ABSTRACT
Background: Histological diagnosis is crucial to the management of breast diseases. It determines the kind of disease, the treatment modalities, and the outcome of management. Our department receives breast biopsies from the northern sector of Ghana constituting over 50% of the Ghanaian population. This study aimed at elucidating the pattern of disease and associated traditional prognostic indices of breast cases in our department over a period of 9 years.
Methods: Information on the demographic characteristics and the histological diagnoses made on all breast cases received and processed in the department were accessed and entered into an Excel spreadsheet. Slides were reviewed and IHC was done on suitable cases. Descriptive statistics were generated using IMB-SPSS version 23.
Results: A total of 4276 breast cases were received by the department within the study period, with 97.6% being female. Age ranged (female/male) from 10 to 98/13 to 102 years, with mean ages of 38.2 years (SD ± 16.7) and 41.15 years (SD ± 21.6), respectively. Cases were evenly distributed in both left and right breasts and 4.3% were bilateral. Inflammatory conditions were seen in 7.5% of cases. The most diagnosed benign tumor was fibroadenoma (54%), followed by fibrocystic change (8.1%). Gynecomastia was diagnosed in 66.3% of males. Malignant cases were 38.6%, with invasive carcinoma NST being the most frequent (87.5%). Histological grades were I = 9.4%, II = 41.6%, and III = 49%. Molecular subtypes were luminal A (19.8%), luminal B (9.9%), Her2 (16%), and TNBC (54.3%).
Conclusion: Our findings show an increase in breast cancer cases compared to previous studies in our center, suggesting increased awareness and improved diagnosis. However, this increase is consistent with most studies in sub-Saharan Africa.
1. Introduction
The series of ductal and lobular growth, lactogenesis, and regression that are consequent to response to menstrual cycle-associated estrogen and progesterone as well as pregnancy- and lactation-related hormones predispose the breast to a myriad of disorders. The majority of these disorders present in the form of breast masses, pain, or nipple discharge and may be accompanied by either changes in overlying skin or lymphadenopathy [Citation1–6].
Even though most breast disorders turn out to be benign, a systemic review and meta-analysis have revealed that the risk of breast cancer increases up to twofolds in individuals who present with benign breast diseases without atypia [Citation7]. More importantly, individuals with benign breast disease with atypia have up to a fivefold increased risk of developing breast cancer. Atypical lobular hyperplasia and atypical ductal hyperplasia are well-known pre-malignant conditions that frequently get upgraded to lobular carcinoma in situ and ductal carcinoma in situ, respectively, following continual pathological assessment [Citation7,Citation8]. This phenomenon therefore stipulates that adequate attention be given to all forms of breast diseases that may be presented at health facilities.
Reporting on the entirety of breast diseases diagnosed is therefore encouraged, in order to provide more information on the pattern of incidence of breast diseases, enhance clinical knowledge, and inform on the best risk assessment models as well as screening and treatment practices. This may further lead to increased research into chemo-preventive therapies to limit the incidence of breast cancer [Citation7]. Accordingly, various reports have been made on the patterns of benign and malignant breast diseases. Through this, it has been realized that there are differences in the commonest types of benign breast diseases as well as the histological and molecular types of the malignant conditions across different populations [Citation9,Citation10].
This study was therefore conducted to assess the patterns of occurrences of benign and malignant breast diseases presented to the PaThology Department of Komfo Anokye Teaching Hospital (KATH) in Kumasi, Ghana. Similar studies [Citation11–14] have previously been conducted in the same department, so this study will assess how the patterns seen have changed over time, and how the current patterns compare with other studies elsewhere in Ghana and other countries.
2. Materials and methods
2.1. Samples
Following ethical approval (Ref: CHRPE/AP/417/18) from the Research and Development Unit, KATH and the Committee on Human Research, Publication and Ethics (CHRPE)-KNUST, information on all breast biopsies that were presented to the Pathology Department of KATH from 2009 to 2017 were retrieved. KATH serves as the main referral center for the northern sector of Ghana. Within the period, breast biopsies for 4276 cases were received and processed. Data obtained on the cases included patient demographics such as age and sex. Site of biopsy, histopathological diagnoses, and histological grades of malignant conditions were also noted.
