ABSTRACT
Perfluoroalkyl acids (PFAAs) are emerging persistent organic pollutants that are globally distributed in the environment. In the present review, the occurrence of PFAAs and their behavior in aquatic ecosystem were summarized, and the health and ecological risk assessment and the multimedia fate simulation were investigated. PFAAs are most likely to exist in the aqueous phase, and PFAAs in atmosphere are also able to enter water bodies through diffusion and wet and dry deposition and eventually become widely distributed in various environmental media. The air-solid partition is considered to be one of the major factors in the long-distance transportation of the pollutants. The pKa values and organic carbon fraction of the sediment could influence the partition of PFAAs between water and sediment. Otherwise, PFAAs have teratogenic, mutagenic and other toxic effects and they could be accumulated by biota, and magnified through trophic level. The ecological and health risks of PFOA and PFOS were assessment. In order to explore the partition mechanism and reduce the uncertainty of the simulation of the transport, transformation and fate, the experimental methods on physicochemical properties of PFAAs should be developed. Moreover, further studies on toxicities of PFAAs are necessary for health and ecological risk assessment.
Introduction
Perfluoroalkyl acids (PFAAs) have been produced and wdely used for more than 60 years (Giesy and Kannan Citation2001) in various applications, such as firefighting foams, carpets, packaging papers, and insulation films (Giesy and Kannan Citation2002; Begley et al. Citation2005; Gewurtz et al. Citation2009).
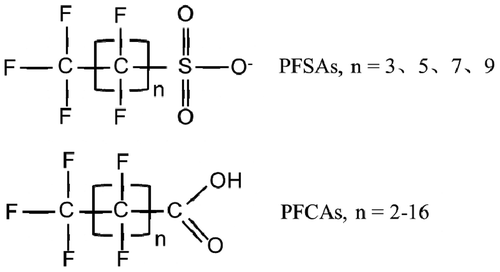
PFAAs, including perfluorosulfonic acids (PFSAs) and perfluoroalkyl carboxylic acids (PFCAs), are a kind of organic acids where all the hydrogen atoms on the carbon chains are replaced by fluorine atoms (). C-F bonds in PFAAs are highly thermally stable, with a bond energy of 105.4 kcal mol−1, the stability of which increases with more carbon atoms. The high electronegativity of fluorine atoms (4.0) makes their three lone pairs barely able to form a resonance structure similar to that for nitrogen and oxygen atoms or become the receptor of hydrogen atoms (O’Hagan Citation2008). Compared to normal carboxylic acids, PFAAs are strongly acidic and are almost completely ionized in water. Furthermore, the low vapor pressure and high solubility in water (Benskin, De Silva, and Martin Citation2010) make the ionized perfluorinated compounds stable to acids, alkali compounds, and strong oxidants, and difficult to be hydrolyzed, photodegraded and biodegraded. In May 2009, the surfactant perfluorooctane sulfonic acid (PFOS), its salts, and perfluorooctane sulfonyl fluoride (PFOSF) were added into Annex B of the Stockholm Convention text (UNEP Citation2009). Then, the Persistent Organic Pollutants Review Committee proposed the addition of PFOA and PFHxS and their salts to Annex A/B/C of the Convention in October 2015 and November 2017 (UNEP Citation2015, Citation2017). In 2017, the use of PFOA and its salts were restricted by European Union (Commission Regulation (EU) Citation2017).
3M company, the main manufacturer of PFOS production, has stopped and recalled all PFOS in 2000 and was completely eliminated in 2002 (3M Citation2010). The content of PFOS in nature has decreased, however, the total amount of perfluorinated compounds has not been significantly reduced (Hart, Gill, and Kannan Citation2009). Studies have shown that PFAAs such as perfluoroundecanoic acid (PFUnA), perfluorodecanoic acid (PFDA), and PFOS have high bioaccumulation factors and food-chain trophic magnification factors (Martin et al. Citation2004a). Degradation of volatile perfluorinated compounds into PFOS resulted in the long-distance migration of PFOS (Martin et al. Citation2004b). PFAAs have certain physiological and ecological toxicity, affecting the population (Boudreau et al. Citation2003; Latala, Nedzi, and Stepnowski Citation2009), physiological metabolism (Austin et al. Citation2003), membrane system (Liu et al. Citation2008), neurotransmission (Slotkin et al. Citation2008), and gene expression (Hickey et al. Citation2009; Mollenhauer et al. Citation2009). Moreover, PFAAs have been detected in a variety of environmental media, including drinking water (Xiao, Simcik, Gulliver Citation2013), surface water (Zhou et al. Citation2012; Zhou et al. Citation2013; Sun et al. Citation2011), groundwater (Murakami et al. Citation2009; Loos, Locoro, and Contini Citation2010), atmosphere (Dreyer et al. Citation2010; Webster and Ellis Citation2012), silt (Clarke and Smith Citation2011), soil (Washington et al. Citation2008, Wang et al. Citation2010), sediments (Benskin et al. Citation2011), indoor and outdoor dust (Haug et al. Citation2011), etc. In present review, we aim to summarize and update the information on the occurrence of PFAAs in multiple environmental media and their source as well as the environmental behaviors, toxic effects, and ecological and health risks of PFAAs. We also attempt to identify gaps in knowledge and suggest possible future research perspectives.
