ABSTRACT
Objective
To evaluate the current state, therapeutic benefit and safety of urethral injection of autologous stem cells for the treatment stress urinary incontinence (SUI).
Materials and methods
A selective database search of PubMed, the Excerpta Medica dataBASE (EMBASE), Cochrane Library and Google Scholar was conducted to validate the effectiveness of stem cell-based therapy. The search included clinical trials published up until 4 January 2020, written in English, and included cohorts of women and men who had received stem cell-based therapy for SUI. The search used the following keywords in various combinations: ‘stem cell therapy’, ‘cell-based therapy for SUI’, ‘regenerative medicine for SUI’, and ‘tissue engineering’. The success rates were assessed according to cough test, urodynamics, pad tests, and International Consultation on Incontinence Questionnaire-Urinary Incontinence. The primary endpoint was continence rate to measure objectively the effect of the treatment.
Results
We identified four clinical trials using local injections of adipose-derived stem cells (ADSCs), 11 trails with muscle-derived stem cells (MDSCs), and two trails with human umbilical cord blood stem cells (HUCBs) and total nucleated cells (TNCs). The median improvement rate of intrinsic sphincter deficiency after ADSCs, MDSCs, TNCs, HUCBs injections were 88%, 77%, 89%, 36% (improvement rate: 1–2 pads) at a mean (range) follow-up of 6 (1–72) months. The cell sources, methods of cell processing, cell number, and implantation techniques differed considerably between studies. Most of the periurethral injections were at the 3, 5, 7, and 9 o’clock positions and for submucosa were at the 4, 6, and 8 o’clock positions. No significant postoperative complications were reported.
Conclusion
Despite many challenges in stem cell-based therapy for treating SUI, it appears to provide, in both male and female patients, acceptable functional results with minimal side-effects and complications. In the future, more clinical trials should be funded in order to optimise stem cell-based therapy and evaluate long-term outcomes.
Abbreviations
ADSC: adipose-derived stem cell; BMSCs: bone marrow-derived mesenchymal stem cell; CLPP: cough leak-point pressure; FPL: functional profile length; HUCB: human umbilical cord blood stem cell; ICIQ-(QOL)(SF)(UI): International Consultation on Incontinence Questionnaire (Quality of life) (-Urinary incontinence Short Form) (-Urinary Incontinence); IIQ-7: Incontinence Impact Questionnaire-short form; I-QOL: Incontinence quality of life questionnaire; ISD: intrinsic urinary sphincter deficiency; MDSC: muscle-derived stem cell; MUCP: maximum urethral closure pressure; NR: not reported; Pdet-max: maximum detrusor pressure; PVR: post-void residual urine volume; Qmax: maximum urinary flow; QOL: quality of life; RP: radical prostatectomy; TNC: total nucleated cell; (S)UI: (stress) urinary incontinence; UDSCs: urine-derived stem cells; UTUS: upper tract ultrasonography; VLPP: Valsalva leak-point pressure
Introduction
Urinary incontinence (UI) is a widespread chronic disease and a growing problem, with significant negative impact on the quality of life (QOL) of those affected. It is estimated that >200 million people worldwide are affected by UI [Citation1]. According to recent studies, UI is found in 20–36% of the population aged >40 years [Citation2,Citation3]. Due to the demographic change of an increasingly ageing population, an increase in stress UI (SUI) is to be expected in the future [Citation4]. UI can isolate patients from their professional, sexual, but especially from their social environment. This problem leads to economic and financial burdens, which will increase in the coming years as the age structure of the population changes. The successful treatment of UI requires a pathophysiological understanding of the underlying causes, as well as orienting diagnostics with therapeutic consequences.
SUI can be attributed to different causes, with differences in both sexes. In general, mechanical and functional reasons can be considered as causes of SUI. Important factors are myogenic, neurogenic, connective tissue and hormonal changes. In addition, muscle cell density decreases as a result of physiological apoptosis due to a decrease in the muscle cells of the rhabdosphincter, with a total volume of 88% immediately after birth decreasing to ~34% in the 90th year of life [Citation5]. Female SUI often has a multifactorial cause with functional defects of the urinary bladder sphincter and morphological nerve damage. This is in contrast to the almost exclusively postoperative prostate resection or radical prostatectomy (RP)-related UI seen in men. In recent years, placement of transvaginal tension-free transobturator tape and retropubic tension-free vaginal tape have become well-established treatment options. The mid-urethral sling has the advantage of a shorter duration of intervention time. The rate of any re-operation, including mesh removal, was 5.5% (95% Cl 5.4–5.7%) at 5 years and 6.9% (95% Cl 6.7–7.1%) at 9 years [Citation6]. However, the USA Food and Drug Administration (FDA) has repeatedly issued warnings on the use of alloplastic material in the treatment of female UI due to >1000 reported severe adverse events [Citation7]. Consequently, alternative treatments are being sought and although stem cell-based therapy has had numerous setbacks, it may well be a concept for treating these disorders. In the last decade, the use of the patient’s own adult stem cells for lower urinary tract dysfunction has been shown to be a promising, causal therapeutic approach [Citation8].
