ABSTRACT
Objective: To evaluate the current literature on the accuracy of fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography (FDG PET-CT) for lymph node (LN) staging in urothelial carcinoma (UC), as robust evidence on the value of this technology in UC is still lacking.
Methods: The Medical Literature Analysis and Retrieval System Online (MEDLINE)/PubMed, Cochrane Library, and Scopus databases were searched for eligible studies. We included all original studies evaluating FDG PET-CT in bladder or upper tract UC. The search results were restricted to the English language, and included prospective and retrospective studies without time restriction. We included only studies reporting the sensitivity and specificity of FDG PET-CT in detecting UC LN metastases.
Results: We identified 23 articles meeting our inclusion criteria. In the preoperative setting, the sensitivity of FDG PET-CT for detecting LN metastases in patients with bladder cancer was widely variable ranging from 23% to 89%; the specificity ranged from 81% to 100%; and the overall accuracy ranged from 65% to 89%. During bladder cancer monitoring the sensitivity for detecting LN metastases ranged from 75% to 92% and the specificity ranged from 60% to 92%. The sensitivity for LN staging in upper tract UC ranged between 82% and 95%, with a specificity of 84–91%.
Conclusion: Despite the inconsistencies in sensitivity between the reports, FDG PET-CT seems to have a high specificity for LN staging in patients with UC. Future prospective, well-designed studies are necessary to evaluate the role of FDG PET-CT in UC management.
Abbreviations: FDG: fluoro-2-deoxy-D-glucose; LN: lymph node; PET: positron emission tomography; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analyses; PSMA: prostate-specific membrane antigen; (N)(P)PV: (negative) (positive) predictive value; QUADAS-2: Quality Assessment of Diagnostic Accuracy Studies-2; SUVmax: maximum standard uptake value; (UT)UC: (upper urinary tract) urothelial carcinoma
Introduction
Urothelial carcinoma (UC) arises from the urothelium of the urinary tract potentially affecting the urinary bladder in 90–95% of cases and, less commonly, the upper urinary tract in 5–10% (i.e. ureters and pyelocalyceal system) [Citation1–3]. In the United States, bladder UC alone accounts for 81 400 cases per year, with 17 980 deaths, while ureteric UC accounted for 3970 cases and 1010 deaths per year [Citation4]. While diagnosis with conventional techniques such as cytology, endoscopy, biopsy, and histopathological evaluation of these tumours is established, accurate UC staging is still challenging [Citation5–8]. Various imaging techniques have been proposed for accurate staging of UC in order to improve patient counselling and treatment planning [Citation9,Citation10]. Conventional imaging techniques, e.g. CT urography and magnetic resonance urography, have poor performance to impact clinical decision-making regarding lymph node (LN) status [Citation1,Citation2]. The overall accuracy of the CT scan was estimated to be between 56% and 90%, with an under- and over-staging rate of 39% and 6%, respectively [Citation11,Citation12]. For MRI, the reported sensitivity for the staging of UC of the bladder in terms of extravesical extension was 60% [Citation13]. Such performance is considered inadequate for proper treatment planning, given the sensitive and varying treatment algorithms.
In the last decade, positron emission tomography (PET) with the glucose analogue fluoro-2-deoxy-D-glucose (FDG) has been used to improve the staging and monitoring of patients with different malignancies, e.g. lung, breast, colorectal, as well as UC [Citation14–16]. PET measures the metabolic activity of the target cells, but suffers from an inaccuracy to capture anatomical details. Thus, a combination of PET and other imaging techniques, e.g. CT, can overcome the limitations of both technologies [Citation17]. Metastatic burden plays an essential role in choosing the appropriate treatment, especially in the era of metastases-targeted therapies and salvage surgery [Citation18]. FDG PET-CT has, indeed, been recently recommended as a non-invasive method for the diagnosis and staging of UCs [Citation19]. However, robust evidence on the accuracy of this technology is still lacking. In the present review, we aimed to summarise the current evidence regarding the accuracy of FDG PET-CT for LN staging and monitoring of bladder and upper tract (UT) UC.
Methods
This systematic review aimed to evaluate the diagnostic accuracy of the FDG PET-CT technique in the staging and monitoring of UC. We performed this review according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [Citation20]. A comprehensive systematic literature search was independently performed by two authors. The search results were restricted to the English language and without time restriction. We excluded case series, case reports, and studies reporting on <10 patients. The participants in the included studies were adult patients with UC of the bladder or UTUC who were clinically followed to obtain a diagnosis, which was considered as the confirmation for FDG PET-CT accuracy in LN detection. Moreover, we included studies that reported the sensitivity and specificity of FDG PET-CT in detecting UC metastases. The outcomes of interest were diagnostic performance, particularly sensitivity, specificity, positive (PPV) and negative predictive value (NPV), and overall accuracy.