2.2. Case review and immunohistochemistry
All consecutive archived tissues in the form of formalin-fixed paraffin-embedded (FFPE) blocks with their corresponding hematoxylin and eosin (H&E)-stained slides were retrieved for subsequent immunohistochemistry on the malignant cases. New H&E slides were made from the available FFPE blocks where necessary. The slides were reviewed, and 203 suitable malignant cases were selected for tissue microarray (TMA) construction and immunohistochemistry. TMA was constructed using a Micatu MicaArray Gen 3.0, after matching corresponding H&E slides and FFPE blocks; two representative cores were taken for each case. Immunohistochemistry was performed using antibodies for estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor II (HER 2), and Ki-67, according to the suppliers’ specifications. Scoring of immunohistochemistry for ER, PR, and HER2 was performed following the American Society of Clinical Oncology/College of American Pathologists guidelines [Citation15,Citation16]. Ki-67 scoring was performed in accordance with the guidelines outlined by Abubakar et al. [Citation17]. shows the details of the antibodies used.
Table 1. Patient demographics and histopathological characteristics of breast cases from 2009 to 2017, KATH
2.3. Statistical analysis
Patient demographics, histological details, and immunohistochemistry scores were analyzed using the Statistical Package for the Social Sciences (SPSS) version 23. Descriptive statistics were computed for all variables.
3. Results
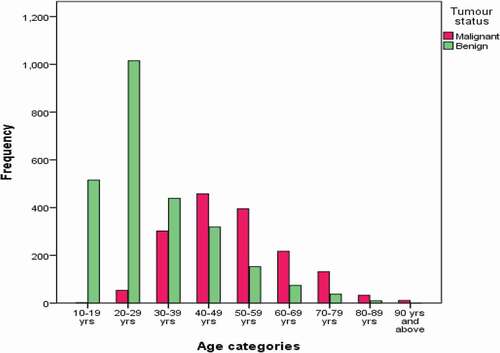
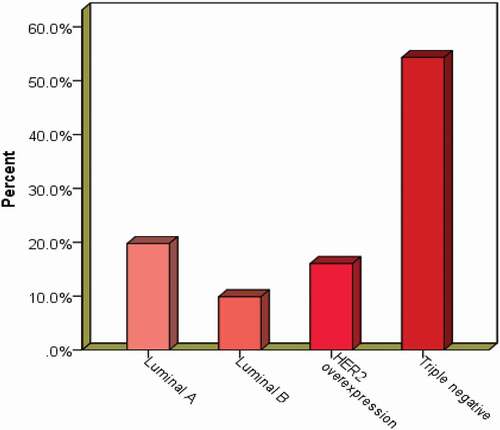
Of the 4276 cases received and processed, 4170 (97.6%) were from female patients. These cases ranged from 10 to 98 years with a mean age of 38.2 years (SD ± 16.7). The male cases ranged from 13 to 102 years with a mean age of 41.15 years (SD ± 21.6). In all, 1824 (47.2%) cases involved the left breast, while 1875 (48.5%) were from the right breast. Only 165 (4.3%) were bilateral conditions. For histological diagnoses, benign breast disorders, including inflammatory conditions, were commonest, making up 61.4% (2596) of all cases. Malignancies were reported on 1631 (38.4%) cases. Among the malignant cases that were graded histologically, 621 (49%) were grade III, 528 (41.6%) were grade II, while 119 (9.4%) were grade I. details a summary of the demographic and histopathological characteristics of the cases. The benign cases were commonly diagnosed in women in the third decade of life (20–29 years), whereas the malignant conditions had a median occurrence in the fifth decade (40–49 years). shows the comparative distribution of the benign and malignant diagnosis among age groups. Fibroadenoma was the modal benign condition, consisting of 1401 cases, making 54% of all benign conditions. Fibrocystic change was the second most frequent benign condition, diagnosed in 209 cases (8.1% of benign cases). Fibrocystic change was followed by abscess. Gynecomastia was diagnosed in 70 (66.3%) males and made up 2.7% of all benign cases. Of the 2596 malignant cases, invasive carcinoma of no special type (NST) was the most frequent diagnosis, with 1427 cases making up 87.5% of all malignant cases. Ductal carcinoma in situ was the second most frequent malignant condition, diagnosed in 60 cases, making up 3.68% of malignant cases. Twenty out of 106 males had malignant diseases within the period. shows the details of the histological diagnoses made within the study period. Immunohistochemistry revealed that the majority of the cases (54.3%) were triple negative, followed by luminal A, with 32 (19.8%) cases. Results of the molecular subtypes are displayed in .