The main source of PFAAs
PFAAs are synthetic compounds, so their wide distribution in the environment mainly comes from industrial emissions and their transportation and transformation afterward. The sources of PFAAs in the environment are usually divided into direct pollution and indirect pollution. The situation is considered direct pollution when PFAAs are directly emitted to the surrounding environment during the production, processing, transportation and deposition of PFAA-related materials. Indirect pollution, however, refers to the PFAAs induced by photodegradation, chemical degradation, and biodegradation as well as the dry and wet deposition from volatile PFAAs and their precursors, such as N-perfluorooctane sulfonamide and fluorine polyalcohol (Zareitalabad et al. Citation2013).
Since 1947, when electrochemistry was first used to produce perfluorooctanoic acid (PFOA) by the 3M Company in the United States, eighty percent of the direct pollution of PFAAs on a global scale has been related to the production of fluoropolymers and the emissions caused by using them (Prevedouros et al. Citation2006). The major products were perfluorooctanoic acid ammonium and perfluorononanoate ammonium, accounting for eighty to ninety percent of direct industrial emissions and representing the dominant precursors of PFOA and perfluorooctanesulfonic acid (PFOS). The emissions of factories producing fluoropolymers and the use of fluoropolymer dispersants and fire extinguisher foam products are all responsible for the direct pollution of PFAAs. In addition, products including PFAAs, such as floor polish (Bultman and Pike Citation1981), cleaning formulas (Asahi Citation1982), hair care products (Cella et al. Citation1976), ink (Zoellner-Braue Citation1995), medical inhalers, fuel additives (Schultz and Quessy Citation1992), paper, air fresheners (Lion-Corporation Citation1988) and textile processing (Enders Citation1961), are all able to result in direct pollution. The main production of perfluorinated compounds was located in European and American developed countries, such as Belgium and Italy, before 2003. By 2004, the global production of perfluorooctanoic acid ammonium was approximately 3600–5700 tons, and the total direct emission was approximately 400–700 tons (Zareitalabad et al. Citation2013). Since companies such as 3M gradually stopped the production of perfluoroalkyl acids, Chinese companies entered the business due to the huge demand both inside and outside of the country, thus becoming the main source of PFAAs in China (Wang et al. Citation2015). The emission intensity of PFOA and PFOS in the Yangtze River, China was estimated to be 39.2 tons per year and 0.807 tons per year (Chen et al. Citation2011), respectively. The emission of PFOS in China in 2010 was estimated to be 70 tons (Xie et al. Citation2013). The indirect pollution of PFAAs comes from a wide variety of sources, the main ones of which are the perfluorooctane sulfonyl products produced by electrochemistry fluorination. According to the difference in synthesis methods and raw materials, the impurities could include homologs, with a carbon number ranging from four to thirteen (Benskin, De Silva, and Martin Citation2010). Fluoropolymers produced by telomerization may include a trace amount of PFAAs as impurities. N-ethyl perfluorooctane sulfonamidoethanol (N-EtFOSE) has been reported to be able to biodegrade to PFOA, or to degrade to PFOS, in activated sludge (Lange Citation2000, Rhoads et al. Citation2008). The raw materials of fluoropolymers may transform to PFAAs during processing. Since the high vapor pressure of fluorine polyalcohol makes them easy to vaporize (Krusic et al. Citation2005), those fluorine polyalcohols in the atmosphere can react with water vapor and nitrogen oxides (NOx) and eventually form PFCAs (Ellis et al. Citation2004), resulting in the long-distance transportation of PFAAs.