Data sources
Electronic databases were searched, including the Medical Literature Analysis and Retrieval System Online (MEDLINE)/PubMed, and Scopus. The databases of systematic reviews, e.g. the Cochrane libraries and Center for Reviews and Dissemination, were screened for eligible primary studies in April 2020, and saved for further screening and in-depth reading. The following keywords were used: ‘transitional cell carcinoma’, ‘urothelial cancer’, ‘urologic neoplasms’, ‘upper tract’, ‘upper tract urothelial cancer’, ‘bladder cancer’, OR ‘urinary tract cancer’, ‘urothelial cancer’, ‘FDG’ OR ‘Fluorodeoxyglucose’, ‘FDG-PET’, ‘PET-CT’.
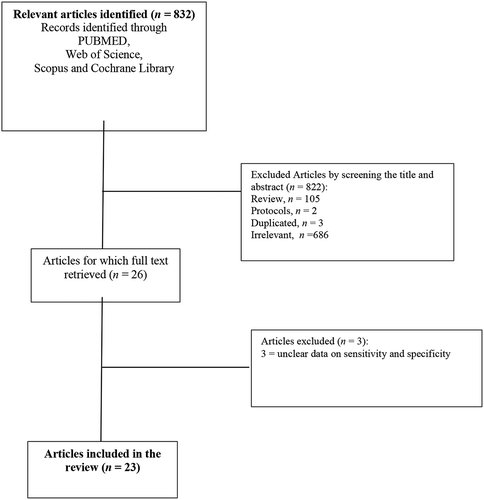
The flow of the information through the different stages of a systematic review (primary screening, secondary screening, and inclusion stage) is shown in the PRISMA flow chart ().
Data collection and analysis
Data from each study were extracted by two independent reviewers. Data were collected from the included studies into data extraction tables, which were designed for this review and contained: author name, year of publication, study design, sample size, characteristics of the imaging technique, performance measures (sensitivity, specificity, PPV and NPV, overall accuracy), and any relevant comment of the included study.
Risk of bias assessment
The risk of bias was evaluated according to the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool. This tool is based on four domains: patient selection, index test, reference standard, and the timing of reference test. Also, the QUADAS-2 tool assessed applicability concerns of (patient selection, index test, and reference standard) ( [Citation10,Citation21–41]).
Table 1. Quality appraisal for included studies using the QUADAS-2 tool. Green = low, i.e. risk of bias low or concerns about applicability low. Red = high or uncertain, i.e. risk of bias high/uncertain or concerns about applicability high/uncertain
Results
The initial search resulted in 822 articles, from which 23 articles met our inclusion criteria: 20 articles evaluated UC of the bladder, whereas three addressed UTUC [Citation10,Citation21,Citation22]. Regarding the main outcome of the included studies, different measures were used to estimate the diagnostic value of the FDG PET-CT technique in the diagnosis or staging of UC. Sensitivity and specificity were reported in all included studies. The summary of the risk of bias and applicability concerns; overall, the quality of the studies was deemed satisfactory.
Role of FDG PET-CT in UC of the bladder
Between 2009 and 2014, several studies reported favourable results of FDG PET-CT for LN detection in patients with bladder UC. Kibel et al. [Citation23] and Apolo et al. [Citation24] evaluated LN detection of FDG PET-CT and showed high sensitivity of (70% and 92%, respectively) and specificity of (94% and 81%, respectively). Likewise, higher sensitivity for FDG PET-CT (57%) as compared with CT alone (33%) for detecting LN metastases was reported by Lodde et al. [Citation25]. Rouanne et al. [Citation26] evaluated 102 patients prospectively and revealed moderate sensitivity and high specificity for detecting LN using FDG PET-CT (50% and 97%, respectively). A similar conclusion was reached by Hitier‐Berthault et al. [Citation27], who prospectively compared FDG PET-CT and CT alone in 52 patients, and reported a higher sensitivity of FDG PET-CT for LN detection (36% vs 9%). A similar pattern of favourable results was obtained by Girard et al. [Citation28] in that FDG PET-CT had significant diagnostic accuracy compared to CT alone, which was 84%, as well as sensitivity and specificity that were 29% and 97%, respectively.
