ABSTRACT
Background: Formaldehyde (FA) is a sensitizing agent that can produce an effective immune system response upon initial exposure. FA that can be used in many industries, medical and anatomical facilities, mainly cadaver-based gross anatomy laboratories, represent a risk to occupational health of professionals and students. The majority of FA exposures occur through inhalation, dermal, and eye contact. FA-induced effects are attributed to site-specific and dose-dependent health impairments in many organs and organ systems. Much progress has been reported on FA-induced health impairments in respiratory tract, but a comprehensive study in different organ systems and the reverse effects of some novel antioxidants is still lacking. This review explores the cytotoxic effects of FA and its role as key signaling molecule, site specific and or dose dependent effects of FA in nasal cavity, carcinogenic effects of FA exposure in nasopharynx, effects of FA in lung macrophage functions and development of pulmonary fibrosis, dose dependent reproductive and genetic effects of FA, neurotoxic effects of FA and potential risk of FA in some beauty salons and cosmetic products. In addition, this study shows the counteracting beneficial role of melatonin, Nigella sativa, rose oils, caffeic acid phenethyl ester (CAPE), vitamin E, and proanthocyanidins (PAs) against FA induced tissue damage.Methods: A comprehensive literature search highlighting the health impairments of FA and counteracting beneficial effects of promising antioxidants was conducted using PubMed, Google Scholar, and Medline Cochrane, to assemble relevant publications from open access international journals published only in English.Results: The search generated 411 articles of which 70 full research articles fulfilled the inclusion criteria and included in the review. The results of this study confirmed relentless toxic effects of FA exposure on various organs of human and other animals. Multiple findings also stated the efficacy of promising antioxidants against FA-induced tissue damage in animal models.Conclusion: Occupational exposure to FA is most likely due to inattention towards its side effects and lack of appropriate air filtering equipment or unmonitored concentration of FA in the working air. Therefore, re-evaluating the concentration of FA, proper ventilation, and assessment of working practices is highly recommended. Proper monitoring is also needed to improve compliance and protection of FA-based reproductive complications in females. Despite complete prevention is not viable, exposed personnel must be aware of FA-induced health effects and require assessing risks and acquiring practical measures in their working environment.
1. Introduction
The occupational exposure of FA and phenol is a public health problem with a large number of underreporting. Among the various chemicals that expose the worker is the FA used in the anatomical facilities, pathology laboratory, funeral services, museums, synthetic fibers industry, and beauty salons.
FA is truly an ancient friend of embalmers and seemingly has been around forever and embalming without FA is, to most embalmers, just a meaningless historical footnote. It is commercially obtainable as formalin, which contains 37% by weight or 40% by volume of FA gas in water [Citation1].
Workers in the anatomy department and students, embalmers in funeral homes, histopathology laboratory workers, and other biological researchers are continually exposed to the toxic vapors of FA [Citation2]. Large numbers of workers are potentially exposed to FA. The highest potential exposure occurs in the FA-based resins industry, where workers may be exposed to high air concentrations and also have dermal exposure from liquid FA. Other types of employees at risk for exposure to FA include dentists, doctors, embalmers, nurses, pathologists, teachers and students who handle preserved specimens in laboratories, veterinarians, and workers in the clothing industry or in furniture factories [Citation3].
FA is readily absorbed following inhalation and ingestion and rapidly metabolized at the initial site of contact into formate by the requirement of glutathione, catalyzed by FA dehydrogenase [Citation4]. FA can react with different macromolecules such as proteins and nucleic acid, or with low molecular weight substances such as amino acids and it is a highly water-soluble compound that can rapidly diffuse into many tissues [Citation5].
FA is a highly reactive molecule that can be directly irritating to tissues when it comes into contact. Animal and human studies suggested that short-term exposure to formaldehyde induces erythema, itchy and burning eyes, runny and stiffy nose, poor appetite, nausea, vomiting, and confusion. Occupational exposure to formaldehyde in professionals using FA as tissue preservative and industrial workers involved in the production and use of FA is mainly via the dermal and inhalation routes, where it is absorbed by lungs and gastrointestinal tract, and effects associated with it occur primarily at sites of contact and these include irritation of eyes and upper respiratory tract [Citation6].
Several studies carried out on Wistar rats showed that inhalation of FA above 3 ppm can result in irritation and damage to the lining of the nose and throat. This indicates that the upper respiratory tract can be responded to low doses of formaldehyde exposure. High concentrations can also affect the lung, and it can be dose-dependent histopathological effects. FA exposure at high concentrations for long periods can impair learning and changes in behavior have been observed in rats.
The exposure to FA remains one of the most prominent occupational and environmental health problems, resulting in the immediate effects as nausea, headache, and ocular irritation that can cause tear overflow and a burning sensation in the throat. Long-term exposure to FA can cause contact dermatitis, congenital defects like low birth weight, isolated heart disease, cytotoxicity in the respiratory tract in the form of acute lung injury, nasal obstruction, pulmonary edema, and cancer. However, the effects of FA on asthmatics may be dependent on previous, repeated exposure to formaldehyde [Citation2,Citation5,Citation7]
Studies done in rats and mice have shown FA exposure at 3–400 ppm decreased food and water intake, decreased body weight, gastrointestinal effect, liver and kidney effects while concentration above 6 ppm in human and animal models showed nasal and eye irritation, throat irritation, change in pulmonary function, decreased body weight, enhanced allergic responses, and neurological effects.
