Abstract
Cluster flies are represented by the genus Pollenia Robineau-Desvoidy, 1830 of the family Polleniidae Brauer and Bergenstamm, 1889. Their larvae are known to be internal parasites or predators of earthworms. Herein, we report for the first time the occurrence of the cluster flies Pollenia rudis Fabricius, 1794 and Pollenia vagabunda (Meigen, 1826) (Diptera: Polleniidae) on carcasses in Algeria and identify them through DNA barcoding. A region of the mitochondrial cytochrome c oxidase I gene (COI) was amplified and sequenced. Genetic distances were determined. A phylogenetic tree was constructed with the maximum parsimony method using 10 000 bootstrap replicates. A total number of 157 adults of P. rudis were collected together with 325 adults of Pollenia vagabunda. The occurrence of Pollenia on animal carcasses does not seem to be correlated with a particular stage of decomposition. All the sequences were correctly identified using the BLASTn tool from the GenBank database and the BOLD identification engine. Intra- and interspecific sequence divergence values were less than 1% and greater than 3%, respectively. COI barcodes obtained from this study were robust enough to identify and distinguish unambiguously between P. rudis and P. vagabunda. In the tree-based analysis, the cluster flies were all assigned to their respective species separately from each other confirming the morphological identification. These results provide DNA barcodes that contribute to the growth of reference databases and allow fast and accurate identification.
Introduction
The family Polleniidae Brauer and Bergenstamm, 1889 has been previously considered a subfamily of Calliphoridae containing several genera and species. Recently, the subfamily Polleniinae has been raised to the family status following phylogenetic analysis [Citation1]. The largest genus is Pollenia Robineau-Desvoidy, 1830 whose species are commonly called cluster flies due to their observed habit of aggregating in crevices and corners of dark parts in buildings [Citation2]. The presence of dull colouration and yellow crinkly hairs on the head and thorax of cluster flies differentiates them from blow flies [Citation3–5]. The adults are common pollinators [Citation6] and can be among the most abundant flower-visiting insects in agricultural and urban areas [Citation3].
Cluster fly larvae are internal parasites or predators of earthworms (Lumbricidae) [Citation2,Citation7,Citation8]. Yet, the biology of this group is relatively poorly known [Citation1] and a few have been observed parasitising other hosts (caterpillars and bees) [Citation3]. Pollenia rudis Fabricius, 1794, the most common species in the genus, is largely distributed in the Palaearctic, Nearctic and Oriental regions [Citation3–5]. Adult P. rudis are mostly herbivores and feed on sap, flowers, fruit, faeces and many different types of decaying organic matter [Citation9]. They are mostly active during the spring and lay their eggs on the soil. After hatching, larvae actively seek earthworm hosts [Citation3]. Pollenia vagabunda (Meigen, 1826) is widespread in Europe [Citation3]. Rognes [Citation2] has recorded P. vagabunda from North Morocco; however, he reported its occurrence in Algeria to be uncertain based on a dubious record. Little is known about the biology of P. vagabunda. This species may overwinter as adults inside buildings. The puparia of P. vagabunda have been found in the stems of corn infested by the moth Sesamia nonagrioides (Lefebvre, 1827). It was therefore suggested that the larvae may parasitise organisms other than lumbricids [Citation2].
Urban entomology is a subfield of forensic entomology which deals with arthropod pests that harm humans and their immediate environment. The species P. rudis has been closely observed due to its tendency to infest buildings and thus its potential as a disease vector. Cluster flies tend to retire indoors in large numbers during the autumn to overwinter. They enter through cracks and holes in houses and other structures [Citation10]. Infestations are often observed in buildings that exist at higher altitudes, as well as in buildings which are older [Citation11]. After entering a building, cluster flies overwinter habitually in isolated areas between the walls and in the ceilings until they emerge in the spring and seek access to the outside [Citation10]. Massive and frequent infestations over the years can damage attics, walls, or windows with fly faeces or bodies, potentially causing allergic reactions, as well as secondary infestations of dermestid beetles that breed on the dead flies [Citation3,Citation11]. Furthermore, Pollenia species can also create a fire hazard when using electric fly killers since dead insects may pile up and eventually touch the killing grid [Citation11].