Table 2. Histological diagnoses of breast cases from 2009 to 2017, KATH
4. Discussion
There is a notable degree of fear and anxiety associated with the observation of symptoms of breast diseases due to the potential of diagnosis with breast cancer or pre-malignant lesions that are associated with an increased risk of developing malignancies later in life. An in-depth knowledge of the pattern of occurrence and the predominant age groups mostly implicated in various forms of breast diseases will afford enhanced clinical diagnosis and effective counseling while waiting for terminal pathologic reports [Citation18]. This will further lead to effective, targeted education on breast diseases, to diffuse the fear and anxiety among age groups which frequently present with benign conditions and stress on the need for early reporting among age groups that are most susceptible to malignant conditions [Citation19].
This audit of breast biopsies received and processed at the Pathology Department of KATH from 2009 to 2017 showed that the ages of persons who reported with breast problems ranged from 10 to 102 years. The age range of cases is wider in the current study, and the proportion of cases above 80 years is higher when compared with studies in the same institution from the year 1998 to 2005 [Citation11,Citation12]. This may support the report of an improvement in life expectancy among the general population [Citation20,Citation21]. Females formed the vast majority of cases, with the younger ones (mean age = 30.39 ± 13.6) presenting predominantly with benign breast diseases, while older women (mean age = 50.26 ± 13.59) presented predominantly with malignant diseases (ANOVA p-value <0.001). Although this relationship is established in the literature [Citation11,Citation22], the mean age of the benign cases recorded in this study is higher than that reported by other studies in Ghana [Citation23] and Nigeria [Citation10].
In keeping with published data, benign breast diseases were the commonest diagnosis made; this study recorded 61.4% of cases as benign. The percentage of cases in this cohort that had benign conditions is, however, lower than that reported by a previous study in the same department, where benign cases made up about 73.8% of the cohort [Citation11]. Again, it is lower compared to studies in Nigeria [Citation24,Citation25] which reported 71.2%, 72.4%, and 78.7% of cases as benign and another in Tanzania [Citation26] which reported 80.4% as benign cases. Other studies, however, reported lower proportions of benign cases [Citation27,Citation28].
Fibroadenoma was the most frequent benign breast disease seen in our cohort (54% of benign conditions), while fibrocystic change came in second (8.1% of benign conditions). Although some disparities exist in the proportions of fibroadenoma and fibrocystic change across different populations and geographical settings with some few studies reporting higher proportions of fibrocystic change [Citation29], it is widely acknowledged that fibroadenoma is the commonest among black-skinned populations [Citation22] as made evident by numerous other studies [Citation10,Citation24–27,Citation30]. More so, there is no breast cancer screening program in Ghana, and cases in this study, like other previous studies, are symptomatic breast cases. Most studies on symptomatic breast cases have higher percentages of fibroadenoma than the other benign breast lesions seen from the screen-detected cases.
The incidence of breast cancer, which is the most common malignancy among females, has been tipped to be on the rise, especially among African populations where incidence was previously low [Citation21,Citation31,Citation32]. Importantly, the breast cancer mortality rates reported among Africans are much higher in comparison to that of Caucasians [Citation33]. Ghana bears its fair share of the low survival rates among breast cancer patients in Africa, with a 5-year survival rate of 47.9% [Citation34]. Consequently, breast cancer is the leading cause of cancer-related deaths among Ghanaian females [Citation35]. This low survival rate/high mortality rate reflects various deficiencies including low levels of education on breast health, delay in health-seeking, delays in diagnosis, high prevalence of aggressive histological and molecular characteristics, and high cost of treatment [Citation19,Citation36–39].
Results from demographic and histological characteristics from this audit generally agree with the literature on African breast cancer. 38.6% of cases in this cohort were diagnosed with various malignant diseases. This figure is notably higher than the 19.9% previously reported by a study on cases presented at the same department in 2005 [Citation11]. Furthermore, it is higher than the 20.9% reported in Accra, Ghana, on cases received from January 2000 to December 2004 [Citation40]. The increase in the proportion of malignant breast diseases in our setting is supportive of an increase in cancer incidence outlined by the rise in age-standardized rates of breast cancer incidence in Kumasi between 2012 and 2015 [Citation41,Citation42]. The proportion of malignant cases reported in the current study is in agreement with the recent studies in Accra [Citation20,Citation34]. The synchronous increase in the breast cancer incidence in our center and others in Accra could be attributed to a number of factors acting in concert. The principal factors among these are changes in population dynamics that favor population growth and improved life expectancy, obesity, and increased use of hormonal contraceptives [Citation43,Citation44]. The 2010 population statistics of Ghana showed a population growth of 30.4% over the 2000 population figure and an increase in the proportion of persons living beyond 50 years in the same period [Citation45]. An analysis of data from the WHO-Study on global AGEing and adult health (WHO-SAGE) waves 1 and 2, along with numerous other studies on different population groups, also revealed a rapid increase in the prevalence of overweightness and obesity among the Ghanaian adult population [Citation46,Citation47]. Similarly, the Performance and Accountability 2020 (PMA2020) surveys indicate that Ghana has one of the highest annual rates of modern contraceptive uptake in Africa [Citation48].