FA can increase the production of reactive oxygen species (ROS) in many tissues, and these ROS including singlet oxygen, hydrogen peroxide, superoxide anions, and hydroxyl radicals are important mediators of cellular injury and play an important role in oxidative damage [Citation8]. FA inhalation inflicts various harms on many organs of living bodies including the liver, testes, and brain [Citation9–11]. Few studies have been conducted and illustrated short-term FA-induced carcinogenic effects in both nose and throat while long-term studies, in laboratory rats, showed induction of nasal cancer only. In the species-specific effect of FA , humans are more prone to develop FA exposure-related cancer than other animals. The Occupational Safety and Health Administration (OSHA) set a legal limit of 0.75 ppm FA in air averaged over an 8-h workday [Citation12]. Although there are multiple specific findings related to FA health effects, extensive investigation and heterogeneous human study and animal models are still limited. The aim of this review is, therefore, to assess occupational FA exposure linked to increased health impairments and counteracting beneficial effects of selected antioxidants.
2. Methods
2.1. Literature search strategy
In this systematic review, we searched different literatures in the databases of Google Scholar, PubMed Central, and Medline Cochrane to explore the occupational FA linked to systemic health impairements and beneficial effects of nobel antioxidants through comparison and discussion of investigation of different scientific works.
2.2. Data extraction and selection criteria
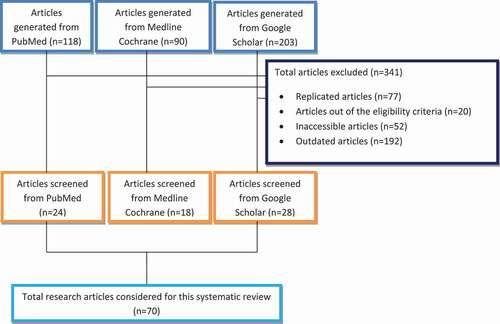
The literature search targeted review and full research articles published only in English with restriction of most publications after 2000. After all the journals filling the inclusion criteria were selected by the authors. The general concepts of FA, FA health impairments (cytotoxicity, site-specific effects of FA on the upper respiratory system, dose-dependent effects of FA on the lower respiratory system, oxidative stress potential of FA on lungs and testis, carcinogenic effects of FA, neurotoxicity of FA, occupational exposure of FA in cosmologists), and antioxidant effects of novel agents were reported and discussed. The flow of information and selection process through the different phases of this review is shown in .
3. Results and discusion
3.1. Qualitative review of formaldehyde exposure health impairments
The search on the detrimental health impairments linked to FA and antioxidant effects against its toxicity yielded 411 scientific articles. From this, 70 articles were screened and included in this review. The study targeted FA-induced tissue damage and novel antioxidant agents on human and animal models like Swiss mice, B6C3F1 mice, Wistar albino rats, F344 rats BN rats, Guinea pigs, and rabbits.
All the investigators have evaluated and directly addressed the toxic effects of FA exposure in various organ systems in human and other animals. In human studies, each participant was interviewed using a questionnaire containing detailed information about their previous occupational and medical history. The doses of FA were expressed in ppm, ppb, percentage, mg/m3, and mg/kg and mol/kg. The minimum and maximum exposure duration was 15 min and 5 years, respectively. The results of this review confirmed the species-specific, site-specific and day-dependent effects of FA, dose-dependent oxidative tissue damage, reproductive and genetic effects of FA, FA neurotoxicity, and occupational effects of FA in cosmologists. Multiple findings also proved that antioxidants are effective in recovering FA-induced tissue damage in rats.
3.2. Cytotoxicity of formaldehyde and its role as key signaling molecule
The study indicated that the cytotoxic effects of FA on osteoblast cells depend on the exposure dose, frequency, and duration of exposure [Citation13]. But, the authors did not hide its intricacy to determine how much FA acts on periapical tissue after leaching from FA-releasing root canal sealers through the apical foramen. In addition, new evidence is also investigated in development of agents that induce the cellular synthesis of GSH level might be useful for protecting periapical tissue against cytotoxicity induced by formaldehyde-releasing root canal sealers. From this, it is true that FA could affect cell activity and even enter cells. Another study showed exposure to FA changed the endogenous FA concentration in cells within 24 h, and this induced expression of FDH for formaldehyde degradation to maintain the FA balance [Citation14]. It can be concluded that the toxicity of FA is not caused by the carbon atoms in the aldehyde, hydroxyl, or carboxyl groups. Therefore, FA is hypothesized to be an important signaling molecule in the regulation of cell growth and maintenance of the endogenous FA level.
Genomic responses with increasing tissue FA are expected to increase more sharply with FA exposures above 3 ppm [Citation15]. Although cell cycle and DNA repair pathway genes were altered at 6 ppm and above at the 1- and 13-week time points, other pathways contributed to tissue responses. The enrichment in immune pathways was mildly evident in the hierarchical clustering at 4 weeks and from pathway enrichment for IL-17 signaling and ETV3 effects on CSF-1–mediated macrophage differentiation. The ETV (ETS family transcriptional repressor) pathway has inhibitory effects on pro-inflammatory mediator production and as the same time, the immune pathways, however, were not significantly enriched at 13 weeks where only the MIF pathway was enriched. Decreased Cxcl13 and STAP1, along with changes in MIF and ETV3 on CSF-1 signaling, may be part of a biological response that blunts immune responses and reduces recruitment of immune cells to the sites of injury at the later exposure times, even though FA-induced necrosis and erosion were still evident [Citation15,Citation16].
3.3. The site-specific and dose-dependent effects of formaldehyde exposure in the nasal cavity
Inspired air is brought high into the nose so that environmental pollutants easily affect the respiratory and olfactory zone of nasal cavity. The study done in the Netherlands showed incidence of nasal lesions characterized by dose, site-specific, and cell type-dependent cytotoxicity after acute exposure of FA[Citation17]. The study was done in male Wistar rats after 6 h/day of FA exposure for 3 days, and it showed itching, stiffy nose, and even epistaxis after long-term exposure. The absence of treatment-related effects in both respiratory and olfactory epithelium after low-dose exposure (1 ppm) was noticed, but marked necrosis and thickening were observed in respiratory epithelium at high-dose (3 ppm) FA-exposed group [Citation18]. The authors attribute this to the significant association between infrequent personal protective equipment in gross anatomy laboratory and noticeable toxic effects of formaldehyde.