Cluster flies’ role in disease transmission as mechanical vectors has been reported in numerous cases. An investigation of mesophilic bacteria carried on P. rudis was conducted after a large infestation of cluster flies in a German hospital in 1973. Results showed that P. rudis was capable of spreading bacteria causing opportunistic and/or nosocomial infections in humans. Consequently, Pollenia species may transmit bacterial pathogens by infesting massively delicate areas such as hospitals [Citation12]. It has also been reported that an entire city’s water reservoir tank, which was infested with cluster flies, was drained in New Zealand in 2002 due to high levels of faecal coliforms [Citation10].
The main application of medicolegal forensic entomology is to estimate the minimum time elapsed since death, or postmortem interval (PMI). It can also be concluded that a corpse has been moved from its original location when an unexpected species is identified, which is more characteristic of a different habitat or geographic region. However, this application depends on the knowledge of the local fauna [Citation13]. Therefore, identifying present insect species is an initial crucial step in death investigations [Citation14]. This step can be problematic when entomologists are confronted with damaged specimens or when partial arthropod remains are the only evidence available [Citation15]. Furthermore, it is challenging to identify intact specimens of immature stages based on morphological criteria due to the presence of similar characteristics shared between different species [Citation14] and the lack of identification keys. Live immature specimens are usually reared to adulthood so that distinguishable features become visible for species identification [Citation16].
Adults of Pollenia are not included in forensically important entomofauna because their larvae are parasites [Citation17]. Nonetheless, they have been found on dead bodies [Citation13], as well as on meat, fish [Citation18], liver [Citation18,Citation19], and squid-baited traps in the past [Citation20,Citation21]. The use of squid as bait has shown promise in decomposition studies, as it retains moisture for much longer than other types of bait [Citation20]. Adult Pollenia can be identified morphologically; however, larvae are mostly undescribed [Citation11]. Some Pollenia species are difficult to identify based on female samples, which are more commonly encountered than males [Citation21]. They have been indistinguishable in many studies based on morphological characteristics and misidentified as P. rudis as a result [Citation3,Citation5]. Molecular techniques have been developed to overcome such challenges, and allow identification regardless of variables such as life stage, sex, and colour morph [Citation22]. DNA barcoding in animal species uses short strands of DNA sequences which are frequently isolated from the mitochondrial cytochrome c oxidase I gene (COI), as it is widely considered the standard gene for such analyses. These sequences are compared to those in various genetic databases, such as Barcode of Life Data Systems (BOLD) or GenBank (the National Center for Biotechnology Information — NCBI), using an alignment programme known as Basic Local Alignment Search Tool (BLAST) [Citation23]. The success of molecular identification depends on the selection of a suitable marker gene and the availability of reference sequences in the databases. Therefore, this study aims to update the distribution records of Polleniidae and test the efficacy of DNA barcoding and the existing databases to identify P. rudis and P. vagabunda. This is the first study in Algeria reporting the occurrence of cluster flies on carcasses.
Material and methods
Samples
Adult flies were collected during spring (between 21 April and 8 May) of 2016 on 18 exposed male rabbit carcasses weighing approximately (2.50 ± 0.42) kg in a semi-urban site in Blida, Algeria (coordinates: 36° 30′ 38.5″ N, 2° 52′ 21.4″ E) at 188 m above sea level using a trap described in Taleb et al. [Citation24]. The carcass and the trap were protected by a metal cage measuring 80 cm × 60 cm × 150 cm. This prevented disturbance by large vertebrate scavengers but permitted arthropod colonization. Carcasses were separated by a minimum distance of 50 m. Sampling was done at 8:00 am and 5:00 pm daily over the course of 2 weeks.
Freezing at −20 °C for 1 h is the recommended method for killing adult insects [Citation16]; however, this method is not possible in the field. It has been shown that DNA barcoding was successful using specimens killed by ethyl acetate vapours [Citation25–28]. Therefore, the flies were killed by exposing them to ethyl acetate vapours for a maximum of 10 min to make sure that the DNA was not damaged. Each specimen was morphologically identified and confirmed multiple times by at least three experts using the keys of Rognes [Citation2,Citation5] and Jewiss-Gaines, et al. [Citation3], with Carl Zeiss® Stemi 2000-C and Leica® EZ4 HD stereo zoom microscopes coupled with digital cameras. The samples were then stored in 70%–95% ethanol and kept at −20 °C.