The mean age of females who presented with malignant diseases was 50.26 years, and invasive carcinoma NST was the predominant malignant disease (87.1%). The age distribution and histological diagnosis are consistent with the published literature on African breast cancer, where invasive carcinoma NST is predominant, and the mean age of malignant female cases was 10–15 years below that of Caucasian women [Citation49]. The majority of the histological types seen among African cases are associated with aggressive phenotypes such as high histological grades. Previous studies made in Ghana [Citation13,Citation14,Citation20,Citation34,Citation50] all reported majority of high-grade carcinomas (grades II and III). In each of these studies, an addition of the proportions of grade II and grade III tumors amounted to more than 80% of cases.
The biological behavior of tumors is inherently dependent on the molecular alterations that drive the tumor characteristics [Citation51]. Disparities have been reported in the molecular characteristics of breast cancer between women of African ancestry and Caucasians, with African and African-American women five times less likely to express estrogen and progesterone receptors. Women of African descent consequently present with triple-negative (lack of ER and PR expression) breast cancer [Citation52]. Epidemiologic and genomic analyses point to genetic differences associated with race/ethnicity as the major reason for this disparity. The results from this study revealed that more than half of the cases (54.3%) were of the triple-negative subtype. Triple-negative breast cancer is known to be associated with aggressive biological behavior, with high rates of proliferation and metastasis. Additionally, a lack of widely recognized and accepted molecular targets as adjuvant therapies leaves cases with this molecular subtype with poor prognosis and unfavorable outcomes [Citation53,Citation54].
Similar to the 54.3% triple-negative cases recorded by this study, a previous study [Citation13] in the same department reported 42.7% as triple-negative cases. On the contrary, other studies [Citation14,Citation55] on samples from the same department reported lower proportions of cases being triple negative. The results of the current study are, however, in agreement with other studies in Accra, Ghana [Citation34,Citation50], and sub-Saharan Africa [Citation56–58].
5. Conclusion
In conclusion, findings from this study show an increase in the incidence of breast cancer compared to previous studies in our center. Similar trends have been observed in recent studies in Accra, Ghana. This supports a rise in breast cancer among Africans but also suggests an increased awareness and improvement in diagnosis. Triple-negative phenotype was predominant, in agreement with other studies in sub-Saharan Africa.
Supplemental Material
Download Rich Text Format File (17 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed here.
References
- Javed A, Lteif A. Development of the human breast. Semin Plast Surg. 2013;27(1):5–12.
- Russo J, Lynch H, Russo IH. Mammary gland architecture as a determining factor in the susceptibility of the human breast to cancer. Breast J. 2001;7(5):278–291.
- Neville MC, Neifert MR, eds. Lactation; physiology, nutrition, and breast-feeding. Boston, MA: Springer US; 1983. DOI:https://doi.org/10.1007/978-1-4613-3688-4
- Chinyama CN. Overview of benign breast lesions. In: Benign breast diseases. 2nd ed. Berlin, Heidelberg: Springer Berlin Heidelberg; 2014. p. 9–16. DOI:https://doi.org/10.1007/978-3-642-41065-9_2
- De Silva NK. Breast development and disorders in the adolescent female. Best Pract Res Clin Obstet Gynaecol. 2018;48(September):40–50.
- Onstad M, Stuckey A. Benign breast disorders. Obstet Gynecol Clin North Am. 2013;40(3):459–473.
- Dyrstad SW, Yan Y, Fowler AM, et al. Breast cancer risk associated with benign breast disease: systematic review and meta-analysis. Breast Cancer Res Treat. 2015;149(3):569–575.
- Sasaki J, Geletzke A, Kass RB, et al. Etiology and management of benign breast disease. In: The breast. 5th ed. Elsevier; 2018. p. 79–92.e5. DOI:https://doi.org/10.1016/B978-0-323-35955-9.00005-2
- Ibrahim I, Iliyasu Y, Mohammed A. Histopathological review of breast tumors in Kano, Northern Nigeria. Sub-Saharan African J Med. 2015;2(1):47.