The study done in North Carolina showed the relative toxicities of FA at the doses of 40, 200, 400, and 800 mM to the rat nasal epithelium. Consequently, determined following intranasal instillation of aqueous solutions into one nostril of male Fischer 344 (F-344) rats was noticed. But, 3 days after treatment, only respiratory epithelium lining the anterior nasal passages confirmed histopathological lesion, and this effect was severe at the concentration above 200 mM of dose exposure [Citation19]. Based on these findings, FA elicits a toxic response at higher dose and not equally responsive at various zones of nasal cavity.
Additionally, long-term inhalation toxicity study with FA mixed with acetaldehyde and acrolein-acetaldehyde was carried out in rats, exposed to 0, 750, 1500, and 3000/1500 ppm of the test compound for 52 weeks and killed after recovery periods of 26 or 52 weeks. Potential histopathological changes like epistaxis, hyperplasia, loss of sensory and sustentacular cells, anosmia, hyper and metaplasia of the respiratory epithelium frequently accompanied by keratinization and occasionally by proliferations of atypical basal cells were appeared to be more extensive in the nasal cavity, and this included both olfactory and respiratory zone of nasal cavity [Citation20]. This is only dose-dependent effect on both olfactory and respiratory epithelia of nasal cavity, but that of other studies in Netherlands and North Carolina indicated dose and cellular type-dependent effects on respiratory epithelium [Citation17,Citation18]. The former effect is site-specific and cellular-dependent potentially marked at respiratory epithelium and more specific to pseudo-stratified ciliated columnar epithelium, but the toxic effects of FA and other aldehydes were observed at both olfactory and respiratory zone of nasal cavity. This proves that olfactory epithelium responds to mixture exposure to aldehydes and combination of different aldehydes at similar duration exposure more severe in nasal cavity.
3.4. Carcinogenic effect of formaldehyde exposure in nasopharynx
The studies showed that humans are more sensitive to indoor and outdoor air pollutants and responded very quickly to low dose of inhaled chemicals like FA. Human study evaluated the histological changes, especially the presence of possible precancerous lesions, in the nasal mucosa of workers exposed to FA . Nasal biopsies of workers occupationally exposed to formaldehyde for more than 5 years showed a higher degree of metaplastic alterations, epithelial dysplasia, recurrent epistaxis, and nasal stenosis [Citation21]. This is long-term effect of formaldehyde exposure, and it indicates that FA may be potentially carcinogenic to human. Workers in the anatomy department, especially the embalmers, are at risk of developing long-term nasal cancer if the concentration of embalming fluid is not monitored regularly. Similarly, the carcinogenic response to the combined and separate exposures to FA and hydrochloric acid (HCI) was investigated and revealed no carcinogenic response in SD rats was with HCI alone, but the mixture of HCHO-HCI showed powerful carcinogenic response. In both studies, FA exposure induced carcinogenic effect in nasal cavity. The combined exposure of formaldehyde with HCL induced no carcinogenic effect in nasal cavity. HCL may induce toxicity when contact with the body, but it is not purely carcinogenic.
The World Health Organization stated the presence of 2.3–6.1 ppm FA in a single cigarette, and it was also proved that smoking of 20 cigarettes per day corresponds to an intake of 1 mg dose of FA [Citation22]. Therefore, the workers who are more close to gross anatomy laboratory and addicted to cigarette smoking are more vulnerable in developing health effects related to FA exposure.
Dose-dependent induction of squamous cell carcinoma of nasal passages [Citation23] is in agreement with the study confirming occurrence of nasal tumors only at high doses of 14 and 15 ppm in rats and also described a potential induction of substantial histopathological derangements directly associated with the development of nasal tumors at 6 ppm and above, with a sufficient duration [Citation24]. Treatment of 10 ppm formaldehyde concentration with sufficient exposure duration also indicated hyperchromatic nasal glands, in rats [Citation25]. This suggests that the facts of squamous cell carcinoma, development of nasal tumors, and hyper-chromatic nasal glands may be the indications of dose-dependent FA toxicity at nasal mucosa. Long-term effects of FA with duration of time and concentration of high-dose exposure most necessarily induce carcinoma in the upper conducting zone of the respiratory system. Increased number of cell proliferation due to FA-induced damage may be potential mechanism for the development of nasal cancer.
High capacity of IgE production and hyper-responsiveness to exposure to allergens and the higher responsive nasal and tracheal mucosa was assessed in BN and F344 rats after the inhalation of aerosol FA [Citation26]. The incidence of clinical signs such as sneezing and abnormal respiration in formaldehyde treated F344 rats was higher than that in FA-treated BN rats, and the mean body weight of FA treated F344 rats also apparently decreased in comparison with control F344 rats, but that of FA treated BN rats was not significantly different from that of control BN rats. Changes such as squamous metaplasia, stratification, degeneration, and desquamation were observed in nasal, tracheal, and bronchial mucosa in the lungs of the FA-treated F344 rats. High lesions were restricted only to the nasal mucosa in BN rats. This finding is in contrary with the site-specific and cellular-dependent effects of FA in nasal cavity [Citation27]. It seems that BN rats have lower responsive to formaldehyde exposure than F344 rats. Therefore, workers having of higher sensitivity to allergies will have increased risk of this serious allergy-induced reaction of FA . So, awareness is mandatory for people occupationally exposed to formaldehyde vapor.
Formalin effects on the nose and throat of personnel of anatomical sciences departments in Iran medical schools showed significant association between smell decrement and FA levels and described the occurrence of nasal irritation at anatomy laboratory even below 0.75 ppm [Citation28]. Employees indicated that runny nose was more severe during the first year of their employment and gradually decreased over time as they get accustomed to the work environment. By evaluating on the students with short time and few sessions contact in gross anatomy laboratory, it also showed that runny nose alone as the significant association with sex (p = 0.049). Females had 2.09 times runny nose than males. The study done in Singapore on medical students’ exposure to 0.5–0.74 ppm formaldehyde in a gross anatomy dissection laboratory suggested no significant differences in the pre- and post-exposure; however, decreased ability to smell, eye irritation, throat irritation, and dry mouth were potentially observed in exposed group [Citation29]. These symptoms were also significantly related to the time and place of occurrence.