Stem cell therapy
The concept of regenerative medicine is based on the regeneration of the damaged rhabdosphincter, with improvements in the function of the external (striated) muscles and internal (smooth) muscles, as well as the blood circulation of the sphincter [Citation9–Citation12]. Over the last 60 years, significant progress has been made in the field of stem cell and tissue engineering. Stem cells are unique, as they have the potential to differentiate between different cell types and to form a directed population from a single cell.
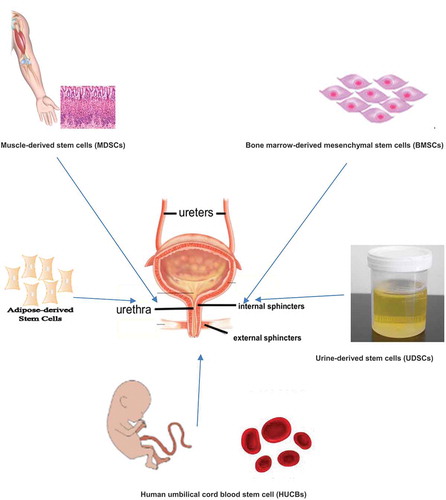
Important roles for stem cells include therapeutic, anti-apoptotic, anti-neoplastic and neovascularisation effects [Citation13]. In contrast to embryonic stem cells, adult stem cells have a lower differentiation capacity and a lower proliferation rate [Citation8]. Due to ethical considerations, the blastocyst stage of a human embryo can already be regarded as a human individual, and thus the isolation of embryonic stem cells is prohibited. The extraction and research with adult stem cells is considered ethically harmless. Adult stem cells can be obtained from various sources including haematopoietic [Citation14], epidermal [Citation15], neuronal [Citation16], and mesenchymal [Citation17] stem cells isolated from bone marrow, muscle tissue, adipose tissue [Citation18] (), and testicular tissue [Citation19]. Adult stem cells from bone marrow, skeletal muscle, mesenchymal and adipose tissue are ideal candidates for regenerative therapy given the low risk of malignant differentiation, the ability of autologous transfer to eliminate the risk of rejection, and the absence of ethical controversy.
Through the implantation of adult stem cells into the damaged rhabdosphincter, the atrophic, damaged musculature is restored to its correct function through the induction of muscle and nerve regeneration. This is a complex process, which involves the re-modelling of the matrix and the restoration of the cells necessary for proper sphincter-complex functioning and continence. This effect is achieved by prior cell differentiation into striated muscle cells or neurones that can replace the injured structures. The aim of the present review was to summarise the clinical trials, effectiveness and safety of stem cell therapy for the treatment of SUI.
Patients and methods
Review criteria
A literature search of PubMed, the National Library of Medicine, the Medical Literature Analysis and Retrieval System Online (MEDLINE), the Excerpta Medica dataBASE (EMBASE), the Cochrane Central Registry of Controlled Trials (CENTRAL), Google Scholar, and ClinicalTrials.gov was undertaken for clinical studies on the treatment of SUI with stem cell therapy. The search included studies published up until 4 January 2020, written in English, and that included cohorts of women and men who had received stem cell-based therapy for UI. The search used the following keywords in various combinations: ‘stem cell therapy’, ‘cell-based therapy for urinary incontinence’, ‘regenerative medicine for urinary incontinence’, and ‘tissue engineering’. In all included studies, the effectiveness of stem cell therapy and continence rate were investigated. The search was limited to clinical studies, fully published articles, systematic reviews, and original papers. Reference lists of articles were also reviewed for relevant articles. The articles were first screened and selected based on their abstracts and then examined in detail. Included articles were selected by consensus of all authors. Two independent researchers reviewed the articles before the final consensus decision was made to include them in the present review.
Data extraction
The following information was extracted from studies that met the inclusion criteria: name of the first author, year of publication, study design, type of UI, type and number of implanted cells, cell sources, subject data, type of intervention, follow-up data, surgical results, and complications.