DNA extraction
The samples were rinsed with deionised water and dried. DNA was extracted from two legs using the DNeasy® Blood and Tissue kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol with over-night incubation. The remaining tissue was retained as a voucher specimen and stored in 95% ethanol. Extracts were kept at 4 °C for future molecular analysis.
Polymerase chain reaction (PCR)
A region of the mitochondrial gene COI was amplified using primers C1–N–2191 (5′-CCCGGTAAAATTAAAATATAAACTTC-3′) and C1-J-1718 (50-GGA GGATTTGGAAATTGATTAGTTCC-3′0) [Citation29]. PCR reactions were carried out in a final volume of 20 µL. Each PCR reaction contained 1× buffer (with 20 mmol/L Mg2+), 2.5 mmol/L of each dNTP, 2.5 U AmpONETM Taq DNA polymerase (all GeneAll®, Seoul, Korea), 10 mmol/L of each primer, and 5 µL of the DNA template. The PCR was carried out in a ProFlexTM 3 × 32-well PCR System thermal cycler (ThermoFisher ScientificTM, Foster City, CA, USA) under the following conditions: 95 °C for 5 min, 35 cycles of 95 °C for 35 s, 54 °C for 35 s and 72 °C for 40 s. The sizes of the amplified fragments were verified using gel electrophoresis in a 1% agarose gel stained with GelRed (Biotium, Fremont, CA, USA). PCR products were purified using the commercial kit AMPure XP (Beckman Coulter, Miami, FL, USA) according to the manufacturer’s instructions.
Sequencing
Sequencing was carried out using the BigDye® Terminator v3.1 sequencing kit (ThermoFisher ScienceTM). The 15 µL reaction mix consisted of 0.5 µL BigDye® Terminator v3.1 Ready Reaction Mix (×2.5), 2.5 µL sequencing buffer BigDye (×5), 0.5 µL primer (3.2 pmol), and 2 µL PCR product. The reactions were performed using a Bio-RAD T100 thermocycler under the following conditions: 96 °C for 1 min, 25 cycles of 96 °C for 10 s, 50 °C for 25 s and 60 °C for 4 min.
Sequencing products were purified using the purification kit Clean-up KitTM (Zymo Research Corp., Irvine, CA, USA) according to the manufacturer’s protocol. Automated sequencing was carried out using ABI 3130 capillary sequencer (Applied Biosystem, Foster City, CA, USA).
Genetic analysis
Raw sequences were quality trimmed and manually aligned using BioEdit v7.2.5 [Citation30]. The obtained sequences were deposited in GenBank (http://www.ncbi.nlm.nih.gov/) and BOLD (http://www.boldsystems.org/). We additionally retrieved publicly available COI sequences referring to Pollenia from the BOLD and GenBank databases to include in the genetic analysis (). The sequences were aligned using MUSCLE [Citation31].
Table 1. List of species used for genetic analysis with their GenBank accession numbers and/or BOLD sequence ID.
Genetic distances in barcoding studies are usually determined using the Kimura’s Two-Parameter (K2P) model [Citation32] following Hebert, et al. [Citation33]. However, it was shown that the use of K2P for closely related COI sequences was inappropriate and unnecessary and that uncorrected p-distances should be used instead [Citation34–36]. Moreover, our preliminary analysis has not revealed any significant difference between the two methods. Thus, uncorrected p-distances were calculated in MEGA7 [Citation37] to study COI intra- and interspecific divergence. The phylogenetic tree was constructed with the maximum parsimony method (MP) using PAUP*4.0 software and 10 000 bootstrap replicates [Citation38]. The COI sequence of the blow fly Calliphora alaskensis (Shannon, 1923) (GenBank accession number JN263392) was used as the outgroup in the phylogenetic analysis.