The presence of link between runny nose and direct contact with FA vapor was indicated in these studies. The symptoms are related to time of exposure and place of occurrence, which means the concentration of FA near the embalming room is completely different from the concentration at the door of dissection room. The reason why females showed higher runny nose than males may suggest the presence of higher susceptibility to formaldehyde vapor in the female students. Exposure of formaldehyde below 0.74 ppm showed gradual decrement of its toxic effect, but at the same dose, medical students showed no significant changes after FA exposure [Citation30]. The gradual over time runny nose reduction in employees in the anatomy department suggests development of FA tolerance, and it may be due to higher duration of time in dissection room. Long-term toxic effect may develop after a long duration of time. The toxicity of FA vapor in B6C3F1 mice was exposed at the doses of 0, 2, 4, 10, 20, or 40 ppm for 13 weeks. From this finding, more squamous metaplasia and inflammation were shown in nasal tissues of male and female mice at the dose of 10, 20, and 40 ppm and in larynx of treated animals at 20 and 40 ppm [Citation31].
Scientific evidences in this study suggested potential effects of FA exposure causing squamous cell carcinoma in nasopharynx, in humans, and animals. These associations between exposure to formaldehyde and induced nasopharyngeal cancer are mostly limited to long-term and high exposure concentrations of formaldehyde.
3.5. Effects of formaldehyde on lung macrophage functions and development of pulmonary fibrosis
There is evidence that FA, at extremely high concentrations, impairs the killing of microorganisms like Streptococcus aureus. Exposing mice to FA and carbon black particles shows no effect on bacterial killing but can depress phagocytosis. This depression is not apparent until 5 days after exposure and is maximal 25 days after exposure [Citation32]. However, when mice is exposed to FAboth before and after inhaling the bacteria, only 1 ppm of FA is needed to impair bacterial killing. The chemical analyses revealed that only 1% of the FA was bound to the carbon black particles, which may account for the absence of an effect of the combined exposure on bacterial killing. It is not known why the combined exposure depressed alveolar macrophage phagocytosis or why this effect was delayed [Citation33].
These experiments demonstrated that inhalation of FA can impair the capacity of mouse alveolar macrophages to kill certain bacteria and that inhalation of FA mixed with carbon black particles can depress alveolar macrophage phagocytosis. However, the detection of these effects depends on the exposure protocol and the time of assay after an exposure.
Despite of the effects of FA in the lung inflammation, it is important to consider that many studies show that the biggest problem of PF is the collagen production and not necessarily inflammatory processes. The increased lung elastance found in the FA group was surprising as well as the increase in the lung inflammation, collagen production, and alveolar enlargement because the dose used in this study was very low and allowed for human exposure [Citation34]. Moreover, in earlier studies this dose of FA did not induce direct effects in the lung of pregnant rats, but it altered the immune system of offspring [Citation35,Citation36]. We attribute such differences to the animal species used in both studies (rats and mice).
One study showed that FA exposure aggravates the lung neutrophils influx and collagen production, but did not alter the lung elastance, mucus production, edema, and interstitial thickening [Citation34]. This work contributes to understanding the effects of pollution on the development of pulmonary fibrosis.
The mechanism of fibrosis induced by FA (10) in exposure duration-dependent was contradicted by the report that interleukin-11 (IL-11), a molecular target for FA , could be involved in inflammatory and fibrogenic pulmonary effects in a dose-dependent manner in cultured lung epithelial cells, in rats [Citation37]. Even though dose-dependent effects were recorded in both studies, the aim of the later study seems to validate induction of IL-11 by the environmental contaminants in lung tissues, which may be due to low IL-11 receptors in the experimental animals.
3.6. Reproductive and genetic effect of formaldehyde exposure
Several potential modes of action of FA for reproductive and developmental outcomes have been suggested by animal studies, including endocrine disruption, genotoxic effects on gametes, and oxidative stress. However, the evidence for causality is weak. In addition, it is not clear that inhaled FA or its metabolites can penetrate fast the portal of entry or cross the placenta, blood–testis barrier, or blood–brain barrier.
Day-dependent oxidative stress of FA in testicles of adult male rats showed disintegration of leydig cells and edematous interstitial tissues with vascular dilations [Citation8]. In this study, animals were also treated with FA mixed with 30 mg/kg doses of vitamin E and the histopathological changes were partially reversed and showed similar normal testicular tissue features. Similarly, atrophication and reduction of seminiferous tubule diameters and decreased amount of Leydig cells were analyzed as long-term effects of testicular tissue in rats and mice [Citation38,Citation39]. The mechanism of testicular damage induced by oxidative stress in testis of rats exposed to formaldehyde [Citation8] is in agreement with the report, which showed that human testis and spermatozoa are extremely sensitive to ROS-induced damage [Citation40]. The histopathological changes in the spermatogenesis process and seminiferous tubules were related to the cytotoxic effect of formaldehyde [Citation10]. It is reported that intraperitoneal administration of FA caused the arrest of nucleic acid synthesis and proteins. The other possible mechanism in oxidative stress may therefore be due to altered levels of trace elements in FA exposure and FA cytotoxicity in testicular tissues. Since FA reacts directly with tissue constituents, cytotoxic effect in testicular tissue is presumably a function of this reactivity.
Although few studies have reported FA provoked spontaneous abortion and low birth weight, a review of adverse pregnancy outcomes and FA exposure in human and animal studies found no concise evidence related to adverse pregnancy outcomes following FA exposure. This study also justified such evidence as reporting biases and publication biases among the epidemiological studies.
