ABSTRACT
Introduction: Gastroesophageal reflux disease is defined as bothersome symptoms and/or complications caused by the reflux of stomach contents. About 40–50% of patients develop refractory gastroesophageal reflux disease (R-GERD), with poor improvement of symptoms with treatment. Many pathogenic mechanisms share in development of R-GERD, among which is the important role of T-helper 1 and T-helper 2 response mediated by cytokines. The interleukin-4 is a cytokine known of its anti-inflammatory effect. In this study, we aimed to evaluate the level of interleukin-4 in Egyptian patients with R-GERD versus those with GERD. PATIENTS AND Methods: Our study included 25 patients with reflux symptoms who received PPIs for less than 8 weeks with improvement of symptoms, versus 25 patients with refractory reflux symptoms who received PPIs for more than 8 weeks without improvement of symptoms. Interleukin-4 levels were assessed in both groups by ELISA.
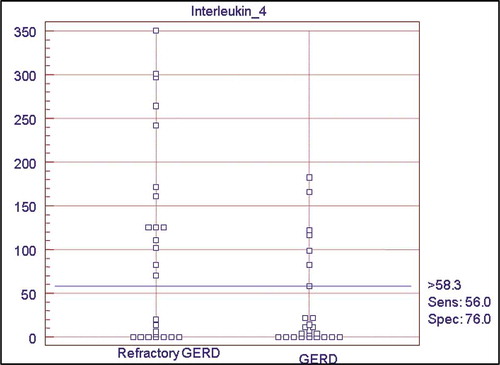
Results: There was a statistically significant difference between the two groups as regard interleukin-4 levels (p < 0.012) which was higher in the patients with (R-GERD), the mean level of IL-4 was 37.31 ± 56.07 in GERD group while in R-GERD group, it was 102.78 ± 112.29. The diagnostic accuracy of interleukin-4 revealed a sensitivity of 56% and specificity of 76% at cutoff value >58.25 pg/ml, with an acceptable accuracy of 0.6.
Conclusions: The present study concluded that IL-4 is significantly higher in patients with R GERD with cut off value > 58.25 pg/ml. Therapeutic strategies that modulate the production of IL-4 may provide a good solution for treatment of R GERD.
1. Background
Gastroesophageal reflux disease (GERD) is considered one of the most prevalent gastrointestinal disordersworldwide[Citation1]. GERD, defined as bothersome symptoms and/or complications caused by the reflux of stomach contents into the esophagus, is known to have a great adverse impacts on patients’ health as well as their quality of life [Citation2]. Chief symptoms of GERD include heartburn and regurgitation. However, reflux of gastric contents may also cause cough, asthma, pharyngitis, laryngitis, hoarseness of voice, sinusitis, otitis media, dental erosions, chest pain, and sleep disturbance, which are considered as atypical or extraesophageal symptoms of GERD [Citation3].
In about 40–50% of patients with GERD, there is a partial or incomplete response to a standard dose of a proton pump inhibitors (PPIs) given once daily for 8 weeks [Citation4,Citation5]. Causes of this condition, also known as refractory GERD (R-GERD), include: PPI compliance issues, weakly acidic or nonacid reflux, nocturnal acid breakthrough, presence of acid pockets, eosinophilic esophagitis, esophageal hypersensitivity, functional heart burn, delayed gastric emptying, rapid PPI metabolism, and genetic polymorphism of isoenzymes CYP3A4 &CYP2C19 of cytochrome P450 [Citation6]. The management of patients with R-GERD is considered a major clinical challenge for gastroenterologists.
In the recent years, the problem of R-GERD has been studied at both tissue and cellular levels. One of the most promising interests in this area is the study of the cytokine profile in such patients. Cytokines, which are peptide signaling molecules, play an important role in the damage of esophageal mucosa, and were found to demonstrate pro-inflammatory as well as anti-inflammatory activity [Citation7].
There are many studies that show the changes in immune response in patients with GERD, which consists of an imbalance between cellular (Th1) and humoral (Th2) immunity, possibly determined by the expression of the cytokines [Citation8,Citation9].Gamma interferon (IFN-γ) and interleukin-4 (IL-4) are central components of the Th1 and the Th2 responses, respectively.