Results and discussion
Occurrence on cadavers
Pollenia species have never been reported from decomposition studies in Algeria. Thus, the current data present the first approach of using molecular tools to identify cluster flies from Algeria (in the context of a decomposition study). A total number of 157 adults of P. rudis were collected together with 325 adults of P. vagabunda (). Pollenia species are usually neglected during carrion decomposition studies and are often identified to the genus level. Specimens of Pollenia, particularly those of P. rudis, have frequently been reported from carrion succession experiments in Europe and North America [Citation17,Citation18,Citation39–41]. Pollenia male samples were completely absent in the collections of the current study. Similarly, Martín-Vega and Baz [Citation21] did not collect any Pollenia male individuals on traps baited with squid. In contrast, Baz, et al. [Citation20] reported both males and females of Pollenia species with female-biased sex ratio, also via the use of squid-baited traps.
Table 2. Absolute abundance of adult Pollenia rudis and Pollenia vagabunda according to the decomposition stages of rabbit carcasses.
Occurrence according to stages of decomposition
Stages of decomposition were defined according to the classification of Reed [Citation42], which establishes four stages; in order of increasing decomposition time, these are named the fresh stage, bloated stage, decay stage, and dry stage. This classification was used in our study because it is more practical to study the decomposition of small carrion. In the current study, P. rudis was recorded in the decay and dry stages whereas P. vagabunda was collected in the bloated stage and continued to appear until the dry stage. Prado e Castro [Citation40] noted the presence of Pollenia spp. in Portugal on pig carcasses during spring in all the stages of decomposition and during summer from the bloated until the dry stage. Tabor [Citation41] reported the occurrence of P. rudis in the USA on pig carcasses during spring in the bloated stage and during summer in every stage of decomposition. Consequently, Pollenia species do not seem to be attracted to a specific stage of decomposition. Decomposing remains are attractive to Pollenia, yet their significance in medicolegal entomology is neglected due to the absence of correlation to a particular stage of decomposition [Citation18].
Temporal occurrence
In the present study, the occurrence of P. rudis and P. vagabunda in spring is consistent with the observations reported by other authors. Pollenia spp. were found to be abundant particularly in spring followed by summer [Citation40,Citation41]. Szpila [Citation43] stated that P. rudis occurred in Poland mainly during spring and summer in very high abundance in grassland. Adult P. vagabunda were recorded in Poland during summer in low numbers [Citation43]. It was found that P. rudis populations in Canada peaked in April and June [Citation3].
Ecological role
The presence of adult Pollenia in high numbers, as observed during this study as well as others, shows that their occurrence on cadavers is unlikely to be accidental. Šuláková and Barták [Citation18] suggested that these species could be attracted to the liquid pouring out of the baits. Arthropod species found on decomposing remains are attributed to ecological categories. These are generally classified into four classes, namely necrophagous species, parasites and predators on the necrophagous species, omnivorous species, and adventive species [Citation44]. A fifth category is sometimes cited; these are the so-called accidental species whose presence on the body is the result of chance [Citation45]. Šuláková and Barták [Citation18] proposed to attribute to Pollenia the status of “predator/parasite on necrophagous species”, following the classification proposed by Smith [Citation44] since earthworms and snails have frequently been observed participating in decomposition in experiments and real cases. Nonetheless, the occurrence of Pollenia spp. on carrion seems to be related to the feeding of the adult flies on cadavers as females feed on liquid and semi-liquid decomposition products. However, the larvae of these true parasites cannot finish their development by feeding on cadavers [Citation43].
Molecular identification
The DNeasy® Blood and Tissue kit (Qiagen) yielded adequate DNA for COI amplification of all the specimens tested. DNA concentrations varied between 4 ng/µL to 8 ng/µL with small variation in the average DNA yield between P. rudis (6.05 ± 1.93 ng/µL) and P. vagabunda (6.13 ± 1.67 ng/µL). Ames, et al. [Citation46] reported DNA concentrations of Calliphora vicina Robineau-Desvoidy, 1830 and Calliphora vomitoria (Linnaeus, 1758) ranging from 0.2 ng/mL from a single wing to 30 ng/mL for a complete adult fly. These authors found little variation in the DNA yield between these two species which is comparable to our result. Data from the literature as well as our results have shown that using minimal fly tissue leads to successful DNA extraction and amplification [Citation47]. This can be useful when only insect parts (legs, wings, etc.) are found at crime scenes. Additionally, it is always helpful to keep the sample for the possibility of a morphological re-examination, if necessary. Fragments of the COI gene of P. rudis and P. vagabunda were amplified and sequenced successfully. The PCR amplification produced fragments of approximately 500 bp.