3.7. Formaldehyde neurotoxicity
Neurotoxicity and cognitive dysfunction have separately been associated with endogenous FA and reduction of acetylcholine signals. However, a limited number of studies have shown a relationship between cholinergic neurotransmitter and FA exposure. The findings suggested the neurotoxic effect of formaldehyde depends on the AChE activity, which is directly affected by metabolism [Citation41]. Its conclusive point showed that cholinergic signal reduction in cases of cognitive dysfunction could be associated with endogenous FA. Another study also addressed FA neurotoxicity, inhalation of FA at concentrations of 6 and 12 ppm, during the early postnatal period (PND30), which resulted in an increase in pyknotic neuron counts in the rat hippocampus pyramidal cell layer [Citation42]. This is inconsistent with another study, which stated that exposure to FA at the dose of 10 mg/kg for 10 days increased pyknosis and decreased neuronal number in the adult rat frontal cortex and hippocampus [Citation43]. Furthermore, FA administration under similar conditions increased apoptosis in the rat prefrontal cortex and caused an increase in the immune-reactivity some pro-apoptotic protein [Citation44]. Similarly, it was found that chronic exposure to low levels of formaldehyde in rats caused an increase in the number of CRH-ir neurons in the hypothalamus [Citation45]. Although FA studies have not focused on the behavioral effects, several symptoms of associated disorders have been observed during studies of FA-exposed rats, such as lethargy, decrease in motor activity, and loss of appetite [Citation40,Citation46,Citation47].
3.8. Effects of formaldehyde in cosmetic products
FA is found in nail polishes, nail hardeners, eyelash glues, hair gels, soaps, makeup, shampoos, lotions, and deodorants, among other products. FA and FA-releasing are also used as preservatives to kill microorganisms or to prevent or inhibit their growth in products.
In Brazil, the study on characterization of FA exposure resulting from the use of four professional hair straightening products was conducted. By using all four different hair treatment brands – Brazilian Blowout, Coppola, Global Keratin, and La Brasiliana – showed in estimated 8-h time-weighted averages ranging from 0.17 to 0.29 ppm. After task sampling, bulk sampling reported formaldehyde concentrations of 11.5% in Brazilian Blowout, 8.3% in Global Keratin, 3% in Coppola, and 0% in La Brasiliana. The study also reported that even those products labeled “FA-free” have the potential to produce formaldehyde concentrations that meet or exceed current occupational exposure limits [Citation48].
FA is found in both hair dye and nail polish. It was reported that hairdressers and nail technicians were predominantly female, and many were of reproductive age [Citation49]. As these workers often started their careers before the age of 20, it was assumed that many had begun working before considering family planning [Citation50]. This assumption possibly raises concerns because these women of reproductive age are at higher risk for the effects of exposure to potential reproductive toxins.
Hair dressing has been reported to be associated with a variety of health issues, including dermatitis, cancer, and respiratory problems [Citation51]. In a meta-analysis of 42 studies, a statistically significant increased risk for bladder cancer was found among hairdressers, specifically those who had held the job for more than 10 years [Citation52]. Alternatively, a review report suggested that occupational exposure to hair dyes poses no carcinogenic or other human health risk. However, this review focused on acute toxicity and health effects of hair dyes [Citation53]. The evidence related to the harmful effects of hair dyes and nail polish on reproductive health is limited. The study done on Spanish hairdressers also showed an increased risk for sub-fertility and menstrual disorders [Citation51].
3.9. Effects of selected antioxidants against formaldehyde toxicity
Antioxidants are endogenous molecules that mitigate any form of oxidative stress or its consequences. They may act from directly scavenging free radicals to inducing antioxidative defense. An antioxidant substance is present in the cell at low concentrations and significantly reduces or prevents oxidation of the oxidizable substrate. Humans have developed highly complex antioxidant systems (enzymatic and non-enzymatic), which work synergistically, and together with each other to protect the cells and organ systems of the body against free radical damage [Citation54]. Antioxidant deficiencies can develop as a result of decreased antioxidant intake, synthesis of endogenous enzymes or increased antioxidant utilization. Antioxidant supplementation has become an increasingly popular practice to maintain optimal body function. However, antioxidants exhibit pro-oxidant activity depending on the specific set of conditions. Of particular importance are their dosage and redox conditions in the cell and most important free radicals in many disease states are oxygen derivatives, particularly superoxide anion (the main source of hydrogen peroxide in vivo) and the hydroxyl radical [Citation55].
ROS are formed continuously in cells as a consequence of external factors, and they become harmful when they are produced in excess under abnormal conditions such as inflammation [Citation56]. Caffeic acid phenethyl ester (CAPE) possesses significant anti-inflammatory and antioxidant properties. It is a potent inhibitor of lipoxygenase, and through this activity, leukocyte chemotaxis and inflammatory activity are abolished and CAPE is effective in protecting the injury of remote organs caused by oxidative stress and neutrophil accumulation [Citation57–59]. CAPE is also effective for reversing histopathological tissue damage in lungs [Citation60].
Nigella sativa (NS) (recognized as black seed or black cumin) is a well-known immune stimulant that protects against many pathological conditions [Citation61,Citation62]. It also has anti-inflammatory activities proven by the fact that NS extracts inhibited the pro-oxidant nitric oxide production. NS aqueous extract demonstrates powerful antioxidative properties on oxidative hepatic damage induced by carbon tetrachloride, proving its hepato-protective role [Citation63].
