RNA-negative cancers demonstrated that HPV DNA existed in only one specimen of every participant, denoting that HPV DNA was unevenly dispersed in the whole tumor tissue, and its existence may be the result of infection in other areas. Such findings reiterate those of previous studies that emphasized the restrictions of considering HPV DNA alone as the evidence of viral causality [Citation14,Citation43].
To further analyze HPV carcinogenic potentiality, the expression of the mRNA-encoded viral oncoproteins (E6/E7) was assessed. In this research, the prevalence of RNA-positive tumors was 52.9%. The expression of E6/E7 mRNA was variable in different studies reaching as high as 78.1% and 66% in some studies [Citation44,Citation45] and as low as 20.8% and 31% in others [Citation46–48]. These discrepancies may be due to the uneven prevalence of HPV-containing tumors, geographical and socio-economical factors, in addition to variations in sample adequacy.
In this study, a statistically significant association was noticeable linking E6/E7 mRNA-positive cases to oropharyngeal location (P- value = 0.007). Similarly, Bishop et al. [Citation49] found a significant association between the transcriptionally active HPV and oropharyngeal location. The oropharynx, predominantly the lingual and palatine tonsils, is lined by reticulated epithelium which may be more predisposed to infection with HPV [Citation12]. By inhibiting virus-specific T-cells, and thus permitting the virus to evade an immune response during preliminary infection and ensuing malignant transformation, the reticulated epithelium of the oropharynx possibly offers an immune-privileged site for early infection and subsequent malignant transformation. Furthermore, the natural breaks in the reticulated epithelium render the basement membrane bare for virus deposition [Citation8].
Overall, in 25 out of 27 (92.6%) HPV DNA-positive tumors the viral transcripts were detected with a substantial concordance between DNA and RNA results (κ = 0.843). Likewise, a study by Schlecht et al. [Citation47] documented a good agreement between HPV16-DNA and E6/E7 RT-PCR (κ = 0.62).
In this research, two of the DNA-positive samples did not show HPV16 viral transcripts. Correspondingly, a study by Palve et al. [Citation50] demonstrated that only 15% of tumors that were HPV DNA-positive had E6/E7 mRNA expressed. This could mirror a transient infection irrelevant to carcinogenesis [Citation41,Citation42,Citation51].
In the current study, two of the HPV DNA-negative samples revealed positive HPV16 viral transcripts. A previous study by Palve et al. [Citation52] also found three cases with HPV E6/E7 RNA and negative HPV DNA results. This was justified by the possibility that the DNA in such tumors was degenerated and hence could not act as a proper template for DNA-dependent tests. Another contributing factor may be the existence of inhibitors of DNA-dependent tests in such tumors.
Our results demonstrated there was no notable association linking HPV-positive HNSCCs to any of age, gender, smoking history, site, stage, or grade. In contrast, many studies found that HPV positive HNSCCs were significantly more prevalent in younger, nonsmoker males, with predominance of the oropharyngeal primary site [Citation53,Citation54].
P16 is usually used as a marker of HPV engrossment elucidated by the observations that the integration of HPV and the transcription of its oncoproteins encourage p16 over-expression. In this research, p16 immunostaining was recognized in 49% of studied positivity. Other studies encountered higher incidence of p16 positivity; Ralli et al. [Citation55] identified p16 expression in 78.7% of their studied patients. Nonetheless, many studies reported fewer incidences of p16-positive patients; being 29.5% in the study by Toman et al. [Citation56], who examined 59 oropharyngeal specimens and 18% in the study by Gomaa et al. [Citation57], who examined 50 cases of laryngeal SCC.
The current study revealed a notable association linking p16 expression to the differentiation degree of SCC which was higher in grade II (92%), while only 8% were in grade III (P- value = 0.021). Previous studies revealed concordant results [Citation21,Citation58]. In divergence to our observations, Shinohara et al. [Citation59] found that p16 overexpression was linked to poorly differentiated tumors. Supporting the former finding, Ralli et al. [Citation55] observed that the over-expression of p16 was more possibly detected in late-stage and advanced grade of a tumor. Still, no significant association was recognized between tumor grade and p16 expression by Dragomir et al. [Citation19] and Yuen et al. [Citation60]. These variabilities could be elucidated via environmental circumstances, racial or genetic factors, and different HPV subtypes in various countries.