IL-4 is an important cytokine that functions as a potent regulator of immune response, and is secreted mainly by mast cells, Th2 cells, eosinophils, and basophils. Initially identified by Howard and Paul [Citation10], IL-4 was shown to be an important player in survival of leukocytes under both physiological and pathological conditions [Citation11]. The earliest studies of IL-4 in macrophages showed that it acted as an anti-inflammatory agent when administered concurrently or shortly after an inflammatory stimulus, and was also capable of downregulating the production of other pro-inflammatory cytokines such as TNF [Citation12]. However, IL-4 is not purely an anti-inflammatory agent, as priming of macrophages with IL-4 followed by pro-inflammatory stimulation can lead to an enhanced inflammatory response [Citation13,Citation14]. These data suggest that the in-vivo effects of IL-4 are complex, well regulated, dependent on the local environment, and that they probably mediate different processes simultaneously in different tissues [Citation15].In this study, we aimed to evaluate the serum level of interleukin-4 in Egyptian patients with GERD and compare it with those with R-GERD.
2. Patients and methods
This cross-sectional study was conducted on 50 age and sex-matched patients who were recruited from gastroenterology and hepatology departments and outpatient clinics of participating institutions in the period between June 2019 and December 2019. This study was performed in accordance with the ethical standards of Ain Shams University Research Committee.
A written informed consent was obtained from all individual participants included in the study.
2.1. Sample size justification
Sampling technique was random. MedCalc® version 12.3.0.0 program was used for calculations of sample size, statistical calculator based on 95% confidence interval and power of the study 80% with α error 5%, According to a previous study, Zhong et al showed that the mean of IL-4 between Barrett’s esophagus and reflux esophagitis (1.28 ± 0.35 and 4.45 ± 1.41) respectively, the mean difference. 3.17, with p-value (<0.001) [Citation9], so it can be relied upon in this study, based on this assumption, sample size was calculated according to these values produced a minimal samples size of 48 cases were enough to find such a difference. Assuming a drop-out ratio of 5%, the sample size will be 25 cases in each group.
Patients were divided into:
Group I: 25 patients with GERD (defined as presence of bothersome symptoms who received treatment for 8 weeks with improvement of symptoms),
Group II: 25 patients with refractory GERD (defined as lack of improvement of symptoms after receiving PPIs for more than 8 weeks). All patients signed an informed consent prior to enrollment in this study. This study was approved by the ethical research committees of participating institutions.
After 8 weeks of therapy with PPI, all patients were subjected to:
History taking with special stress on GERD symptoms (typical, atypical), drug history especially PPIs, NSAIDS, special habits (smoking, alcohol, addiction) and medical history especially esophageal varices and liver cirrhosis.
Clinical examination to exclude unfit patients for biopsy such as patients with liver cirrhosis and/or signs of portal hypertension.
Upper gastrointestinal endoscopy with biopsy from lower end of esophagus.
Full routine laboratory investigations including Complete blood count (CBC), prothrombin time (PT), partial thromboplastin time (PTT), International Normalized Ratio (INR), Aspartate Aminotrasferase(AST), Alanine aminotransferase (ALT), Total bilirubin (direct&indirect), serum creatinine, blood urea, sodium (Na), potassium (K).
The serum IL-4 level was assessed by enzyme-linked immunosorbent assay (ELISA); detection range was 31.2–2000 pg/ml.
5 ml of whole blood has been collected in a covered test tube without anticoagulants and allowed to clot by leaving it undisturbed at room temperature for 20 min. Clot has been then removed by centrifuging at 1500 ×g for 10 minutes in a refrigerated centrifuge. The resulting supernatant was designated serum. Following centrifugation, the serum has been immediately transferred into a clean polypropylene tube using a Pasteur pipette. The samples were maintained at 2–8°C while handling and immediately analyzed.
2.2. Statistical analysis
Statistical presentation and analysis of the results from the present study was conducted using SPSS version 20 computer software. The data were described using frequency, mean, and standard deviation and expressed in tables and figures. Data were compared using unpaired student t-test, analysis of variance (ANOVA) test, chi-square, and linear correlation coefficient [r] tests. The cutoff level of IL-4 with the highest sensitivity and specificity rates was chosen using the receiver operating characteristic (ROC) curve. A p-value less than 0.05 was considered statistically significant.
3. Results
This cross-sectional study involved 50 Egyptian patients made of two groups, GERD, and R-GERD each comprising 25 patients. GERD patients (group 1) included 8 (32%) male patients and 17 (68%) female patients with mean age 45.36 ± 13.42, while R-GERD patients group (group 2) included 40 (56%) male patients, and 11 (44%) female patients with mean age 48.36 ± 14.59, .
Table 1. Comparison of age and gender between the patients with GERD and R-GERD
There was no statistically significant gender or age difference between the two groups (p < 0.382, p < 0.453, respectively) .
As regard the co-morbidities there was no statistically significant difference between the two groups, .