However, several studies have reported less promising results. Drieskens et al. [Citation29] found a comparable sensitivity for FDG PET-CT and CT alone in detecting LN metastasis (50% and 42%, respectively). Swinnen et al. [Citation30] observed similar results, a sensitivity of 46% for both FDG PET-CT and CT alone, as well as no considerable benefit of specificity for detecting LN metastasis (97% and 92%, respectively). In addition, a large study that included 233 patients [Citation31], showed 69% sensitivity of FDG PET-CT as compared with 41% for CT alone, which was not significant enough to justify the additional cost according to the authors’ opinion. Aljabery et al. [Citation32], also showed no advantage of PET-CT in terms of sensitivity and specificity (41% and 86%, respectively) as compared to CT alone (41% and 89%, respectively). Along with the previous study, Pichler et al. [Citation33] found comparable sensitivity and specificity results for FDG PET-CT (64% and 86%, respectively) and CT alone (46% and 92%, respectively). Moreover, in 2020, Dason et al. [Citation34] retrospectively evaluated 208 patients and found very poor sensitivity of FDG PET-CT for detecting LN metastases, which was between 7% and 23%, even though they found high specificity from 89% to 99%.
Vind-Kezunovic et al. [Citation35] evaluated the maximum standard uptake value (SUVmax) on FDG-PET scans in 131 patients with bladder cancer. The SUVmax >2 FDG-PET scans had a sensitivity and specificity of 79% and 66%, respectively. In contrast, a SUVmax of >4 FDG-PET had a sensitivity and specificity of 61% and 84%, respectively, which may help in identifying patients with a poor prognosis ( [Citation23–35,Citation39-41]).
Table 2. 18F-FDG PET-CT in LN staging in patients with UC
Role of FDG-PET in monitoring
Jadvar et al. [Citation36] retrospectively studied 35 patients and found that the sensitivity of PET-CT was double that of CT alone in detecting metastatic LN and changed the management of 17% of patients. Overall, these findings are consistent with the Apolo et al. [Citation24] study that concluded that PET-CT affected the management in 68% of patients, with a sensitivity of 75% and specificity of 92%. Yang et al. [Citation37] also reported sensitivity and specificity of PET-CT in patients with recurrent bladder UC of 87% and 89%, respectively. Likewise, Öztürk et al. [Citation38] reported a sensitivity of 92% and specificity of 83% using PET-CT in the detection of recurrence after radical cystectomy ( [Citation24,Citation36–38]).
Table 3. 18F-FDG PET-CT in re-staging of LN in UC
Role of FDG PET-CT in UTUC
The performance of FDG PET-CT in the evaluation of UTUC has been examined in three retrospective studies [Citation10,Citation21,Citation22]. Tanaka et al. [Citation21] compared the diagnostic accuracy of FDG PET-CT and CT alone in 53 patients for detecting UTUC metastases in primary and recurrent diseases. The authors found that the sensitivity and specificity of FDG PET-CT were comparable to CT alone (95% and 91% respectively for FDG PET-CT, and 82% and 85% respectively for CT). Asai et al. [Citation22] investigated the performance of FDG PET-CT in the detection of LNs in a subgroup of 28 patients with UTUC and reported a sensitivity of 60%, with no data on specificity or other measures. Finally, in 2019, Voskuilen et al. [Citation10] evaluated the role of FDG PET-CT for the detection of LN metastases in 117 patients with UTUC. Overall, the authors reported a sensitivity and specificity of FDG PET-CT of 82% and 84%, respectively ( [Citation10,Citation21,Citation22]).
Table 4. 18F-FDG PET-CT for patients with upper tract UC
Discussion
The introduction of any technique to clinical practice requires proving the diagnostic accuracy with a low risk of harm. Non-invasive diagnostic techniques such as CT or MRI were found to be inadequate in the diagnosis and staging of UC [Citation11]. New techniques with high diagnostic performance will improve the detection of tumours, as well as metastatic deposits guiding surgical intervention and improving survival.