All the sequences were correctly identified using the BLASTn tool from the GenBank database and the BOLD identification engine. Meiklejohn, et al. [Citation23] considered molecular identification reliable when multiple independent records with the same top match statistics had the correct taxonomic, name which is in agreement with our findings. The GenBank percent identity of the first match was (99.16 ± 0.13)%, whereas the BOLD percent of similarity of the first match was (99.43 ± 0.09)%.
Genetic variation
The intra- and interspecific divergence is shown in . No intraspecific variation was observed within P. rudis sequences while the divergence between P. vagabunda sequences varied from 0 to 0.33%. No intraspecific divergence amongst the samples of P. rudis and P. vagabunda from different countries was found. As for the genetic variation between P. rudis and P. vagabunda specimens sequenced in this study, an average value of 9.59% was found. Many studies have attempted to determine the standard threshold for intra- and interspecific divergences. For instance, Hebert, et al. [Citation48] reported a rule for the intraspecific variation of being <3% and interspecific divergence at least 10 times greater than the intraspecific divergence. Wells and Sperling [Citation49] who studied the genetic variation of necrophagous fly species reported the percentage of intraspecific divergence being less than or equal to 1% for the sequences of cytochrome c oxidase I and II (COI + COII) and the percentage of intraspecific divergence being greater than 3% as adequate thresholds. In general, intraspecific variations are commonly less than 1% and rarely greater than 2% [Citation50]. Intra- and interspecific divergence values of our sequences were found in agreement with the permissible limits, i.e. <1% and >3%, respectively [Citation48,Citation49]. COI barcodes obtained from this study were robust enough to identify and distinguish unambiguously between P. rudis and P. vagabunda. Nonetheless, these rules cannot be generalised to all groups as COI barcoding may fail to discriminate between sister species [Citation51].
Table 3. Intra- and interspecific P-distances of the analysed cytochrome c oxidase I gene (COI) expressed as percentages.
The divergence between P. rudis sequences from Algeria to the ones from different parts of the world was higher (0.66%). Likewise, the Algerian sequences of P. vagabunda showed greater divergence to those of different countries (0.71%). These findings may be a result of a gene pool fragmentation caused by geographical isolation and genetic disparity [Citation48]. Furthermore, silent substitutions not affecting the amino-acid sequence have been reported as the source of haplotype diversity amongst fly species of forensic importance [Citation52].
Phylogenetic analysis
Based on our preliminary analysis of the submitted COI sequences, the most reliable sequences within the genus Pollenia were chosen for the phylogenetic analysis in order to verify our species identification accuracy. Some of the P. rudis sequences from Spain, Portugal, and Norway (GenBank accession numbers KX161505, JX438050, and MG673730, respectively) may have been wrongly identified. For this reason, they were excluded from the analysis.
In the tree-based analysis, the Algerian cluster flies were all assigned to their respective species. At the species level, the nine Pollenia species formed monophyletic clusters with very robust supportive values (). Thus, P. rudis and P. vagabunda individuals from Algeria clustered separately from each other confirming the morphological identification. This shows that the COI marker was sufficient to differentiate between P. rudis and P. vagabunda.
Figure 1. Maximum parsimony consensus phylogram of 41 cytochrome c oxidase I (COI) sequences from nine Pollenia species and one outgroup (Calliphora alaskensis) based on 10 000 bootstrap replicates.