Table 2. Comparison of co-morbidities between the patients with GERD and R-GERD
The IL-4 was higher in patients with refractory GERD with statistically significant difference (p value = 0.012), the mean level of IL-4 in group 1 was 37.31 ± 56.07, while it was 102.78 ± 112.29 in group 2, .
Table 3. Comparison of serum levels of IL-4 in the patients with GERD and R-GERD
There was no statistically significant difference between the two groups of patients as regard routine laboratory investigations .
Table 4. Comparison of results of routine laboratory investigations in the patients with GERD and R-GERD
shows statistically significant difference between the two groups as regard reflux esophagitis (p value = 0.004), as 100% of patients in GERD group had reflux esophagitis versus 72% in R-GERD group. As regard Barrett’s esophagitis it was higher in R-GERD group with statistically significant difference (p value = 0.004). While there was no statistically significant difference between the two groups as regard other endoscopic features, .
Table 5. Comparison of Endoscopic features in the patients with GERD and R-GERD
The pathological examination of biopsies obtained endoscopically from patients in GERD group showed findings consistent with mild, moderate, and severe esophagitis in 10 (40%), 13 (52%), and 2 (8%) patients, respectively. On the contrary, R-GERD group patients showed pathological features consistent with moderate, severe esophagitis, and Barrett’s esophagitis in 7 (28%) patients in R-GERD group only but in GERD group. There was a highly significant difference between the two groups as regard pathological findings (p < 0.001), .
Table 6. Comparison of Pathological Findings in the patients with GERD and R-GERD
Among the patients with GERD, the levels of IL-4 increased progressively according to severity of reflux esophagitis. Accordingly, mean IL-4 levels in group 1 were 11.51 ± 25.33, 41.3 ± 56.36, and 140.37 ± 58.88 in mild, moderate, and severe reflux esophagitis subgroups, respectively. There was a statistically significant difference between the three subgroups as regard mean IL-4 levels (p < 0.006), .
Table 7. Relation between Pathological findings and (IL-4 with Routine Laboratory investigations) in the patients with GERD
On the contrary, mean IL-4 levels in group II showed greater diversity between the moderate and severe reflux esophagitis subgroups and the Barrett’s esophagus subgroup, where the mean IL-4 levels were 64.23 ± 73.83 and 49.06 ± 56.24 for the moderate and severe subgroups, respectively, while the Barrett’s esophagus subgroup showed a mean IL-4 level of 232.25 ± 116.54. There was a highly significant statistical difference between the three subgroups in this aspect (p < 0.001), .
Table 8. Relation between Pathology Findings and IL-4 with Routine Laboratory investigations in the patients with R-GERD
The diagnostic accuracy of IL-4 as a discriminatory marker between GERD and R-GERD patients was assessed using ROC curve which revealed a sensitivity 56% and specificity of 76% at cutoff value >58.25 pg/ml, with an acceptable discriminative accuracy of 0.6 ( and ).
Table 9. Statistical analysis of IL-4 levels between GERD and Refractory GERD patients for ROC curve
Figure 1. ROC curve (Receiver operator characteristic curve); Accuracy (area under ROC curve) of Interleukin-4 among GERD and R-GERD
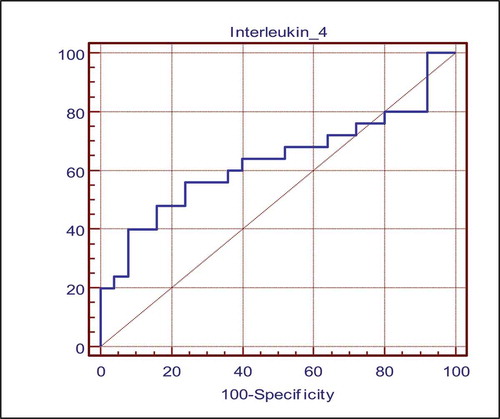
Acceptable discriminative and predictive ability of Interleukin-4 between patients with GERD and Refractory GERD as indicated by the area under the ROC curve (the distance between the curve and diagonal) or accuracy of 0.6, .
4. Discussion
GERD is considered a significant health problem that affects the quality of life of many patients [Citation1]. Moreover, it represents a challenge for gastroenterologists, since many patients develop R-GERD, making the symptoms harder to control [Citation16]. Many pathogenic mechanisms share in development of R-GERD. Among these mechanisms is the prominent role of TH1 and Th2 immune responses controlled by various cytokines, including IL-4 [Citation17]. Our study aimed to evaluate the serum level of IL-4 in patient with GERD versus those with R-GERD and it showed that IL-4 levels are significantly increased in cases of R-GERD versus those of GERD group. This finding agrees with the findings of Major et al. study which showed that IL-4, though having anti-inflammatory effect, can also promote pro-inflammatory response through stimulation of Th1 response [Citation13]. Another study by Fort et al. showed similar findings as our study, stressing on the ability of IL-4 to mount Th1 immune response, promoting inflammatory process. These studies can be viewed as evidence proving the dual nature of IL-4 as both anti-inflammatory in addition to pro-inflammatory cytokine [Citation14].