Regarding the LN staging of UC, the variability in the sensitivity measures was more evident than in the monitoring phase. The highest sensitivity was estimated at 95%, while the lowest sensitivity was 7–23% [Citation34]. On the other hand, specificity was generally higher than sensitivity with more consistent values among the included studies, which ranged from 81% [Citation24] to 100% [Citation25]. The PPV, which is more important from a clinical perspective, ranged from 25% to 37% [Citation34], to 100% [Citation25]. Likewise, the NPV ranged from 65% [Citation27] to 92% [Citation33]. Regarding the monitoring of LN in UC, the sensitivity measures among the included studies were higher than those in the staging phase. It ranged from 75% [Citation24] to 90% [Citation38]. The specificity for monitoring of LN in UC was generally lower than that reported in the staging phase. It ranged from 83% [Citation38] to 92% [Citation24]. Generally, the sample size in the monitoring studies was small, except in a study conducted by Lu et al. [Citation42], where 236 patients were recruited.
Our present result is consistent with what has been found with the use of PET-CT with radiolabelled prostate-specific membrane antigen (PSMA) for diagnostic assessment of patients with prostate cancer [Citation43]. PSMA PET/CT has revealed a higher sensitivity than traditional methods in detecting metastases in primary staging to detect metastases [Citation44]. A meta-analysis included 298 patients with prostate cancer who underwent traditional imaging studies and PSMA PET-CT, the detection of metastatic LNs was higher by PSMA PET-CT than with CT and MRI (71% sensitivity and 95% specificity) [Citation45].
The ability to exclude false positives in the staging is an advantage of this technique. While in staging tumours, specificity is more important than sensitivity because false positives can be subjected to unnecessary invasive investigations such as biopsies. Nevertheless, missing cancer cases due to low sensitivity may result in delayed treatment and associated adverse effects. Thus, several sequential diagnostic modalities are recommended to improve the net sensitivity of staging LN of UC. These findings demonstrate that studies assessing the role of PET-CT in LN monitoring revealed comparatively high sensitivity and specificity values of PET-CT in surveillance and proved its capability to impact individual treatment plans and change the clinical decision.
Generally, FDG PET-CT has higher diagnostic values in cancer monitoring than in cancer staging [Citation46]. This can be attributed to the anatomical locations and surrounding tissues, which makes it challenging to diagnose LN involvement using imaging techniques. A possible explanation for the low accuracy is the high flow of fluids in the urinary system that interferes with image interpretation. The importance of the scanning protocol was highlighted by Mertens et al. [Citation47], who found a sensitivity for cancer detection of 38% in the empty bladder protocol, and 63% in the filled bladder protocol. Adjunct to FDG PET imaging, which detects highly metabolic metastatic LN to the anatomical assessment provided by CT, increases the sensitivity for detecting LNs that are not large enough to be recognised by CT alone. Nevertheless, FDG PET-CT sensitivity is, indeed, adversely affected by minor metastatic lesions that cannot reach the metabolic activity threshold [Citation48]. Furthermore, the low specificity of FDG PET is mainly related to a high rate of false-positive LNs, where the uptake of 18F-FDG is caused by inflammatory states and not by cancer.
The approach utilised suffers from the limitation that the included studies are mostly retrospective. Other limitations include heterogeneous patient populations, non-standardisation of techniques, variable follow-up durations, and different confirmatory tests. Furthermore, most of the included studies lacked statistical power due to small samples, which are far lower than the sample size recommended for diagnostic studies. There is a need for further well-designed studies to accurately determine the diagnostic accuracy of FDG PET-CT in patients with UC.
Conclusions
The use of FDG PET-CT appears to have a high sensitivity for both UTUC and bladder UC in the detection of LN metastasis. Despite the high specificity of FDG PET-CT, the detection of LNs is still in the range of conventional images. Albeit the role of PET-CT could be considered as promising, more research is required to establish it as the imaging modality of choice, or at least to recommend its routine use in a selected group of patients.
References
- Green DA, Rink M, Xylinas E, et al. Urothelial carcinoma of the bladder and the upper tract: disparate twins. J Urol. 2013;189:1214–1221.
- Rink M, Ehdaie B, Cha EK, et al. Stage-specific impact of tumor location on oncologic outcomes in patients with upper and lower tract urothelial carcinoma following radical surgery. Eur Urol. 2012;62:677–684.
- Soria F, Shariat SF, Lerner SP, et al. Epidemiology, diagnosis, preoperative evaluation and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J Urol. 2017;35:379–387.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.
- Remzi M, Haitel A, Margulis V, et al. Tumour architecture is an independent predictor of outcomes after nephroureterectomy: a multi-institutional analysis of 1363 patients. BJU Int. 2009;103:307–311.
- Favaretto RL, Shariat SF, Savage C, et al. Combining imaging and ureteroscopy variables in a preoperative multivariable model for prediction of muscle-invasive and non-organ confined disease in patients with upper tract urothelial carcinoma. BJU Int. 2012;109:77–82.