In the current study, no statistically significant association was noticeable between the expression of p16 and each of smoking or alcohol intake. This was consistent with a study by Ralli et al [Citation55]. who found no significant association linking p16 expression to tobacco use. However, these results differ from an earlier study by Smith et al [Citation61] who demonstrated a notable association of the expression of p16 with tobacco use and alcohol consumption.
Of note, hypermethylation of p16 with loss of p16 expression has been linked to smoking, whereas up-regulation of p16 with over-expression has been associated with active HPV [Citation55]. As most of our patients were smokers, loss of p16 expression was expected. Though statistically non-significant; still, the presence of p16 expression in 25 of 51 cases (49%) points toward an additional risk factor, which might be HPV. The difference in p16 expression in our study compared to other studies can be attributed to the geographic variations and the presence of disparate risk determinants.
In this study, HPV16 immunoreactivity had no correlation with age, sex, tumor site or stage. These results corroborated the findings of Gomaa et al [Citation57]. who did not find a correlation between p16 IHC results and other parameters. On the other hand, some studies have documented more HPV16 prevalence in younger participants suffering from cancer of the larynx [Citation62].
Comparing HPV16 DNA results with p16 IHC results, there was a good agreement between the two tests (κ = 0.608). However, there were 10 discordant cases; six cases had positive HPV16 DNA and negative p16 and the remaining four cases were negative for HPV16 DNA but positive for p16. A previous study by Stephen et al. [Citation37] also revealed discordant p16 and HPV16 DNA cases.
In the meantime, there was a good agreement in this study between E6/E7 mRNA detection and p16 IHC results (κ = 0.608). The finding that p16 was not expressed in HPV-positive HNSCC can be accredited to the possibility that HPV in the cancer tissue was inactive, and its existence was either as a contaminant or as a passenger virus [Citation63].
Exposure to a certain carcinogen, e.g. tobacco, may result in the alteration of p16 expression. The hypermethylation provoked by smoking or other factors could result in non-expressed p16, which may precede HPV infection. In such incident, HPV would not trigger the accumulation of p16 in HNSCC [Citation64]. In the same context, Halec et al. [Citation14] suggested that the expanding chromosomal instability prompted by oncoproteins of HPV may elicit p16 loss in such tumors.
Alternatively, the over-expression of p16 in absence of HPV, could be due to p16 activation by a mechanism that is irrelevant to HPV, or due to other subtypes of HPV eliciting such an activation [Citation65]. Hence, p16 alone may not be satisfactory for researches that aim to accurately investigate the prevalence of HPV.
In this research, the mean length of follow up of our participants was 17.29 months. At the end of the study period, 86.3% of the patients were alive, and 13.7% of patients experienced recurrence. Further analysis of survival data could not be performed owing to the small sample and the relatively short-term following up of patients. Most of the published data documented a statistically significant correlation between better overall survival and HPV positivity and that difference was more pronounced in the oropharyngeal sub-site [Citation12,Citation66].
5. Conclusion
This study lays out novel insights on the contribution of HPV16 in the development of HNSCC in Egypt. HPV16 E6 and/or E7 mRNA was expressed in most of HPV16 DNA-positive tumors, indicating an important role of HPV16 in the carcinogenesis of HNSCC.
More research is warranted to investigate how HPV infection can impact HNSCC prognosis, and to recognize how viral oncogene expression impacts the patients’ outcome.
Data availability
All datasets generated or analyzed during this study are included in the manuscript.
Disclosure statement
Authors declare there are no conflicts of interest.
Additional information
Notes on contributors
Reham Ali Dwedar
Reham Ali Dwedar Associate Professor of Medical Microbiology and Immunology, Kasralainy Faculty of Medicine, Cairo University.
Noha Mohamed Omar
Noha Mohamed Omar Assistant Lecturer of Medical Microbiology and Immunology, Kasralainy Faculty of Medicine, Cairo University.
Somaia Abdel-Latif Eissa
Somaia Abdel-Latif Eissa Emeritus Professor of Medical Microbiology and Immunology, Kasralainy Faculty of Medicine, Cairo University.
Abdelrahman Younes Aly Badawy
Abdelrahman Younes Aly Badawy Associate Professor of Otorhinolaryngology, Kasralainy Faculty of Medicine, Cairo University.