Melatonin is also one of the novel antioxidants. Numerous investigators have suggested the antioxidant effects of melatonin in the nervous system and stated that melatonin as pronounced neuroprotective effect and stimulation of the immune system [Citation64–66]. Similarly, Skaper and coworkers have reported that melatonin prevents aging-related neuronal damage [Citation67]. Another study also showed the protective effects of melatonin against formaldehyde in prefrontal cortex at immune-histochemical and biochemical levels [Citation68]. Melatonin has been documented as a direct free radical scavenger and an indirect antioxidant, as well as an important immunomodulatory agent [Citation46]. Another study also reported that melatonin prevented FA-induced neuronal damage in the prefrontal cortex of rats in our study. Zararsiz and his coworkers were also demonstrated the reversible effects of melatonin on FA-induced apoptosis in the prefrontal cortex [Citation69]. Multiple investigations demonstrated that melatonin and NSare beneficial in counteracting formaldehyde-induced oxidative stress.
Proanthocyanidins (PAs) are also natural antioxidants whose polyphenolic properties are known and are commonly found in fruits and vegetables. A study showed degenerative changes in mitochondria and the ER in FA-induced testicular tissue damage and recoveries in the cristae of mitochondria and ER sacs, which are important for both the synthesis of the basement membrane and the components of the extracellular matrix, after PA treatment [75]. Backer E and his coworkers also investigated total recovery of degeneration in chromatin material, membrane damage in mitochondria, and losses in mitochondrial cristae in hepatocytes in the FA + vit E and FA + PA-treated groups, in liver tissue of Wistar albino rats [Citation70]. It is clear and promising that PA and vit E have protective effects against FA-induced testis and liver toxicity. Their use is suggested as a pre-treatment agent in FA toxicity, and combinations of both PA and vit E are powerful novel antioxidants in reversing FA-induced tissue damage.
4. Conclusion
The review highlighted the enormous health effects of FA exposure. People are affected by FA when they work with products made with FA such as cosmetic products, and accidentally ingesting or occupationally inhaling FA vapors from outdoor or indoor air. Occupational exposure to formaldehyde most likely results in detrimental health effects, which is most likely due to inattention of its side effects and lack of appropriate air filtering equipment, or unmonitored concentration of formalin in the working air. Moreover, the effectiveness of powerful antioxidants and/or anti-inflammatory such as vitamin E, PAs, NS, rose oil, fish omega-3, melatonin, and CAPE is observed in the treatment of the harmful effects of formaldehyde-induced tissue impairments. Moreover, deep studies are required in formulation, dosage, and route of application of these antioxidants. Therefore, reevaluating the concentration of formalin, proper ventilation, and assessment of working practices is highly recommended. Industry monitoring is also needed to improve compliance and protection of FA-based reproductive complications in females. Those who have experienced severe FA exposure must undergo close medical monitoring as required. Despite complete prevention is not viable, exposed personnel must be aware of FA-induced health effects and require assessing risks and acquiring practical measures in their working environment.
Ethical statement
The authors of this study confirm that they have written original work, suitably citing the work and/or words of other investigators.
Authors’ contributions
The authors contributed equally to the work:
ST: conceptualization, investigation, writing original draft, reviewing and editing,
NH: investigation, writing original draft, reviewing and editing.
AG: structuring of the manuscript, investigation, reviewing and editing
ZN: structuring of the manuscript, investigation, reviewing and editing
List of abbreviations
PA: proanthocyanidins, NS: Nigella sativa, PPM: parts per million, FA: formaldehyde, HCL: hydrochloric acid, OSHA: Occupational Safety and Health Administration, WHO: World Health Organization, CAPE: caffeic acid phenethyl ester, IL: interleukin, µmol/kg: micro-mole per kilogram, IgE: immunoglobulin E, HUD: Housing and Urban Development, mg/m3: milligram per cubic meter, ROS: reactive oxygen species, CRH: corticotropin-releasing hormone, GIT: gastrointestinal tract, ER: endoplasmic reticulum, BPD: beginning on postnatal day, CRH: corticotropin-releasing hormone, BN rats: Brown Norway rats, SCF: colony-stimulating factor
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors
Availability of data and material
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the manuscript.
References
- Bedino JH. Formaldehyde exposure hazards and health effects: a comprehensive review for embalmers. Expand EncyclMortuPract. 2004;650:2653–2657.
- Raja DS, Sultana B. Potential health hazards for students exposed to formaldehyde in the gross anatomy laboratory. J Environ Health. 2012;74(6):36–41.
- Kim K-H, Jahan SA, Lee J-T. Exposure to formaldehyde and its potential human health hazards. J Environ Sci Health Part C. 2011;29(4):277–299.
- Golden R. Identifying an indoor air exposure limit for formaldehyde considering both irritation and cancer hazards. Crit Rev Toxicol. 2011;41(8):672–721.
- Rovira J, Roig N, Nadal M, et al. Human health risks of formaldehyde indoor levels: an issue of concern. J Environ Sci Health Part A. 2016;51(4):357–363.
- Liteplo RG, Beauchamp R, Chénier R, et al. Formaldehyde. World Health Organization; 2002. Royal Ottawa Mental Health Center
- Public Review Draft, (2007). Formaldehyde reference … - Google Scholar. Accessed 2021 Feb 27.
- Zhou D-X, Qiu S-D, Zhang J, et al. The protective effect of vitamin E against oxidative damage caused by formaldehyde in the testes of adult rats. Asian J Androl. 2006;8(5):584–588.
- Cortés O, Fernández J, Boj J, et al. Effect of formaldehyde on rat liver in doses used in pulpotomies. J Clin Pediatr Dent. 2007;31(3):179–182.
- Golalipour MJ, Azarhoush R, Ghafari S, et al. Formaldehyde exposure induces histopathological and morphometric changes in the rat testis. Department of Histology and Embryology, Gorgan University of Medical Sciences, Ian. Folia Morphol. 2007;66(3):167–171.
- Arici S, Karaman S, Dogru S, et al. Central nervous system toxicity after acute oral formaldehyde exposure in rabbits: an experimental study. Hum Exp Toxicol. 2014;33(11):1141–1149.
- Standard OBP. OSHA Fact Sheet.; 2012.