- Rouprêt M, Babjuk M, Compérat E, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. 2018;73:111–122.
- Babjuk M, Burger M, Compérat EM, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and Carcinoma In Situ) - 2019 update. Eur Urol. 2019;76:639–657.
- Sevcenco S, Ponhold L, Heinz-Peer G, et al. Prospective evaluation of diffusion-weighted MRI of the bladder as a biomarker for prediction of bladder cancer aggressiveness. Urol Oncol. 2014;32:1166–1171.
- Voskuilen CS, Schweitzer D, Jensen JB, et al. Diagnostic value of (18)F-fluorodeoxyglucose positron emission tomography with computed tomography for lymph node staging in patients with upper tract urothelial carcinoma. Eur Urol Oncol. 2020;3:73–79.
- Paik ML, Scolieri MJ, Brown SL, et al. Limitations of computerized tomography in staging invasive bladder cancer before radical cystectomy. J Urol. 2000;163:1693–1696.
- Crozier J, Papa N, Perera M, et al. Comparative sensitivity and specificity of imaging modalities in staging bladder cancer prior to radical cystectomy: a systematic review and meta-analysis. World J Urol. 2019;37:667–690.
- Nishimura K, Hida S, Nishio Y, et al. The validity of magnetic resonance imaging (MRI) in the staging of bladder cancer: comparison with computed tomography (CT) and transurethral ultrasonography (US). Jpn J Clin Oncol. 1988;18:217–226.
- Vali R, Loidl W, Pirich C, et al. Imaging of prostate cancer with PET/CT using 18F-Fluorocholine. Am J Nucl Med Mol Imaging. 2015;5:96–108.
- Grigsby PW, Siegel BA, Dehdashti F. Lymph node staging by positron emission tomography in patients with carcinoma of the cervix. J Clin Oncol. 2001;19:3745–3749.
- Gupta T, Master Z, Kannan S, et al. Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2011;38:2083–2095.
- Cervino AR, Cuppari L, Reccia P, et al. The role of PET/CT in the evaluation of patients with urothelial cancer: a systematic review and meta-analysis. Clin Transl Imaging. 2018;6:77–89.
- Abufaraj M, Dalbagni G, Daneshmand S, et al. The role of surgery in metastatic bladder cancer: a systematic review. Eur Urol. 2018;73:543–557.
- Zhang H, Xing W, Kang Q, et al. Diagnostic value of [18 F] FDG-PET and PET/CT in urinary bladder cancer: a meta-analysis. Tumour Biol. 2015;36:3209–3214.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100.
- Tanaka H, Yoshida S, Komai Y, et al. Clinical value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in upper tract urothelial carcinoma: impact on detection of metastases and patient management. Urol Int. 2016;96:65–72.
- Asai S, Fukumoto T, Tanji N, et al. Fluorodeoxyglucose positron emission tomography/computed tomography for diagnosis of upper urinary tract urothelial carcinoma. Int J Clin Oncol. 2015;20:1042–1047.
- Kibel AS, Dehdashti F, Katz MD, et al. Prospective study of [18F]fluorodeoxyglucose positron emission tomography/computed tomography for staging of muscle-invasive bladder carcinoma. J Clin Oncol. 2009;27:4314–4320.
- Apolo AB, Riches J, Schöder H, et al. Clinical value of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in bladder cancer. J Clin Oncol. 2010;28:3973–3978.
- Lodde M, Lacombe L, Friede J, et al. Evaluation of fluorodeoxyglucose positron-emission tomography with computed tomography for staging of urothelial carcinoma. BJU Int. 2010;106:658–663.
- Rouanne M, Girma A, Neuzillet Y, et al. Potential impact of 18F-FDG PET/CT on patients selection for neoadjuvant chemotherapy before radical cystectomy. Eur J Surg Oncol. 2014;40:1724–1730.
- Hitier-Berthault M, Ansquer C, Branchereau J, et al. 18F-fluorodeoxyglucose positron emission tomography–computed tomography for preoperative lymph node staging in patients undergoing radical cystectomy for bladder cancer: A prospective study. Int J Urol. 2013;20:788–796.
- Girard A, Rouanne M, Taconet S, et al. Integrated analysis of (18)F-FDG PET/CT improves preoperative lymph node staging for patients with invasive bladder cancer. Eur Radiol. 2019;29:4286–4293.