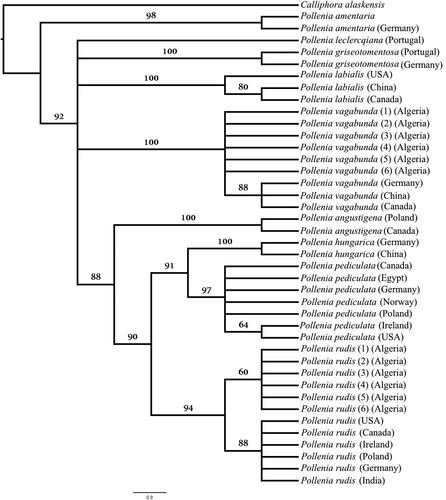
The phylogenetic analysis of Pollenia COI sequences was congruent with the monophyletic species-groups described by Rognes [Citation2,Citation5,Citation7,Citation8]. Rognes [Citation5] divided the P. rudis species-group into seven distinct species: P. angustigena Wainwright, 1940, P. hungarica Rognes, 1987, P. paupera Rondani, 1862 (P. longitheca Rognes, 1987), P. luteovillosa Rognes, 1987, P. pediculata Macquart, 1834 (P. pseudorudis Rognes, 1985), P. rudis and Pollenia sp. Rognes [Citation2] described five species in the vagabunda species-group: P. bezziana Rognes, 1992, P. stigi Rognes, 1992, P. verneri Rognes, 1992, P. contempta Robineau-Desvoidy, 1863 and P. vagabunda. Nevertheless, only P. vagabunda COI sequences are available online. Sequences of P. angustigena, P. hungarica, P. pediculata, and P. rudis formed a unique clade. Therefore, the present study supports the monophyly of the rudis species-group. Our study has also showed P. hungarica forming a sister clade with P. pediculata (bootstrap value of 91%). P. rudis has formed two separate subclades within its clade showing the Algerian P. rudis as a separate group from the rest of the world. Stevens, et al. [Citation53] have also reported the presence of intraspecific divergence and geographical disparities between blow fly (Calliphoridae) populations. However, it is important to use multiple genetic markers and a large number of specimens to thoroughly examine the population assembly and the accurate genetic divergence within a species [Citation28].
Conclusion
The present study was the first molecular approach to identify cluster flies from Algeria and record their occurrence on carcasses. Pollenia species are found on carrion particularly in spring without being restricted to a particular stage of decomposition. Although they are not considered primary species in cadaver analysis, cluster flies should be included in carrion entomofauna. COI barcodes obtained from this study were robust enough to identify and distinguish unambiguously between P. rudis and P. vagabunda. Taxonomic identification through DNA barcoding should be supported by accurate morphological identification and proper genetic analyses to submit reliable DNA sequences to reference databases. Our results provide DNA barcodes that contribute to the growth of reference databases and allow fast and accurate identification. More studies are required to increase the availability of DNA barcodes and new molecular markers should be tested.
Authors’ contributions
Meriem Taleb and Halide Nihal Açıkgöz designed the study and did the morphological identification and the molecular analyses. Ghania Tail supervised the study and edited the manuscript. Meriem Taleb gathered the fly samples, analysed the data and wrote the manuscript. All the authors read and approved the final manuscript.
Compliance with ethical standard
This article does not contain any studies with human participants or animals performed by any of authors.
Acknowledgements
We thank the National Institute of Criminalistics and Criminology, Algeria for support. We express our gratitude to Prof. Dr. H. Sinan Süzen, Forensic Science Institute, Ankara University, Turkey, for his support. We thank Dr. K.B. Rebijith, molecular entomologist, MPI, New Zealand, for his help and valuable comments. We are grateful to the three anonymous reviewers for their constructive comments and suggestions.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Cerretti P, Stireman JO, Badano D, et al. Reclustering the cluster flies (Diptera: Oestroidea, Polleniidae). Syst Entomol. 2019;44:957–972.
- Rognes K. Revision of the cluster-flies of the Pollenia vagabunda species-group (Diptera: Calliphoridae). Insect Syst Evol. 1992;23:95–114.
- Jewiss-Gaines A, Marshall SA, Whitworth TL. Cluster flies (Calliphoridae: Polleniinae: Pollenia) of North America. Can J Arthropod Identif. 2012;19:1–22.
- Moon RD. Muscid flies (Muscidae). In: Mullen GR, Durden LA, editors. Medical and veterinary entomology. 3rd ed. London: Academic Press; 2019. p. 345–368.
- Rognes K. The taxonomy of the Pollenia rudis species‐group in the Holarctic Region (Diptera: Calliphoridae). System Entomol. 1987;12:475–502.
- Samkari BA. Distribution of cluster fly species (Pollenia spp., Diptera: Calliphoridae) across Canada including range extensions and first provincial records. [Dissertation]. Canada: Trent University; 2018.