Statistical analysis and data synthesis
The primary outcome of the review was the percentage of continent, partially continent and incontinent patients after surgery. Furthermore, we recorded type and number of implanted cells, cell sources, patient data, type of intervention, follow-up data, outcomes, and complications. Pertaining to UI, outcome measures were summarised based on preoperative UI status, as we considered this the most important factor in postoperative assessment. The treatment outcome was categorised as: ‘healing’, ‘improvement’ or ‘failure’. The ‘objective healing’ was defined as negative cough stress and/or pad testing. ‘Objective improvement’ was defined as the reduction of pads (≥50%). However, if the study did not report the mean and standard deviation (SD), median and sample size were used to estimate the mean and variance. All analyses were performed using the Statistical Analysis Software Comprehensive version 2.0 (Biostat Inc., Englewood, NJ, USA).
Results of the review
Study selection, study characteristics and outcomes
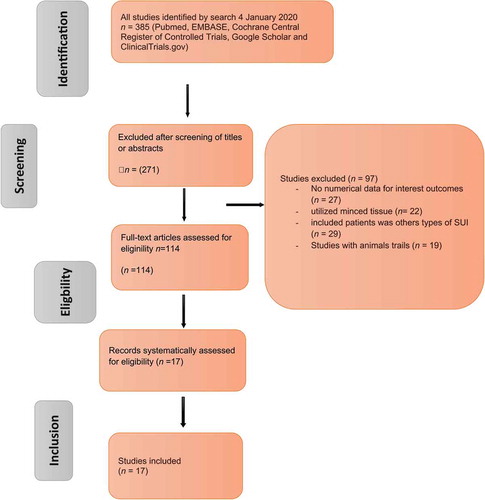
The search strategy and the results of the present literature review are shown in . After de-duplication, 114 articles were screened for further analysis and 17 studies identified (– [Citation20,Citation21,Citation22,Citation23,Citation24,Citation25,Citation26,Citation27,Citation28,Citation29,Citation30,Citation31,Citation32,Citation33,Citation34,Citation35,Citation37,Citation32]), which included cohorts of patients undergoing injection of stem cells. In particular, the studies that adhered to the Population, Intervention, Control, Outcome (PICO) quality assessment criteria were included in this analysis. Of the 114 relevant articles, 17 clinical trials (715 patients) met the criteria for inclusion in this analysis (, –). The evaluated study designs were prospective analyses of cohorts of patients who underwent injection of stem cells into the urinary sphincter. The number of operating surgeons was not quantified in the included studies. The primary endpoint for the included studies at the last follow-up was continence, categorised as: ‘complete continence’ (no leaking, no pads used), ‘social continence’ (1–2 pads/day), ‘improved UI’ (>50% decrease in number of pads used), or ‘failure’ (<50% improvement, persistent or increased leaking). The secondary endpoint was any complication. The outcomes of included studies were based on urodynamic studies.
Table 1. Clinical studies using ADSCs for SUI.
Table 2. Clinical studies using MDSCs for SUI.
Table 3. Clinical studies using other types of stem cells, HUCBs and TNCs.
Excluded studies
Initially, our search yielded 385 publications. After removal of the duplicates, 271 articles remained and were screened using their title and abstract; leaving 114 articles selected for full-text review. Another 29 studies were excluded from the review because an endpoint (SUI) was not defined and mentioned in the context (n = 29) or they utilised minced tissue (n = 22). While, 27 studies were excluded because the results were not quantitative outcomes. Another 19 studies were excluded due to being trails in animals. The flow of studies through the selection process is presented in .
Adipose-derived stem cells (ADSCs) implantation
ADSCs are one of the most commonly used stem cell types in autoplastic transplantations. Using ADSCs is advantageous due to the easy recovery of adipose tissue. This has led to large quantities being recovered safely with minimal morbidity [Citation38]. Several studies have shown that ADSCs are multi-potent cells and can differentiate into adipogenic, chondrogenic, myogenic and osteogenic cells [Citation38,Citation39]. All this makes adipose tissue an attractive source for stem cell therapy. Adipose tissue has a high content of ADSCs, with ~15 million ADSCs/g of adipose tissue. In addition, ADSCs have the ability to proliferate rapidly, even at low serum levels. Rodriguez et al. [Citation40] showed that ADSCs can be obtained from processed lipoaspirates and can differentiate into functional urothelial and smooth muscle cells, which can be contracted with cholinergic stimulation. Adipose tissue can therefore be an important cell source for the treatment of injured tissues in which smooth muscle plays an important role. ADSCs therapy is not only used in female UI, but also plays an important role in the treatment and restoration of male UI. Gotoh et al [Citation20], Yamamoto et al [Citation41] and Choi et al. [Citation21] provide evidence that periurethral injection of autologous ADSCs is a safe and technically feasible treatment for men with moderate SUI after RP and holmium laser enucleation of the prostate. In total, we found five clinical trials (40 patients) using adult ADSCs (three in men and two in women). The largest patient scope was the Gotoh et al. [Citation20] study with 13 male patients, showing that leakage volume (in frequency and amount of UI) decreased by 59.8% (from 260.7 to 152.7 g) and improved QOL. They showed that the mean maximum urethral closure pressure (MUCP) increased by 9 cmH2O from baseline, the functional profile length (FPL) increased by 6 mm, and the post-void residual urine volume (PVR) decreased after treatment from 52 to 4.5 mL. In addition, they reported an increase in urethral length of 2.2 mm on MRI after injection (). Choi et al. [Citation21] used ADSCs to treat persistent UI after RP in six men. They showed a decrease in the amount of UI and a significant increase in MUCP by 19.5 cmH2O from baseline. The Arjmand et al. [Citation22] study of 10 female patients showed significant short-term improvement in UI after ADSCs injection [2 weeks after injection (P < 0.001) and after 24 weeks (P = 0.018)]. The interpretation and direct comparison of studies was difficult due to variability in surgical techniques and the number of injected cells. The median postoperative improvement rate was 88% after ADSCs injection (improvement rate: 1–2 pads).