Dalia Abd El-Kareem
Dalia Abd El-Kareem Lecturer of Pathology, Kasralainy Faculty of Medicine, Cairo University.
Lamiaa Abd El-Fattah Ahmed Madkour
Lamiaa Abd El-Fattah Madkour Associate Professor of Medical Microbiology and Immunology, Kasralainy Faculty of Medicine, Cairo University.
References
- Wang HF, Wang SS, Tang YJ, et al. The double-edged sword–how human papillomaviruses interact with immunity in head and neck cancer. Front Immunol. 2019;10:653.
- Alsbeih G, Al-Harbi N, Bin Judia S, et al. Prevalence of human papillomavirus (HPV) infection and the association with survival in saudi patients with head and neck squamous cell carcinoma. Cancers (Basel). 2019;11(6):820.
- Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550.
- Kobayashi K, Hisamatsu K, Suzui N, et al. A review of HPV-related head and neck cancer. J Clin Med. 2018;7(9):241.
- Gillespie MB, Rubinchik S, Hoel B, et al. Human papillomavirus and oropharyngeal cancer: what you need to know in 2009. Curr Treat Options Oncol. 2009;10(5–6):296–307.
- Lowy DR, Schiller JT. educing HPV-associated cancer globally. Cancer Prev Res. 2012;5(1):18–23.
- Westra WH. Detection of human papillomavirus (HPV) in clinical samples: evolving methods and strategies for the accurate determination of HPV status of head and neck carcinomas. Oral Oncol. 2014;50(9):771–779.
- Westra WH. Detection of human papillomavirus in clinical samples. Otolaryngol Clin North Am. 2012;45(4):765–777.
- Saleh K, Eid R, Haddad FG, et al. New developments in the management of head and neck cancer–impact of pembrolizumab. Ther Clin Risk Manag. 2018;14:295.
- Head and Neck Cancer Study Group (HNCSG), Monden N, Asakage T, Kiyota N, et al. A review of head and neck cancer staging system in the TNM classification of malignant tumors. Jpn J Clin Oncol. 2019;49(7):589–595.
- Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116(9):2166–2173.
- Shankar A, Crouch DH, Macluskey M. Ezrin–radixin–moesin binding phosphoprotein 50: a potential novel biomarker in human papilloma virus-associated head and neck squamous cell carcinomas. Head Neck Pathol. 2019;13(2):188–197.
- Mansour A, Helmy H, Ali M, et al. Head and neck cancer association to CIAP-2 expression due to human papilloma virus-16 (HPV-16) infection. Egypt J Biochem Mol Biol. 2011;29(2). DOI:10.4314/ejbmb.v29i2.72443
- Halec G, Schmitt M, Dondog B, et al. Biological activity of probable/possible high‐risk human papillomavirus types in cervical cancer. Int J Cancer. 2013;132(1):63–71.
- Wang‐Johanning F, Lu DW, Wang Y, et al. Quantitation of human papillomavirus 16 E6 and E7 DNA and RNA in residual material from Thin Prep Papanicolaou tests using real‐time polymerase chain reaction analysis. Cancer. 2002;94(8):2199–2210.
- Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35.
- Deng Z, Hasegawa M, Kiyuna A, et al. Viral load, physical status, and E6/E7 mRNA expression of human papillomavirus in head and neck squamous cell carcinoma. Head Neck. 2013;35(6):800–808.
- Attar E, Dey S, Hablas A, et al. Head and neck cancer in a developing country: a population-based perspective across 8 years. Oral Oncol. 2010;46(8):591–596.
- Dragomir LP, Simionescu C, Mărgăritescu C, et al. P53, p16 and Ki67 immunoexpression in oral squamous carcinomas. Rom J Morphol Embryol. 2012;53(1):89–93.
- Olimid DA, Simionescu CE, Mărgăritescu C, et al. Immunoexpression of Ki67 and cyclin D1 in oral squamous carcinomas. Rom J Morphol Embryol. 2012;53(3):795–798.
- El-Azeem MAA, Wasfy RE, Shareef MM. Immunohistochemical expression of p16 and p53 in oral and oropharyngeal squamous cell carcinoma: a pilot study in Egyptian patients. Egypt J Pathol. 2015;35(1):81–86.