- Rognes K. Revision of the cluster-flies of the Pollenia venturii species-group with a cladistic analysis of Palaearctic species of Pollenia Robineau-Desvoidy (Diptera: Calliphoridae). Insect Syst Evol. 1992;23:233–248.
- Rognes K. Revision of the species of Pollenia Robineau-Desvoidy described by Camillo Rondani (Diptera: Calliphoridae). Entomol Scand. 1991;22:65–67.
- Richards PG, Morrison FO. Summary of published information on the cluster fly, Pollenia rudis (Fabricius) (Diptera: Calliphoridae). Phytoprotection. 1972;53:103–111.
- Heath A. Cluster Fly, Pollenia rudis (Fabricius) and P. pseudorudis Rognes (Diptera: Calliphoridae). In: Capinera JL, editor. Encyclopedia of entomology. Dordrecht: Springer; 2008. p. 932–935.
- Reynolds JW, Reeves WK, Olayemi OP, et al. A new look at earthworms (Oligochaeta: Lumbricidae) in Colorado, USA as hosts of the cluster fly Pollenia rudis F. (Diptera: Calliphoridae). Megadrilogica. 2020;25:107–112.
- Faulde M, Sobe D, Burghardt H, et al. Hospital infestation by the cluster fly, Pollenia rudis sensu stricto Fabricius 1794 (Diptera: Calliphoridae), and its possible role in transmission of bacterial pathogens in Germany. J Hyg Environ Health. 2001;203:201–204.
- Gennard D. Forensic entomology: an introduction. Chichester, West Sussex (UK): Wiley-Blackwell; 2012.
- Cainé L, Corte Real F, Lima G, et al. Genetic identification of forensically important Calliphoridae in Portugal. Int Congr. 2006;1288:846–848.
- Chimeno C, Moriniere J, Podhorna J, et al. DNA barcoding in forensic entomology — establishing a DNA reference library of potentially forensic relevant arthropod species. J Forensic Sci. 2019;64:593–601.
- Amendt J, Campobasso CP, Gaudry E, European Association for Forensic Entomology, et al. Best practice in forensic entomology-standards and guidelines. Int J Legal Med. 2007;121:90–104.
- Benbow ME, Lewis AJ, Tomberlin JK, et al. Seasonal necrophagous insect community assembly during vertebrate carrion decomposition. J Med Entomol. 2013;50:440–450.
- Šuláková H, Barták M. Forensically important Calliphoridae (Diptera) associated with animal and human decomposition in the Czech Republic: preliminary results. Casopis Slezkeho Zemskeho Muzea. 2013;62:255–266.
- Hwang C-C, Turner BD. Spatial and temporal variability of necrophagous Diptera from urban to rural areas. Med Vet Entomol. 2005;19:379–391.
- Baz A, Cifrián B, Díaz-Äranda LM, et al. The distribution of adult blow-flies (Diptera: Calliphoridae) along an altitudinal gradient in Central Spain. Ann Soc Entomol Fr. 2007;43:289–296.
- Martín-Vega D, Baz A. Sex-biased captures of sarcosaprophagous Diptera in carrion-baited traps. J Insect Sci. 2013;13:14–12.
- Rebijith KB, Asokan R, Kumar NKK. Molecular identification of mealybugs. In: Mani M, Shivaraju C, editors. Mealybugs and their management in agricultural and horticultural crops. New Delhi (India): Springer; 2016. p. 75–86.
- Meiklejohn KA, Damaso N, Robertson JM. Assessment of BOLD and GenBank — their accuracy and reliability for the identification of biological materials. PLoS One. 2019;14:e0217084
- Taleb M, Tail G, Açıkgöz HN. First Record of Chrysomya megacephala (Fabricius, 1794) (Diptera: Calliphoridae) in Algeria. Entomol News. 2018;128:78–86.
- Schmidt O, Hausmann A, Cancian de Araujo B, et al. A streamlined collecting and preparation protocol for DNA barcoding of Lepidoptera as part of large-scale rapid biodiversity assessment projects, exemplified by the Indonesian Biodiversity Discovery and Information System (IndoBioSys). BDJ. 2017. doi: https://doi.org/10.3897/BDJ.5.e20006
- Willows-Munro S, Schoeman MC. Influence of killing method on Lepidoptera DNA barcode recovery. Mol Ecol Resour. 2015;15:613–618.