It is worth noting that the overall data situation is not sufficient for assessing the long-term safety of the results. The published studies have small populations and short-term results. Further scientific studies with a longer follow-up period and more patients are required. However, the available results indicate a regenerative potential of ADSCs for the treatment of SUI.
Muscle-derived stem cells (MDSCs) implantation
Despite technical difficulties in the past, MDSC-based therapy has proven to be a promising option for patients with UI in recent years, with the cultivation of these cells and the selection of stem cells [Citation42]. As such, MDSC-based therapy is an important and easily accessible source of adult stem cells for the treatment of UI. The procurement of muscle tissue is associated with minimal morbidity by the easy sampling of muscle biopsy under local anaesthesia [Citation43]. However, the isolated autologous MDSCs must first be expanded in vitro and cultured for a short time before injection into the urethral sphincter [Citation42,Citation43,Citation44]. Deasy et al. [Citation45] and Wu et al. [Citation46] have shown that MDSCs have great potential for tissue regeneration through their proliferation and self-renewal. MDSC-injection therapy offers the advantage that the cells injected into the rhabdosphincter form so-called myotubes and myofibres, which are not only regarded as blocking agents, but also are physiologically capable of improving the function of the sphincters [Citation47]. In all the clinical studies published on UI, autologous MDSCs were injected directly into the rhabdosphincter either transurethrally or periurethrally.
Overall, we identified 11 clinical trials (637 patients) using MDSCs; nine included only female patients (n = 352) and two studies included male patients (n = 285) (). The first clinical study of MDSCs (42 women and 21 men) with autologous myoblasts and fibroblasts was conducted by Strasser et al. [Citation48] between 2002 and 2004. The fibroblasts were injected into the submucosa of the urethra, whereas the myoblasts were implanted in the rhabdosphincter. However, it must be mentioned that this clinical study of the Austrian research group was later withdrawn due to scientific irregularities, violations of ethical guidelines, and protocol irregularities. The Austrian research group reported an improvement in QOL and contractility of the urethral sphincter, with a success rate of >90% for women and >50% for men.
The subsequent study by Carr et al. [Citation49] in 2008 was a follow-up at 1 year in eight female patients. Each patient received MDSC-based therapy under local anaesthesia. Five of the eight women showed a realistic improvement in symptoms and one achieved complete continence. The same research group published another study in 2013 with 38 female patients who had been treated with a low MDSCs dose (1, 2, 4, 8 or 16 × 106) and a high MDSCs dose (32, 64 or 128 × 106) [Citation23]. The autologous MDSCs were injected ultrasonically into the sphincter muscle. A total of 32 patients (20 with a low-cell dose and 12 with a high-cell dose) had a significant reduction in the number of wet pads (pad test) at the 3-month follow-up. It should be mentioned that the results in the high-dose group were better than those in the patients treated with a low dose (88.9% vs 61.5%, 77.8% vs 53.3%). In a similar population, the Mitterberger et al. [Citation24] study of 123 female patients, with an average follow-up of 62.8 months, provided evidence of a significant improvement in SUI after injections of myoblasts and fibroblasts in the middle of the urethra. At the 1-year follow-up after implantation of the cells, 94 women (79%) were continent and 16 women (13%) had a significant improvement.
A clinical study by Stangel-Wojcikiewicz et al. [Citation25] in 2014 included 16 female patients and showed an improvement in UI in four patients (25%), at a follow-up of 12 months based on clinical parameters (Gaudenz questionnaire) and urodynamic parameters [cough leak detection pressure (CLPP); Valsalva leak-point pressure (VLPP)]. Eight of the 16 patients (50%) were continent after 8 months (). In 2016, Sharifiaghdas et al. [Citation26] published their prospective, small cohort study of 10 female patients receiving autologous MDSC therapy for severe SUI. At a follow-up of 36 months, three patients showed full continence based on the cough stress test, 1-h pad test and UI impact questionnaire. Four had significant improvement in UI, and three patients did not respond to the treatment.