- Alexander Femi A. Profile of benign breast diseases in an African population. J Surg. 2016;4(2):35.
- Ohene-Yeboah MOK. An audit of excised breast lumps in Ghanaian women. West Afr J Med. 2005;24(3):252–255.
- Ohene-Yeboah M, Amaning E. Spectrum of complaints presented at a specialist breast clinic in Kumasi, Ghana. Ghana Med J. 2009;42(3):110–112.
- Adjei E, Ohene-Yeboah M. Breast cancer in Kumasi, Ghana. Ghana Med J. 2012;46(1):13.
- Thomas AS, Osei-bonsu E. Breast cancer in Ghana: demonstrating the need for population-based cancer registries in low- and middle-income countries. J Glob Oncol. 2017;3(6):765–772.
- Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38(12):1346–1366.
- Wolff AC, Elizabeth Hale Hammond M, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/ College of American Pathologists Clinical Practice guideline focused update. J Clin Oncol. 2018;36(20):2105–2122.
- Abubakar M, Orr N, Daley F, et al. Prognostic value of automated KI67 scoring in breast cancer: a centralised evaluation of 8088 patients from 10 study groups. Breast Cancer Res. 2016;18(1):1–13.
- Torous VF, Schnitt SJ, Collins LC. Benign breast lesions that mimic malignancy. Pathology. 2017;49(2):181–196.
- Brinton L, Figueroa J, Adjei E, et al. Factors contributing to delays in diagnosis of breast cancers in Ghana, West Africa. Breast Cancer Res Treat. 2017;162(1):105–114.
- Edmund DM, Naaeder SB, Tettey Y, et al. Breast cancer in Ghanaian women. Am J Clin Pathol. 2013;140(1):97–102.
- Vanderpuye V, Grover S, Hammad N, et al. An update on the management of breast cancer in Africa. Infect Agent Cancer. 2017;12(1):13.
- Gyan E, Derkyi-Kwarteng L, Brown AA, et al. Benign breast conditions: an eight-year single-centre histopathological review of women presenting with mass lesions at the Korle-Bu Teaching Hospital, Ghana. Ann Diagn Pathol. 2019;42:33–38.
- Forae G, Igbe A, Ijomone E, et al. Benign breast diseases in Warri Southern Nigeria: a spectrum of histopathological analysis. Ann Niger Med. 2014;8(1):28.
- Ugiagbe E, Olu-Eddo A. Benign breast lesions in an African population: a 25-year histopathological review of 1864 cases. Niger Med J. 2011;52(4):211.
- Nwafor CC, Keshinro S. The pathology of breast biopsies in a sample of Nigerian patients: review and analysis. Ann AFRICAN Surg. 2015;12(2):89–94.
- Chalya PL, Manyama M, Rambau PF, et al. Clinicopathological pattern of benign breast diseases among female patients at a tertiary health institution in Tanzania. Tanzan J Health Res. 2016;18(1):1–9.
- Nwafor C, Udo I. Histological characteristics of breast lesions in Uyo, Nigeria. Niger J Surg. 2018;24(2):76.
- Siddiqui MS, Kayani N, Pervez S, et al. Breast diseases: a histopathological analysis of 3279 cases at a tertiary care center in Pakistan. J Pakistan. 2003;53(March):94–97.
- Ciatto S, Bonardi R, Ravaioli A, et al. Benign breast surgical biopsies: are they always justified? Tumori J. 1998;84(5):521–524.
- Khan A, Jan H, Rafiq MS, et al. Types of breast lumps in a tertiary care hospital. J Med Sci. 2016;24(3):133–135.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A J Clin. 2018;1–31. DOI:https://doi.org/10.3322/caac.21492
- Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends – an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27.
- Joko-fru WY, Miranda-filho A, Soerjomataram I, et al. Breast cancer survival in sub-Saharan Africa by age, stage at diagnosis and Human Development Index: a population-based registry study. Int J Cancer. 2020;146(May2019):1208–1218.
- Mensah AC, Yarney J, Nokoe SK, et al. Survival outcomes of breast cancer in Ghana: an analysis of clinicopathological features. Open Access Libr J. 2016;3(e2145):1–11.
- Wiredu EK, Armah HB. Cancer mortality patterns in Ghana: a 10-year review of autopsies and hospital mortality. BMC Public Health. 2006;6(159):1–7.