- Drieskens O, Oyen R, Van Poppel H, et al. FDG-PET for preoperative staging of bladder cancer. Eur J Nucl Med Mol Imaging. 2005;32:1412–1417.
- Swinne G, Maes A, Pottel H, et al. FDG-PET/CT for the preoperative lymph node staging of invasive bladder cancer. Eur Urol. 2010;57:641–647.
- Goodfellow H, Viney Z, Hughes P, et al. Role of fluorodeoxyglucose positron emission tomography (FDG PET)-computed tomography (CT) in the staging of bladder cancer. BJU Int. 2014;114:389–395.
- Aljabery F, Lindblom G, Skoog S, et al. PET/CT versus conventional CT for detection of lymph node metastases in patients with locally advanced bladder cancer. BMC Urol. 2015;15:87.
- Pichler R, De Zordo T, Fritz J, et al. Pelvic lymph node staging by combined (18)F-FDG-PET/CT imaging in bladder cancer prior to radical cystectomy. Clin Genitourin Cancer. 2017;15:e387–e395.
- Dason S, Wong NC, Donahue TF, et al. Utility of routine preoperative 18F-fluorodeoxyglucose positron emission tomography/computed tomography in identifying pathological lymph node metastases at radical cystectomy. J Urol. 2020;204:254–259.
- Vind-Kezunovic S, Bouchelouche K, Ipsen P, et al. Detection of lymph node metastasis in patients with bladder cancer using maximum standardised uptake value and 18F-fluorodeoxyglucose positron emission tomography/computed tomography: results from a high-volume centre including long-term follow-up. Eur Urol Focus. 2019;5:90–96.
- Jadvar H, Quan V, Henderson RW, et al. [F-18]-Fluorodeoxyglucose PET and PET-CT in diagnostic imaging evaluation of locally recurrent and metastatic bladder transitional cell carcinoma. Int J Clin Oncol. 2008;13:42–47.
- Yang Z, Pan L, Cheng J, et al. Clinical value of whole body fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography in the detection of metastatic bladder cancer. Int J Urol. 2012;19:639–644.
- Öztürk H, Karapolat I. Efficacy of (18)F-fluorodeoxyglucose-positron emission tomography/computed tomography in restaging muscle-invasive bladder cancer following radical cystectomy. Exp Ther Med. 2015;9:717–724.
- Soubra A, Hayward D, Dahm P, et al. The diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography and computed tomography in staging bladder cancer: a single-institution study and a systematic review with meta-analysis. World J Urol. 2016;34:1229–1237.
- Jeong IG, Hong S, You D, et al. FDG PET-CT for lymph node staging of bladder cancer: a prospective study of patients with extended pelvic lymphadenectomy. Ann Surg Oncol. 2015;22:3150–3156.
- Jensen TK, Holt P, Gerke O, et al. Preoperative lymph-node staging of invasive urothelial bladder cancer with 18F-fluorodeoxyglucose positron emission tomography/computed axial tomography and magnetic resonance imaging: correlation with histopathology. Scand J Urol Nephrol. 2011;45:122–128.
- Lu YY, Chen JH, Liang JA, et al. Clinical value of FDG PET or PET/CT in urinary bladder cancer: a systemic review and meta-analysis. Eur J Radiol. 2012;81:2411–2416.
- von Eyben FE, Baumann GS, Baum RP. PSMA diagnostics and treatments of prostate cancer become mature. Clin Transl Imaging. 2018;6:145–148.
- Corfield J, Perera M, Bolton D, et al. 68Ga-prostate specific membrane antigen (PSMA) positron emission tomography (PET) for primary staging of high-risk prostate cancer: a systematic review. World J Urol. 2018;36:519–527.
- Kim SJ, Lee SW, Ha HK. Diagnostic performance of radiolabeled prostate-specific membrane antigen positron emission tomography/computed tomography for primary lymph node staging in newly diagnosed intermediate to high-risk prostate cancer patients: a systematic review and meta-analysis. Urol Int. 2019;102:27–36.
- Shvarts O, Han KR, Seltzer M, et al. Positron emission tomography in urologic oncology. Cancer Control. 2002;9:335–342.
- Mertens LS, Bruin NM, Vegt E, et al. Catheter-assisted 18F-FDG-PET/CT imaging of primary bladder cancer: a prospective study. Nucl Med Commun. 2012;33:1195–1201.
- Li Y, Diao F, Shi S, et al. Computed tomography and magnetic resonance imaging evaluation of pelvic lymph node metastasis in bladder cancer. Chin J Cancer. 2018;37:3.