In one of the largest clinical trials done, Gerullis et al. [Citation27] obtained four muscles biopsies in 222 patients; 192 with UI following RP, nine after TURP, and 21 after radical cystoprostatectomy with neobladder. The autologous MDSCs were expanded in vitro after successful cultivation and directly injected into the sphincter under endoscopic view in a cell dose of 5.2 × 106. At a follow-up of 6–12 months, only 26 patients (12%) were continent, 94 patients (42%) had improved, and 102 patients (46%) reported persistent UI.
Mitterberger et al. [Citation28] and his team used a mixture of autologous myoblasts and fibroblasts from muscle biopsies in patients with UI after RP. The fibroblasts were suspended in small amounts of collagen as carrier material and injected into the urethral submucosa, while the myoblasts were implanted into the rhabdosphincter. The injection was ultrasonically controlled with a specially developed injection device and injected into the rhabdosphincter (15–18 doses, 50–100 μL/dose) at the 5 and 7 o’clock positions in two different stages. Then, 25–30 doses of fibroblasts/collagen were injected circumferentially into the submucosa. At the 1-year follow-up, 41 men (65%) were completely continent, while 17 (27%) had a significant improvement ().
The interpretation and direct comparison between clinical trails with MDSCs was difficult due to variability in surgical techniques and the number of injected cells. The median postoperative improvement rate was 77% after MDSCs injection (improvement rate: 1–2 pads).
The studies published using MDSC-based therapy show potential for improving sphincter function by re-modelling damaged sphincter muscle and bulking the urethra with new tissue [Citation29,Citation50]. However, MDSC-injection therapy does have certain disadvantages including migration and absorption of cells, as is the case with other commercial injectable agents. It will require the development of new strategies, such as tissue engineering, to improve the retention and survival of transplanted stem cells.
Other types of stem cells: bone marrow-derived mesenchymal stem cell (BMSCs), urine-derived stem cells (UDSCs), human umbilical cord blood stem cell (HUCBs), and total nucleated cells (TNCs)
Bone marrow contains heterogeneous cell populations, including erythrocytes, macrophages, endothelial cells, fibroblasts, adipocytes, and haematopoietic/mesenchymal stem cells [Citation51]. BMSCs develop in the bone marrow and can renew themselves and differentiate into different cell types. The first BMSCs were described as osteogenic progenitor cells in 1966. Later, the BMSCs were used in tissue engineering. The procurement of BMSCs can be achieved with sufficient density for stem cell-based therapy. Disadvantageous for the use of BMSCs in clinical use is the painful bone aspiration under local anaesthesia, thus requiring general anaesthesia for the procedure. The isolated BMSCs must also be expanded in vitro and cultivated for a short time prior to the final injection. To our knowledge, no BMSCs have been used in clinical urology to date. Some animal model studies have shown that the injected BMSCs were able to restore damaged urethral sphincter tissue and significantly improve UI [Citation52]. However, the results of other animal studies were very contradictory to these findings.
UDSCs can easily be recovered from human urine and expanded in vitro. The advantage of using UDSCs as a cell source for treatment lies in the easy removal without invasive measures. This represents a considerable financial advantage compared to extraction of other cell types by means of biopsy. To the best of our knowledge, few experimental studies using UDSCs for the treatment of UI currently exist. Zhang et al. [Citation53] collected 55 urine samples from 15 healthy individuals and eight patients with VUR. The UDSCs were then successfully isolated and expanded. The authors showed that the individual clones of UDSCs have the ability to differentiate into cell lines that express urothelium, smooth muscle, endothelial and interstitial cell markers [Citation50,Citation53].
Human umbilical cord blood stem cells (HUCBs) can be extracted from human umbilical cord blood. HUCBs are not considered embryonic cells, which is a great advantage compared to other cell sources. Stem cells from elderly people have a low differentiation potential compared to stem cells from young people [Citation54]. Another benefit of using stem cells from umbilical cord blood is the ease of harvesting it without invasive measures. The Lee et al. [Citation30] clinical study is the only one that used HUCBs in 39 women with UI after failure of conservative and surgical therapy. The umbilical cord vein was punctured after normal delivery and human umbilical cord blood was then collected in an aseptic collection bag containing 23 mL citrate-phosphate-dextrose-adenine (CPDA-1) and stored at – 70°C.
Lee et al. [Citation30] injected HUCBs under local anaesthesia into the submucosal area of the proximal urethra at the 4 and 8 o’clock positions (430 ± 190 × 106 cells/2 mL). At the 12-month follow-up, 13 patients (36%) were completely satisfied and 13 patients (36%) had significant improvement of their UI (). However, 10 patients (27%) in this study remained incontinent. Urodynamic examination at 3 months in 10 of the patients showed that the MUCP almost doubled after cell injection.