- Sanuade OA, Ayettey H, Hewlett S, et al. Understanding the causes of breast cancer treatment delays at a teaching hospital in Ghana. Journal of Health Psychology 2018. DOI:https://doi.org/10.1177/1359105318814152
- Zelle SG, Nyarko KM, Bosu WK, et al. Costs, effects and cost-effectiveness of breast cancer control in Ghana. Trop Med Int Heal. 2012;17(8):1031–1043.
- Aziato L, Clegg-Lamptey JNA. Breast cancer diagnosis and factors influencing treatment decisions in Ghana. Health Care Women Int. 2015;36(5):543–557.
- Obrist M, Osei-Bonsu E, Ahwah B, et al. Factors related to incomplete treatment of breast cancer in Kumasi, Ghana. Breast. 2014;23(6):821–828.
- Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378–397.
- Pace LE, Shulman LN. Breast cancer in sub‐Saharan Africa: challenges and opportunities to reduce mortality. Oncologist. 2016;21(6):739–744.
- Ghana Statistical Service. Ghana population and housing census 2010; 2012.
- Ofori-Asenso R, Agyeman AA, Laar A, et al. Overweight and obesity epidemic in Ghana – a systematic review and meta-analysis. BMC Public Health. 2016;16(1):1239.
- Lartey ST, Magnussen CG, Si L, et al. Rapidly increasing prevalence of overweight and obesity in older Ghanaian adults from 2007-2015: evidence from WHO-SAGE Waves 1 & 2. Ahmad R, ed. PLoS One. 2019;14(8):e0215045.
- Ahmed S, Choi Y, Rimon JG, et al. Trends in contraceptive prevalence rates in sub-Saharan Africa since the 2012 London summit on family planning: results from repeated cross-sectional surveys. Lancet Glob Heal. 2019;7(7):e904–e911.
- Quayson SE, Wiredu EK, Adjei DN, et al. Breast cancer in Accra, Ghana. J Med Biomed Sci. 2014;3(3):21–26.
- Laryea DO, Awuah B, Amoako YA, et al. Cancer incidence in Ghana, 2012: evidence from a population-based cancer registry. BMC Cancer. 2014;14(1):362.
- Amoako YA, Awuah B, Larsen-Reindorf R, et al. Malignant tumours in urban Ghana: evidence from the city of Kumasi. BMC Cancer. 2019;19(1):267.
- Evans M, Shaaban AM. Breast cancer in sub-Saharan Africa. In: Adedeji OA, editor. Cancer in sub-Saharan Africa; current practice and future. Springer; 2016. p. 81–93. DOI:https://doi.org/10.1007/978-3-319-52554-9
- Seshie B, Adu-Aryee NA, Dedey F, et al. A retrospective analysis of breast cancer subtype based on ER/PR and HER2 status in Ghanaian patients at the Korle Bu Teaching Hospital, Ghana. BMC Clin Pathol. 2015;15(1):14.
- Makanjuola S, Ayodele S, Javid F, et al. Breast cancer receptor status assessment and clinicopathological association in Nigerian women: a retrospective analysis. J Cancer Res Ther. 2014;2(8):122–127.
- Newman LA. Disparities in breast cancer and African ancestry: a global perspective. Breast J. 2015;21(2):133–139.
- Bianchini G, Balko JM, Mayer IA, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674–690.
- Denkert C, Liedtke C, Tutt A, et al. Molecular alterations in triple-negative breast cancer – the road to new treatment strategies. Lancet. 2017;389(10087):2430–2442.
- Adjei EK, Owusu-Afriyie O, Awuah B, et al. Hormone receptors and Her2 expression in breast cancer in sub-Saharan Africa. A comparative study of biopsies from Ghana and Norway. Breast J. 2014;20(3):308–311.
- Titiloye NA, Foster A, Omoniyi-Esan GO, et al. Histological features and tissue microarray taxonomy of Nigerian breast cancer reveal predominance of the high-grade triple-negative phenotype. Pathobiology. 2016;83(1):24–32.
- Minoza KG, Yawe KT, Mustapha Z, et al. Hormonal and HER2 receptor immunohistochemistry of breast cancer in North-Eastern Nigeria: a preliminary report. IOSR J Dent Med Sci. 2016;15(6):18–23.
- Usman A, Iliyasu Y, Atanda AT. Molecular subtyping of carcinoma of the female breast in a tertiary teaching hospital in Northern Nigeria. Ann Trop Pathol. 2019;10(1):20–26.