Also, our study findings are similar to the report from another study by Ivashkin et al. which showed that IL-4 has an anti-inflammatory effect only, mainly in cases of Barrett’s esophagus. This elucidates the ongoing debate about the precise role of IL-4 and its relation to both Th1 and Th2 responses [Citation18]. Comparison between the two groups as regard endoscopy findings and confirmation by pathology, in the group of GERD there is reflux esophagitis in all cases and in the group of refractory GERD there is reflux esophagitis in 72% of cases and Barrett’s esophagitis in 28% of cases of refractory GERD and 14% from all cases participating in the study, and there is statistically significant difference between the two groups as regard Reflux esophagitis and Barrett’s esophagitis.
Our findings were corroborated by the observation in the study by Westhof which involved consecutive patients that presented to the endoscopy unit of a Veterans Affairs Medical Center for a first upper endoscopy with the indication of GERD. A total of 378 consecutive patients with GERD were evaluated. A diagnosis of BE was made in 50 patients (13.2%) [Citation19]. Another study was carried out in Egypt by Gado et al and disagrees with the findings from our study. It reported that the prevalence of Barrett’s esophagitis was 1% from all cases of GERD [Citation20].
Also we found in the group of refractory GERD the pathology reported that 28% of patients have Barrett’s esophagitis and the other 72% have reflux esophagitis (moderate 16%, and severe 56%). Relation between pathology and endoscopy was statistically high significant as regard reflux esophagitis and Barrett’s esophagitis (p-value <0.001).
A previous cross-sectional study by Piqué et al study disagrees with our findings as it revealed that severe reflux esophagitis was less in patients with refractory GERD than patients with GERD, but mild and moderate reflux esophagitis were found in the patients with refractory GERD more than patients with GERD [Citation21].
Relation between serum IL4 and pathological findings in the two groups included in the study was reported. We couldn’t find any study designed to evaluate the relation between IL4 and refractory GERD, all previous studies were designed to assess the relation of IL4 with Reflux esophagitis and Barrett’s esophagus.
Comparison between the two groups as regard routine investigations (CBC, Liver Enzymes, Bilirubin, PT, PTT, INR, Creatinine, Urea, Na, K) revealed no statistically significant difference. Also the relation between pathological features and routine investigations revealed no statistically significant difference between both groups and these findings are in contrast to a study by Loke et al which revealed that metabolic syndrome, impaired liver function, and a higher ratio of total cholesterol to HDL-C were associated with erosive esophagitis [Citation22].
Finally, there is acceptable discriminative and predictive ability of Interleukin-4 between GERD and R GERD groups as indicated by the area under the ROC curve (the distance between the curve and diagonal) or accuracy of 0.6 with cut off value of Interleukin-4 by sensitivity and specificity between GERD and R-GERD, Interleukin-4 had sensitivity 56% and specificity 76% at cut off level of >58.25 pg/ml to discriminate between GERD and R-GERD.
Limitations of this study include small sample sizes of the groups and their subgroups limiting sound conclusions, hence calling for further study with higher population of patients to elucidate on the findings.
5. Conclusion
The present study concluded that IL-4 is significantly higher in patients with refractory GERD with cut off value > 58.25 pg/ml compared to the patients with GERD and also it was high in patients with BE. Therapeutic strategies that modulate the production of IL-4 may provide a good solution for treatment of refractory GERD and also to prevent the development of BE.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Tarek M. Yosef
Tarek M. Yosef is a Professor of Gastroenterology and Hepatology at Faculty of Medicine, Ain Shams University in Cairo, Egypt.
Ahmed ElMetwally Ahmed
Ahmed ElMetwally Ahmed is a Consultant and Lecturer of Gastroenterology and Hepatology at Faculty of Medicine, Ain Shams University in Cairo, Egypt.
Ahmed Mansour
Ahmed Mansour is a Consultant and Lecturer of Gastroenterology and Hepatology at Faculty of Medicine, Ain Shams University in Cairo, Egypt.
Mahmoud Ahmed AbuFayyoud
Mahmoud Ahmed AbuFayyoud is a Specialist Gastroenterologist at Kafr El-Shiekh General Hospital in Kafr El-Shiekh, Egypt.