Multipotent endothelial cells (EPCs) can be taken from peripheral blood to produce autologous TNCs and used for the treatment of UI. The role of growth factors and the effects of platelet-rich plasma have been investigated with in vitro and in vivo studies. This effect has been associated with several growth factors produced by platelets (platelet-derived growth factor, transforming growth factor β1, fibroblast growth factors, vascular endothelial growth factors, etc.) [Citation54]. We found a single clinical pilot study using platelet-mixed TNCs for the treatment of severe female UI [Citation31]. In that study, 120 mL of peripheral blood was drawn for the separation of platelets and nucleated cells. After centrifugation, the total volume of the platelets and TNCs was equal. A mixture of 10 mL was then injected into the periurethral tissue. Eight of nine patients had a clinically significant improvement according to the International Consultation on Incontinence Questionnaire-Urinary Incontinence (ICIQ-UI) and ICIQ-QOL at the 3- and 6-month follow-ups (). One patient had an improvement in UI, but was not cured.
Challenges with stem cells in the treatment of SUI
Although the clinical studies on stem cell therapy for the treatment of SUI show encouraging results with great potential, these short-term results must be interpreted with caution. The results of various clinical trials are controversial. One challenge is the lifespan of stem cells. The reabsorption of autologous fat is rapid. Cell membrane damage is caused by suction and only 10–30% of the adipose cells can be detected 6 months after application. Myeloblasts, theoretically, have the potential to develop functional muscular structures. However, at present it can only be speculated whether the myeloblasts can actually form new fibres or contribute to the contractility of an atrophic sphincter. It is also unclear how the necessary nerve supply of the ‘neosphincter cells’ takes place. The very high success rates in most published clinical studies with very small numbers of patients require critical consideration. After an initial 90%, only 70% success rates are published with filling materials after appropriate selection. Although the filling volume is only ~3–4 mL of substance, an additional bulking effect must be assumed, and thus as persistent obstruction effect could result. The lifespan of implanted stem cells is relatively short. In addition, cell death due to ischaemia, inflammation, or apoptosis may occur within the first week [Citation55]. The size and function of the biotechnological muscle decreases with increasing age [Citation56]. This certainly plays a further role in the lifespan of the stem cells. Furthermore, one of the major challenges for stem cell treatment is not only the selection of stem cells with great differentiation potential, but also optimisation of the technical processes. Some studies were even withdrawn after publication due to ethical and regulatory concerns [Citation32,Citation48].
As shown in , most clinical studies have been conducted with autologous MDSCs. Treatment with autologous MDSCs requires an optimal lengthy isolation/cultivation work process prior to injection. In all published UI clinical studies, autologous MDSCs were injected either transurethrally or periurethrally directly into the rhabdosphincter, but the number of transplanted cells varied greatly. The different cell doses used are due to the lack of a clear understanding of stem cell-based therapy. However, it is indisputable that the concept of regenerative medicine leads to regeneration of the damaged rhabdosphincter with improvement in function of the external (striated muscle) and internal (smooth muscle) sphincters, as well as the blood circulation of the urethral sphincter.
Both differentiated cell and mesenchymal stem cell therapies have become attractive instruments to improve biocompatibility, tissue integration and minimise adverse inflammatory responses. However, the available studies are very heterogeneous, making it difficult to make comparisons between cell types or cell coating processes. In addition, only a few studies have been conducted in clinically relevant animal models, leading to contradictory results. Finally, a comprehensive understanding of the biological mechanisms of MDSCs associated with the foreign body reaction is required.
Conclusion
Despite many challenges stem cell-based therapy for treating SUI appears to provide, in both male and female patients, acceptable functional results with minimal side-effects and complications.
In summary, future studies should focus on the following points:
(1) The standardised selection of stem cells.
(2) Compliance with regulatory guidelines for clinical trials.
(3) Establishment of a long-term, clinically relevant animal model with long-term outcomes.
(4) A feasibility study in a multicentre clinical trial with more patients and longer follow-ups.
Author contributions
Barakat Bara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Manuscript writing/editing
Protocol/project development: Barakat Bara.
Data collection or management: Barakat Bara, Knut Franke
Data analysis: Barakat Bara, Knut Franke
Drafting of the manuscript: Barakat Bara, Sameh Hijazi
Critical revision of the manuscript: Sameh Hijazi, Samer Schkaki, Hasselhof Viktoria. Thomas-Alexander Vögeli
Administrative, technical, or material support: Barakat Bara.