- Shariff MA. Anatomical site-wise distribution of upper aerodigestive tract malignancies in a rural population. Int J Otolaryngol Head Neck Surg. 2018;4(6):1394.
- Mehanna H, Beech T, Nicholson T, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—systematic review and meta‐analysis of trends by time and region. Head Neck. 2013;35(5):747–755.
- Chen AM, Chen LM, Vaughan A, et al. Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int J Radiat Oncol Biol Phys. 2011;79(2):414–419.
- El-Mofty SK, Patil S. Human papillomavirus (HPV)-related oropharyngeal nonkeratinizing squamous cell carcinoma: characterization of a distinct phenotype. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):339–345.
- Heck JE, Berthiller J, Vaccarella S, et al. Sexual behaviours and the risk of head and neck cancers: a pooled analysis in the international head and neck cancer epidemiology (INHANCE) consortium. Int J Epidemiol. 2009;39(1):166–181.
- Lindel K, Beer KT, Laissue J, et al. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92(4):805–813.
- El-Naggar AK, Chan JKC, Grandis JR, et al. WHO classification of head and neck tumours. Lyon: IARC; 2017. p. 347.
- Lewis JS Jr, Khan RA, Masand RP, et al. Recognition of nonkeratinizing morphology in oropharyngeal squamous cell carcinoma–a prospective cohort and interobserver variability study. Histopathology. 2012;60(3):427–436.
- Gunnell AS, Tran TN, Torrång A, et al. Synergy between cigarette smoking and human papillomavirus type 16 in cervical cancer in situ development. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2141–2147.
- Gillison ML, Chaturvedi AK, Anderson WF, et al. Epidemiology of human papillomavirus–positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3235.
- Mena M, Taberna M, Monfil L, et al. Might oral human papillomavirus (HPV) infection in healthy individuals explain differences in HPV-attributable fractions in oropharyngeal cancer? A systematic review and meta-analysis. J Infect Dis. 2018;219(10):1574–1585.
- Näsman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral‐induced carcinoma? Int J Cancer. 2009;125(2):362–366.
- Goodman MT, Saraiya M, Thompson TD, et al. Human papillomavirus genotype and oropharynx cancer survival in the United States of America. Eur J Cancer. 2015;51(18):2759–2767.
- Carlander ALF, Larsen CG, Jensen DH, et al. Continuing rise in oropharyngeal cancer in a high HPV prevalence area: a Danish population-based study from 2011 to 2014. Eur J Cancer. 2017;70:75–82.
- Posner MR, Lorch JH, Goloubeva O, et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011;22(5):1071–1077.
- Stephen JK, Divine G, Chen KM, et al. Significance of p16 in site-specific HPV positive and HPV negative head and neck squamous cell carcinoma. Cancer Clin Oncol. 2013;2(1):51.
- Chen XJ, Sun K, Jiang WW. Absence of high-risk HPV 16 and 18 in Chinese patients with oral squamous cell carcinoma and oral potentially malignant disorders. Virol J. 2016;13(1):81.
- Blumberg J, Monjane L, Prasad M, et al. Investigation of the presence of HPV related oropharyngeal and oral tongue squamous cell carcinoma in Mozambique. Cancer Epidemiol. 2015;39(6):1000–1005.
- Hauck F, Oliveira-Silva M, Dreyer JH, et al. Prevalence of HPV infection in head and neck carcinomas shows geographical variability: a comparative study from Brazil and Germany. Virchows Arch. 2015;466(6):685–693.
- Ndiaye C, Mena M, Alemany L, et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15(12):1319–1331.
- Gheit T, Anantharaman D, Holzinger D, et al. Role of mucosal high‐risk human papillomavirus types in head and neck cancers in central India. Int J Cancer. 2017;141(1):143–151.
- Chernock RD, Wang X, Gao G, et al. Detection and significance of human papillomavirus, CDKN2A (p16) and CDKN1A (p21) expression in squamous cell carcinoma of the larynx. Mod Pathol. 2013;26(2):223.
- Ukpo OC, Flanagan JJ, Ma XJ, et al. High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35(9):1343–1350.
- Shi W, Kato H, Perez-Ordonez B, et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol. 2009;27(36):6213–6221.