Ahmed M ElGhandour
Ahmed M ElGhandour is a Consultant and Lecturer of Gastroenterology and Hepatology at Faculty of Medicine, Ain Shams University in Cairo, Egypt.
References
- El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut. 2014;63(6):871–880.
- Tack J, Becher A, Mulligan C, et al. Systematic review: the burden of disruptive gastro-oesophageal reflux disease on health-related quality of life. Aliment Pharmacol Ther. 2012;35(11):1257–1266.
- Vakil N. A global evidence-based consensus definition and classification of gastroesophageal reflux disease. Ther Res. 2006;27(5):805–812.
- Bytzer P, Van Zanten SV, Mattsson H, et al. Partial symptom-response to proton pump inhibitors in patients with non-erosive reflux disease or reflux oesophagitis - A post hoc analysis of 5796 patients. Aliment Pharmacol Ther. 2012;36(7):635–643.
- Cicala M, Emerenziani S, Guarino MPL, et al. Proton pump inhibitor resistance, the real challenge in gastro-esophageal reflux disease. World J Gastroenterol. 2013;19(39):6529–6535.
- Mermelstein J, Mermelstein AC, Chait MM. Proton pump inhibitor-refractory gastroesophageal reflux disease: challenges and solutions. Clin Exp Gastroenterol. 2018;11:119–134.
- Isomoto H, Inoue K, Kohno S. Interleukin-8 levels in esophageal mucosa and long-term clinical outcome of patients with reflux esophagitis. Scand J Gastroenterol. 2007;42(3):410–411.
- Kohata Y, Fujiwara Y, MacHida H, et al. Role of Th-2 cytokines in the development of Barrett’s esophagus in rats. J Gastroenterol. 2011;46(7):883–893.
- Zhong YQ, Lin Y, Xu Z. Expression of IFN-γ and IL-4 in the esophageal mucosa of patients with reflux esophagitis and Barrett’s esophagus and their relationship with endoscopic and histologic grading. Dig Dis Sci. 2011;56(10):2865–2870.
- Howard M, Farrar J, Hilfiker M, et al. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982;155(3):914–923.
- Hu-Li J. B cell stimulatory factor 1 (interleukin 4) is a potent costimulant for normal resting T lymphocytes. J Exp Med. 2004;165(1):157–172.
- Hart PH, Vitti GF, Burgess DR, et al. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci U S A. 1989;86(10):3803–3807.
- Major J, Fletcher JE, Hamilton TA. IL-4 pretreatment selectively enhances cytokine and chemokine production in lipopolysaccharide-stimulated mouse peritoneal macrophages. J Immunol. 2014;168(5):2456–2463.
- Fort MM, Lesley R, Davidson NJ, et al. IL-4 exacerbates disease in a Th1 cell transfer model of colitis. J Immunol. 2014;166(4):2793–2800.
- Gadani SP, Cronk JC, Norris GT, et al. IL-4 in the Brain: A Cytokine To Remember. J Immunol. 2012;189(9):4213–4219.
- Hershcovici T, Fass R. Step-by-step management of refractory gastresophageal reflux disease. Dis Esophagus. 2013;26(1):27–36.
- Li J, Chen X, Shaker A, et al. Contribution of immunomodulators to gastroesophageal reflux disease and its complications: stromal cells, interleukin 4, and adiponection. Ann N Y Acad Sci. 2016;1380(1):183–194.
- Ivashkin V, Evsyutina Y, Trukhmanov A, et al. Systemic inflammatory response in patients with gastroesophageal reflux disease. Am J Clin Med Res. 2015;3(4):64–69.
- Westhoff B, Brotze S, Weston A, et al. The frequency of Barrett’s esophagus in high-risk patients with chronic GERD. Gastrointest Endosc. 2015;61(2):226–231.
- Gado A, Ebeid B, Abdelmohsen A, et al. Prevalence of reflux esophagitis among patients undergoing endoscopy in a secondary referral hospital in Giza, Egypt. Alexandria J Med. 2015;51:89–94.
- Piqué N, Ponce M, Garrigues V, et al. Prevalence of severe esophagitis in Spain. Results of the PRESS study (Prevalence and Risk factors for Esophagitis in Spain: A cross-sectional study). United European Gastroenterol J. 2016;4(2):229–235.
- Loke SS, Kuender D, Yang KD, et al. Erosive esophagitis associated with metabolic syndrome, impaired liver function, and dyslipidemia. World J Gastroenterol. 2013;19(35):5883–5888.