- Nakahama N, Isagi Y, Ito M. Methods for retaining well-preserved DNA with dried specimens of insects. Eur J Entomol. 2019;116:486–491.
- Taleb M, Tail G, Acikgoz HN. DNA barcoding of Stearibia nigriceps (Meigen) and Piophila casei (Linnaeus) (Diptera: Piophilidae) from Algeria and the first African report of Stearibia nigriceps. Int J Legal Med. 2020;134:895–902.
- Simon C, Frati F, Beckenbach A, et al. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am. 1994;87:651–701.
- Hall T. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98.
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797.
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120.
- Hebert PD, Cywinska A, Ball SL, et al. Biological identifications through DNA barcodes. Proc Biol Sci. 2003;270:313–321.
- Collins RA, Boykin LM, Cruickshank RH, et al. Barcoding's next top model: an evaluation of nucleotide substitution models for specimen identification. Mol Biol Evol. 2012;3:457–465.
- Collins RA, Cruickshank RH. The seven deadly sins of DNA barcoding. Mol Ecol Resour. 2013;13:969–975.
- Srivathsan A, Meier R. On the inappropriate use of Kimura-2-parameter (K2P) divergences in the DNA-barcoding literature. Cladistics. 2012;28:190–194.
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874.
- Swofford DL. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland (MA): Sinauer Associates; 2002.
- Arnaldos MI, Romera E, Presa JJ, et al. Studies on seasonal arthropod succession on carrion in the southeastern Iberian Peninsula. Int J Legal Med. 2004;118:197–205.
- Prado e Castro C. Seasonal carrion Diptera and Coleoptera communities from Lisbon (Portugal) and the utility of forensic entomology in legal medicine [Dissertation]. Lisbon (Portugal): University of Lisbon; 2011.
- Tabor KL. Succession and development studies on carrion insects of forensic importance [Dissertation]. Blacksburg (VA): Virginia Polytechnic Institute and State University; 2004.
- Reed HB. A study of dog carcass communities in Tennessee, with special reference to the insects. Am Midl Nat. 1958;59:213–245.
- Szpila K. Annotated list of blowflies (Diptera: Calliphoridae) recorded during studies of insect succession on large carrion in Poland. Dipteron. 2017;33:85–93.
- Smith KGV. A manual of forensic entomology. London (UK): The British Museum (Natural History); 1986.
- Arnaldos MI, Garcia MD, Romera E, et al. Estimation of postmortem interval in real cases based on experimentally obtained entomological evidence. Forensic Sci Int. 2005;149:57–65.
- Ames C, Turner B, Daniel B. The use of mitochondrial cytochrome oxidase I gene (COI) to differentiate two UK blowfly species — Calliphora vicina and Calliphora vomitoria. Forensic Sci Int. 2006;164:179–182.
- Chen W-Y, Hung T-H, Shiao S-F. Molecular identification of forensically important blow fly species (Diptera: Calliphoridae) in Taiwan. J Med Entomol. 2004;41:47–57.
- Hebert PD, Ratnasingham S, deWaard JR. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc Biol Sci. 2003;270:S96–S99.
- Wells JD, Sperling FAH. DNA-based identification of forensically important Chrysomyinae (Diptera: Calliphoridae). Forensic Sci Int. 2001;120:110–115.
- Avise JC. Phylogeography: the history and formation of species. Cambridge (MA): Harvard University Press; 2000.
- Reibe S, Schmitz J, Madea B. Molecular identification of forensically important blowfly species (Diptera: Calliphoridae) from Germany. Parasitol Res. 2009;106:257–261.
- Wells JD, Introna F, Jr., D, Vella G, et al. Human and insect mitochondrial DNA analysis from maggots. J Forensic Sci. 2001;46:685–687.
- Stevens JR, Wall R, Wells JD. Paraphyly in Hawaiian hybrid blowfly populations and the evolutionary history of anthropophilic species. Insect Mol Biol. 2002;11:141–148.