- Salazar CR, Smith RV, Garg MK, et al. Human papillomavirus-associated head and neck squamous cell carcinoma survival: a comparison by tumor site and initial treatment. Head Neck Pathol. 2014;8(1):77–87.
- Schlecht NF, Brandwein-Gensler M, Nuovo GJ, et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol. 2011;24(10):1295.
- Lam EW, Chan JY, Chan AB, et al. Prevalence, clinicopathological characteristics, and outcome of human papillomavirus–associated oropharyngeal cancer in southern Chinese patients. Cancer Epidemiol Biomarkers Prev. 2016;25(1):165–173.
- Bishop JA, Ma XJ, Wang H, et al. Detection of transcriptionally active high risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36(12):1874.
- Palve V, Bagwan J, Krishnan NM, et al. High-risk human papillomavirus in oral cavity squamous cell carcinoma. BioRxiv. 2018;082651. DOI:10.1101/082651
- Castellsagué X, Mena M, Alemany L. Epidemiology of HPV-positive tumors in Europe and in the world. In: HPV infection in head and neck cancer. Cham: Springer; 2017. p. 27–35.
- Palve V, Bagwan J, Krishnan NM, et al. Detection of high-risk human papillomavirus in oral cavity squamous cell carcinoma using multiple analytes and their role in patient survival. J Glob Oncol. 2018;4:1–33.
- Ni G, Huang K, Luan Y, et al. Human papillomavirus infection among head and neck squamous cell carcinomas in southern China. PloS One. 2019;14(9). DOI:10.1371/journal.pone.0221045
- Zaravinos A. An updated overview of HPV-associated head and neck carcinomas. Oncotarget. 2014;5(12):3956.
- Ralli M, Singh S, Yadav SPS, et al. Assessment and clinicopathological correlation of p16 expression in head and neck squamous cell carcinoma. J Cancer Res Ther. 2016;12(1):232.
- Toman J, Von Larson S, Umeno H, et al. HPV-positive oropharyngeal cancer via p16 immunohistochemistry in Japan. Ann Otol Rhinol Laryngol. 2017;126(2):152–158.
- Gomaa MAM, El Gindy KE, Nabi UGA, et al. Human Papillomavirus Subtype 16 And The Pathologic Characteristics Of Laryngeal Cancer. OTO open. 2017;1(2):2473974X17707925.
- Natarajan E, Omobono JD II, Jones JC, et al. Co-expression of p16INK4A and laminin 5 by keratinocytes: a wound-healing response coupling hypermotility with growth arrest that goes awry during epithelial neoplastic progression. J Investig Dermatol Symp Proc. 2005;10(2):72–85.
- Shinohara S, Kikuchi M, Tona R, et al. Prognostic impact of p16 and p53 expression in oropharyngeal squamous cell carcinomas. Jpn J Clin Oncol. 2014;44(3):232–240.
- Yuen PW, Man M, Lam KY, et al. Clinicopathological significance of p16 gene expression in the surgical treatment of head and neck squamous cell carcinomas. J Clin Pathol. 2002;55(1):58–60.
- Smith EM, Rubenstein LM, Hoffman H, et al. Human papillomavirus, p16 and p53 expression associated with survival of head and neck cancer. Infect Agent Cancer. 2010;5(1):4.
- Maier M, Kraft K, Steinestel K, et al. Human papillomavirus in squamous cell cancer of the head and neck. A study at the Ulm Military Hospital, Germany. HNO. 2013;61(7):593–601.
- Ursu RG, Danciu M, Spiridon IA, et al. Role of mucosal high-risk human papillomavirus types in head and neck cancers in Romania. PloS One. 2018;13(6):e0199663.
- Takeshima M, Saitoh M, Kusano K, et al. High frequency of hypermethylation of p14, p15 and p16 in oral pre‐cancerous lesions associated with betel‐quid chewing in Sri Lanka. J Oral Pathol Med. 2008;37(8):475–479.
- Gao G, Chernock RD, Gay HA, et al. A novel RT‐PCR method for quantification of human papillomavirus transcripts in archived tissues and its application in oropharyngeal cancer prognosis. Int J Cancer. 2013;132(4):882–890.
- D’Souza G, Anantharaman D, Gheit T, et al. Effect of HPV on head and neck cancer patient survival, by region and tumor site: a comparison of 1362 cases across three continents. Oral Oncol. 2016;62:20–27.