Supervision: Knut Franke, Thomas-Alexander Vögeli
Compliance with ethical standards
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Research Ethics Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Financial disclosures
I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g., employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Informed consent statement
Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Disclosure statement
The authors declare that they have no relevant conflict of interest.
References
- Corcos J, Beaulieu S, Donovan J, et al. Symptom quality of life assessment committee of the first international consultation on incontinence, quality of life assessment in men and women with urinary incontinence. J Urol. 2002;168:896–905.
- Hunskaar S, Arnold EP, Burgio K, et al. Epidemiology and natural history of urinary incontinence. In: Abrams P, Khoury S, Wein Aeditors. Incontinence. Plymouth: Plymbridge Distributors Ltd; 1999. p. 197–226.
- Brocklehurst JC. Urinary incontinence in the community: analysis of a MORI poll. Br Med J. 1993;306:832–834.
- Hannestad YS, Rortveit G, Sandvik H, et al. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT Study. J Clin Epidemiol. 2000;53:1150–1157.
- Strasser H, Tiefenthaler M, Steinlechner M, et al. Urinary incontinence in the elderly and age-dependent apoptosis of rhabdosphincter cells. Lancet. 1999;354:918–919.
- Ashok K, Wang A. Recurrent urinary stress incontinence: an overview. J Obstet Gynaecol Res. 2010;36:467–473.
- Food and Drug Administration (FDA). FDA Public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. 2008 [cited 2020 Feb]. Available from: http://www.fda.gov/cdrh/safety/102008- surgicalmesh.html
- Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49.
- Furuta A, Jankowski R, Honda M, et al. State of the art of where we are at using stem cells for stress urinary incontinence. Neurourol Urodyn. 2007;26:966–971.
- Wang H, Chuang Y, Chancellor M. Development of cellular therapy for the treatment of stress urinary incontinence. Int Urogynecol J. 2011;22:1075–1083.
- Goldman H, Sievert K, Damaser M. Will we ever use stem cells for the treatment of SUI?—ICI-RS 2011. Neurourol Urodyn. 2012;31:386–389.
- Vaegler M, Lenis A, Daum L, et al. Stem cell therapy for voiding and erectile dysfunction. Nat Rev Urol. 2012;9:435–447.
- Gnecchi M, Zhang Z, Ni A, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219.
- Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287:1442–1446.
- Watt FM. Epidermal stem cells: markers, patterning and the control of stem cell fate. Philos Trans R Soc Lond B Biol Sci. 1998;353:831–837.
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438.
- Caplan AI. The mesengenic process. Clin Plast Surg. 1994;21:429–435.
- Park SR, Oreffo RO, Triffitt JT. Interconversion potential of cloned human marrow adipocytes in vitro. Bone. 1999;24:549–554.
- Guan K. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199–1203.
- Gotoh M, Yamamoto T, Kato M, et al. Regenerative treatment of male stress urinary incontinence by periurethral injection of autologous adipose-derived regenerative cells: 1-year outcomes in 11 patients. Int J Urol. 2014;21:294–300.
- Choi JY, Kim TH, Yang JD, et al. Adipose-derived regenerative cell injection therapy for postprostatectomy incontinence: a Phase I clinical study. Yonsei Med J. 2016;57:1152–1158.
- Arjmand B, Safavi M, Heidari R, et al. Concomitant transurethral and transvaginal-periurethral injection of autologous adipose derived stem cells for treatment of female stress urinary incontinence: a Phase One clinical trial. Acta Med Iran. 2017;55:368–374.
- Carr LK, Robert M, Kultgen PL, et al. Autologous muscle derived cell therapy for stress urinary incontinence: a prospective, dose ranging study. J Urol. 2013;189:595–601.
- Mitterberger M, Marksteiner R, Margreiter E, et al. Autologous myoblasts and fibroblasts for female stress incontinence: A 1-year follow-up in 123 patients. BJU Int. 2007;100:1081–1085.
- Stangel-Wojcikiewicz K, Jarocha D, Piwowar M, et al. Autologous muscle-derived cells for the treatment of female stress urinary incontinence: a 2-year follow-up of a Polish investigation. Neurourol Urodyn. 2014;33:324–330.
- Sharifiaghdas F, Tajalli F, Taheri M, et al. Effect of autologous muscle-derived cells in the treatment of urinary incontinence in female patients with intrinsic sphincter deficiency and epispadias: A prospective study. Int J Urol. 2016;23:581–586.
- Gerullis H, Eimer C, Georgas E, et al. Muscle-derived cells for treatment of iatrogenic sphincter damage and urinary incontinence in men. ScientificWorldJournal. 2012;2012:898535.
- Mitterberger M, Marksteiner R, Margreiter E, et al. Myoblast and fibroblast therapy for post-prostatectomy urinary incontinence: 1-year followup of 63 patients. J Urol. 2008;179:226–231.