- Ho Y-C, Huang F-M, Chang Y-C. Cytotoxicity of formaldehyde on human osteoblastic cells is related to intracellular glutathione levels. J Biomed Mater Res B Appl Biomater. 2007;83(2):340–344.
- Ke YJ, Qin XD, Zhang YC, et al. In vitro study on cytotoxicity and intracellular formaldehyde concentration changes after exposure to formaldehyde and its derivatives. Hum Exp Toxicol. 2014;33(8):822–830.
- Andersen ME, Clewell III HJ, Bermudez E, et al. Formaldehyde: integrating dosimetry, cytotoxicity, and genomics to understand dose-dependent transitions for an endogenous compound. Toxicol Sci. 2010;118(2):716–731.
- Shah AV, Birdsey GM, Randi AM. Regulation of endothelial homeostasis, vascular development and angiogenesis by the transcription factor ERG. VasculPharmacol 2016;86:3–13.
- St. Clair MBG, Gross EA, Morgan KT. Pathology and cell proliferation induced by intra-nasal instillation of aldehydes in the rat: comparison of glutaraldehyde and formaldehyde. ToxicolPathol. 1990;18(3):353–361.
- Sammeta N, McClintock TS. Chemical stress induces the unfolded protein response in olfactory sensory neurons. J Comp Neurol. 2010;518(10):1825–1836.
- Swenberg JA, Kerns WD, Mitchell RI, et al. Induction of squamous cell carcinomas of the rat nasal cavity by inhalation exposure to formaldehyde vapor. Cancer Res. 1980;40(9):3398–3402.
- Albert RE, Sellakumar AR, Laskin S, et al. Gaseous formaldehyde and hydrogen chloride induction of nasal cancer in the rat. J Natl Cancer Inst. 1982;68(4):597–603.
- Majumder PK, Kumar VL. Inhibitory effects of formaldehyde on the reproductive system of male rats. Ind J Physiol Pharmacol. 1995;39(1):80–82.
- Mercury M. International programme on chemical safety. In: Environ Health Criteria. 1990. p. 118.
- Monticello TM, Miller FJ, Morgan KT. Regional increases in rat nasal epithelial cell proliferation following acute and subchronic inhalation of formaldehyde. ToxicolApplPharmacol. 1991;111(3):409–421.
- Purchase IFH, Paddle GM. Does formaldehyde cause nasopharyngeal cancer in man? Cancer Lett. 1989;46(2):79–85.
- Moss OR, Gross EA, James RA, et al. Respiratory tract toxicity in rats exposed to Mexico City air. Res Rep-Health Eff Inst. Volume 8 93): 212-219 March 1, 2001.
- Ohtsuka R, Shuto Y, Fujie H, et al. Response of respiratory epithelium of BN and F344 rats to formaldehyde inhalation. Exp Anim. 1997;46(4):279–286.
- Sobre La Mucosa EDF, enRatas R. Effects of formaldehyde on respiratory mucosa in rats. Int J Morphol. 2012;30(2):521–523.
- Namavar MR. Formalin effects on the nose and throat of personnel of anatomical sciences departments in Iran medical schools. Department of Anatomy, Iran Medical Schools. Published online; 2012.
- Chia SE, Ong CN, Foo SC, et al. Medical students’ exposure to formaldehyde in a gross anatomy dissection laboratory. J Am Coll Health. 1992;41(3):115–119.
- Oosthuizen JDV. An evaluation of the exposure of students and staff to formaldehyde vapour in the human anatomy laboratory of the faculty of medicine, University of Natal, South Africa. SEMANTIC SCHOLAR. Published online; 1996.
- Wilbur S, Wohlers D, Paikoff S, et al. ATSDR evaluation of health effects of benzene and relevance to public health. Toxicol Ind Health. 2008;24(5–6):263–398.
- Clarke RW, Antonini JM, Hemenway DR, et al. Inhaled particle-bound sulfate: effects on pulmonary inflammatory responses and alveolar macrophage function. InhalToxicol. 2000;12(3):169–186.
- Jakab GJ. Relationship between carbon black particulate-bound formaldehyde, pulmonary antibacterial defenses, and alveolar macrophage phagocytosis. InhalToxicol. 1992;4(4):325–342.
- Leal MP, Brochetti RA, Ignácio A, et al. Effects of formaldehyde exposure on the development of pulmonary fibrosis induced by bleomycin in mice. Toxicol Rep. 2018;5:512–520.
- Ibrahim BS, Da Silva CM, Barioni ÉD, et al. Formaldehyde inhalation during pregnancy abolishes the development of acute innate inflammation in offspring. Toxicol Lett. 2015;235(2):147–154.
- Maiellaro M, Correa-Costa M, Vitoretti LB, et al. Exposure to low doses of formaldehyde during pregnancy suppresses the development of allergic lung inflammation in offspring. ToxicolApplPharmacol. 2014;278(3):266–274.
- Lecureur V, Arzel M, Ameziane S, et al. MAPK-and PKC/CREB-dependent induction of interleukin-11 by the environmental contaminant formaldehyde in human bronchial epithelial cells. Toxicology. 2012;292(1):13–22.
- Vosoughi S, Khavanin A, Salehnia M, et al. Effects of simultaneous exposure to formaldehyde vapor and noise on mouse testicular tissue and sperm parameters. Health Scope. 2012;1(3):110–117.
- Vosoughi S, Khavanin A, Salehnia M, et al. Adverse effects of formaldehyde vapor on mouse sperm parameters and testicular tissue. Int J FertilSteril. 2013;6(4):250.
- Sorg BA, Bailie TM, Tschirgi ML, et al. Exposure to repeated low-level formaldehyde in rats increases basal corticosterone levels and enhances the corticosterone response to subsequent formaldehyde. Brain Res. 2001;898(2):314–320.