- Cannon TW, Lee JY, Somogyi G, et al. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003;62:958–963.
- Lee CN, Jang JB, Kim JY, et al. Human cord blood stem cell therapy for treatment of stress urinary incontinence. J Korean Med Sci. 2010;25:813–816.
- Shirvan MK, Alamdari DH, Mahboub MD, et al. A novel cell therapy for stress urinary incontinence, short-term outcome. Neurourol Urodyn. 2013;32:377–382.
- Cornu JN, Lizee D, Pinset C, et al. Long-term follow-up after regenerative therapy of the urethral sphincter for female stress urinary incontinence. Eur Urol. 2014;65:256–258.
- Sebe P, Doucet C, Cornu JN, et al. Intrasphincteric injections of autologous muscular cells in women with refractory stress urinary incontinence: a prospective study. Int Urogynecol J. 2011;22:183–189.
- Gräs S, Klarskov N, Lose G. Intraurethral injection of autologous minced skeletal muscle: a simple surgical treatment for stress urinary incontinence. J Urol. 2014;192:850–855.
- Peters KM, Dmochowski RR, Carr LK, et al. Autologous muscle derived cells for treatment of stress urinary incontinence in women. J Urol. 2014;192:469–476.
- Kuismanen K, Sartoneva R, Haimi S, et al. Autologous Adipose Stem Cells in Treatment of Female Stress Urinary Incontinence: results of a Pilot Study. Stem Cells Transl Med. 2014;3:936–941.
- Blaganje M, Lukanović A. Intrasphincteric autologous myoblast injections with electrical stimulation for stress urinary incontinence. Int J Gynaecol Obstet. 2012;117:164–167.
- Roche R, Festy F, Fritel X. Stem cells for stress urinary incontinence: the adipose promise. J Cell Mol Med. 2010;14:135–142.
- Nikolavsky D, Chancellor MB. Stem cell therapy for stress urinary incontinence. Neurourol Urodyn. 2010;29:S36–S41.
- Rodriguez LV, Alfonso Z, Zhang R, et al. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci USA. 2006;103:12167–12172.
- Yamamoto T, Gotoh M, Hattori R, et al. Periurethral injection of autologous adipose-derived stem cells for the treatment of stress urinary incontinence in patients undergoing radical prostatectomy: report of two initial cases. Int J Urol. 2010;17:75–82.
- Li Y, Pan H, Huard J. Isolating stem cells from soft musculoskeletal tissues. J Vis Exp. 2011;2010(41):pii.
- Usas A, Huard J. Muscle-derived stem cells for tissue engineering and regenerative therapy. Biomaterials. 2007;36:5401–5406.
- Zhou S, Zhang K, Atala A, et al. Stem cell therapy for treatment of stress urinary incontinence: the current status and challenges. Stem Cells Int. 2016;2016:7060975.
- Deasy BM, Jankowski RJ, Huard J. Muscle-derived stem cells: characterization and potential for cell-mediated therapy. Blood Cell Mol Dis. 2001;27:924–933.
- Wu XY, Wang SL, Chen BL, et al. Muscle-derived stem cells: isolation, characterization, differentiation, and application in cell and gene therapy. Cell Tissue Res. 2010;340:549–667.
- Chancellor MB, Yokoyama T, Tirney S. Preliminary results of myoblast injection into the urethra and bladder wall: a possible method for the treatment of stress urinary incontinence and impaired detrusor contractility. Neurourol Urodyn. 2000;19:279–287.
- Strasser H, Marksteiner R, Margreiter E, et al. Transurethral ultrasonography-guided injection of adult autologous stem cells versus transurethral endoscopic injection of collagen in treatment of urinary incontinence. World J Urol. 2007;25:385–392.
- Carr LK, Steele D, Steele S, et al. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:881–883.
- Liu Z, Wu Y, Chen BG. Myoblast therapy: from bench to bedside. Cell Transplant. 2006;15:455–462.
- Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596.
- Gunetti M, Tomasi S, Giammò A, et al. Myogenic potential of whole bone marrow mesenchymal stem cells in vitro and in vivo for usage in urinary incontinence. PLoS One. 2012;7:e45538.
- Zhang Y, Mcneill E, Tian H, et al. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008;180:2226–2233.
- Roobrouck VD, Ulloa-Montoya F, Verfaillie CM. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008;314:1937–1944.
- Qin D, Long T, Deng J, et al. Urine-derived stem cells for potential use in bladder repair. Stem Cell Res Ther. 2014;5:69.
- Delo DM, Eberli D, Williams JK, et al. Angiogenic gene modification of skeletal muscle cells to compensate for ageing-induced decline in bioengineered functional muscle tissue. BJU Int. 2008;102:878–884.