- Neurotoxicity effect of formaldehyde on occupational exposure and influence of individual susceptibility to some metabolism parameters - PubMed. cited Feb 28, 2021. https://pubmed.ncbi.nlm.nih.gov/27796833/
- Gurel A, Coskun O, Armutcu F, et al. Vitamin E against oxidative damage caused by formaldehyde in frontal cortex and hippocampus: biochemical and histological studies. J Chem Neuroanat. 2005;29(3):173–178.
- Zararsiz I, Kus I, Akpolat N, et al. Protective effects of $ømega$-3 essential fatty acids against formaldehyde-induced neuronal damage in prefrontal cortex of rats. Cell BiochemFunct Cell Biochem Its Modul Act Agents Dis. 2006;24(3):237–244.
- Sari DK, Kuwahara S, Tsukamoto Y, et al. Effect of prolonged exposure to low concentrations of formaldehyde on the corticotropin releasing hormone neurons in the hypothalamus and adrenocorticotropic hormone cells in the pituitary gland in female mice. Brain Res. 2004;1013(1):107–116.
- Ozen OA, Yaman M, Sarsilmaz M, et al. Testicular zinc, copper and iron concentrations in male rats exposed to subacute and subchronic formaldehyde gas inhalation. J Trace Elem Med Biol. 2002;16(2):119–122.
- Zararsiz I, Kus I, Ogeturk M, et al. Melatonin prevents formaldehyde-induced neurotoxicity in prefrontal cortex of rats: an immunohistochemical and biochemical study. Cell BiochemFunct Cell Biochem Its Modul Act Agents Dis. 2007;25(4):413–418.
- Pierce JS, Abelmann A, Spicer LJ, et al. Characterization of formaldehyde exposure resulting from the use of four professional hair straightening products. J Occup Environ Hyg. 2011;8(11):686–699.
- Halliday-Bell JA, Gissler M, Jaakkola JJ. Work as a hairdresser and cosmetologist and adverse pregnancy outcomes. Occup Med. 2009;59(3):180–184.
- Baste V, Moen BE, Riise T, et al. Infertility and spontaneous abortion among female hairdressers: the Hordaland Health Study. J Occup Environ Med. 2008;50(12):1371–1377.
- McCall EE, Olshan AF, Daniels JL. Maternal hair dye use and risk of neuroblastoma in offspring. Cancer Causes Control. 2005;16(6):743–748.
- Ronda E, García AM, Sánchez-Paya J, et al. Menstrual disorders and subfertility in Spanish hairdressers. Eur J ObstetGynecol Reprod Biol. 2009;147(1):61–64.
- Harling M, Schablon A, Schedlbauer G, et al. Bladder cancer among hairdressers: a meta-analysis. Occup Environ Med. 2010;67(5):351–358.
- Nohynek GJ, Fautz R, Benech-Kieffer F, et al. Toxicity and human health risk of hair dyes. Food ChemToxicol. 2004;42(4):517–543.
- Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84.
- Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2015;15(1):1–22.
- Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol. 2012;2012:1–14.
- Çalikoglu M, Tamer L, Sucu N, et al. The effects of caffeic acid phenethyl ester on tissue damage in lung after hindlimb ischemia-reperfusion. Pharmacol Res. 2003;48(4):397–403.
- Mirzoeva OK, Calder PC. The effect of propolis and its components on eicosanoid production during the inflammatory response. Prostaglandins LeukotEssent Fatty Acids. 1996;55(6):441–449.
- Koksel O, Ozdulger A, Tamer L, et al. Effects of caffeic acid phenethyl ester on lipopolysaccharide-induced lung injury in rats. PulmPharmacolTher. 2006;19(2):90–95.
- Türkoğlu AÖ, Sarsılmaz M, Çolakoğlu N, et al. Formaldehyde-induced damage in lungs and effects of caffeic acid phenethyl ester: a light microscopic study. Eur J Gen Med. 2008;5(3):152–156.
- Fararh KM, Atoji Y, Shimizu Y, et al. Mechanisms of the hypoglycaemic and immunopotentiating effects of Nigella sativa L. oil in streptozotocin-induced diabetic hamsters. Res Vet Sci. 2004;77(2):123–129.
- Corder C, Benghuzzi H, Tucci M, et al. Delayed apoptosis upon the treatment of Hep-2 cells with black seed. Biomed SciInstrum. 2003;39:365–370.
- Kabuto H, Yokoi I, Ogawa N. Melatonin inhibits iron-induced epileptic discharges in rats by suppressing peroxidation. Epilepsia. 1998;39(3):237–243.
- Cabrera J, Reiter RJ, Tan D-X, et al. Melatonin reduces oxidative neurotoxicity due to quinolinic acid:: in vitro and in vivo findings. Neuropharmacology. 2000;39(3):507–514.
- Skaper SD, Floreani M, Ceccon M, et al. Excitotoxicity, oxidative stress, and the neuroprotective potential of melatonin. Ann N Y Acad Sci. 1999;890(1):107–118.
- Mason RP, Leeds PR, Jacob RF, et al. Inhibition of excessive neuronal apoptosis by the calcium antagonist amlodipine and antioxidants in cerebellar granule cells. J Neurochem. 1999;72(4):1448–1456.
- Mahmood MS, Gilani AH, Khwaja A, et al. The in vitro effect of aqueous extract of Nigella sativa seeds on nitric oxide production. Phytother Res Int J Devoted PharmacolToxicolEval Nat Prod Deriv. 2003;17(8):921–924.
- Longoni B, Salgo MG, Pryor WA, et al. Effects of melatonin on lipid peroxidation induced by oxygen radicals. Life Sci. 1998;62(10):853–859.
- Ulucam E, Bakar E. The effect of proanthocyanidin on formaldehyde-induced toxicity in rat testes. Turk J Med Sci. 2016;46(1):185–193.
- Bakar E, Ulucam E, Cerkezkayabekir A. Investigation of the protective effects of proanthocyanidin and vitamin E against the toxic effect caused by formaldehyde on the liver tissue. Environ Toxicol. 2015;30(12):1406–1415.