The multi-media distribution of PFAAs
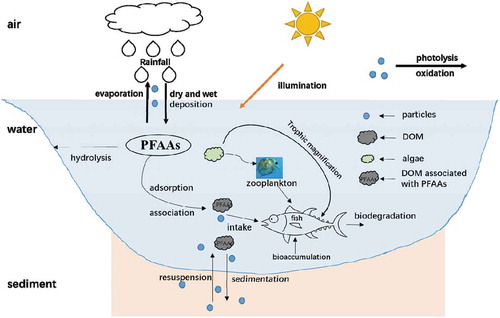
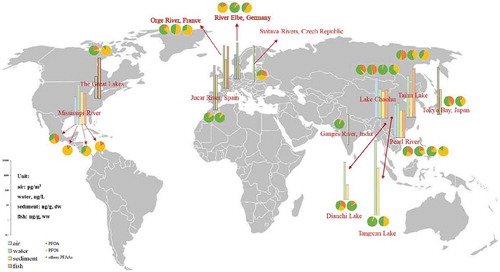
Due to the physicochemical characteristic of PFAAs, they are most likely to exist in the aqueous phase as ions, thus making their transportation with water flow relatively slow. However, the precursor materials of PFAAs, such as chlorodifluoromethane (HCFC), hydrofluorocarbons (HFCs), and fluorine polyalcohol, are able to vaporize and form PFAAs through photodegradation and oxidation; then, these compounds enter water bodies and sediments through diffusion and wet and dry deposition and eventually become widely distributed in various environmental media. shows the global distributions of PFAAs in environmental media. (Table S1 for more details).
Figure 2. The occurrence of PFAAs in the global environmental media (including air, water, sediment, and fish)
Distribution of PFAAs in the atmosphere
PFAAs almost always exist as ions in the aqueous phase and are difficult to vaporize. However, the wide distribution of PFAAs in the atmosphere has been reported in large amount of research. Perfluoroaldehyde (CxF2x+1CHO) and fluorine polyaldehyde (CxF2x+1CH2CHO) have been reported to form PFCAs through photodegradation (Chiappero et al. Citation2006). Volatile HCFCs and N-methyl perfluorobutane sulfonamidoethanol (NMeFBSE) can produce perfluorobutanesulfonic acid (PFBS) and PFCAs through atmospheric oxidation (D’eon et al. Citation2006). Fluorotelomer alcohols (FTOHs) can produce homologous PFCAs through degradation in the atmosphere, resulting in the wide spread of PFCAs (Ellis et al. Citation2004). Moreover, the degradation of PFAAs in the atmosphere is not only influenced by gas phase reactions but is also related to the rate of dry and wet deposition (Hurley et al. Citation2004).
Considering the low volatility of PFAAs, previous research on perfluorinated compounds in the atmosphere has been focused on their volatile precursors, such as fluorine polyalcohol, methyl perfluorobutyl sulfonamide, methyl perfluorooctane sulfonamide and ethyl perfluorooctane sulfonamide (Shoeib et al. Citation2010, Ahrens et al. Citation2011a, Cai et al. Citation2012, Muller et al. Citation2012, Lai et al. Citation2016). The research on PFCAs and PFSAs is relatively limited. Barber et al. reported the levels of PFAAs and fluorine polyalcohol in the atmosphere in the northwest of Europe (England, Ireland and Norway) (Barber et al. Citation2007). Kim et al. reported the level of PFAAs in the United States (Kim and Kannan Citation2007). Dreyer et al. reported the concentrations of PFAAs and their precursors in the atmosphere of Hamburg City, Germany, where PFAAs were only detected in the particle phase (Dreyer et al. Citation2009). Wang et al. investigated the perfluorinated compounds and fluorotelomer in the atmosphere of Büsum, a town in northern Germany (Wang et al. Citation2014). Liu et al. reported the distribution of gaseous PFAAs in the atmosphere of Shenzhen, China (Liu et al. Citation2015a). In addition, focusing on the potential risks to human health, the levels of PFOA and PFOS in indoor and outdoor dust have also been studied (Moriwaki, Takata, and Arakawa Citation2003, Murakami and Takada Citation2008, Strynar and Lindstrom Citation2008, Zhang et al. Citation2010, Fraser et al. Citation2013).
According to these results, the PFAAs in the atmosphere are mainly composed of short carbon chain PFCAs. The concentration of PFAAs in the atmosphere of Manchester and Hazelrigg in the United Kingdom were much higher (up to 574 pg/m3, with PFOA as predominant contaminant). In addition, the PFAAs levels in the atmosphere of Lake Chaohu and the city of Tianjin were also higher, while the concentrations of PFAAs in the rest of the regions were lower. This might be due to the fact that there is a fluoropolymer production plant near Manchester and Hazelrigg. Although there are no fluorination plants around Chaohu and Tianjin, the two large fluorine industrial parks approximately 300 km away could be the source of pollutants in their atmosphere. Inland or rural areas, which are far from point source emissions, may be affected by long-range atmospheric transmission.
Distribution of PFAAs in water bodies
PFAAs are most likely to exist as ions in the aqueous phase. In addition, PFAAs in the atmosphere can enter water bodies through dry and wet deposition and air-water exchange. PFAAs in soils can be washed away through precipitation and end up in all types of water bodies following surface runoff. PFAAs in water bodies have been investigated worldwide, especially in rivers near point sources of pollution, such as the Tennessee River in the United States (Hansen et al. Citation2002); the River Po in Italy (Loos et al. Citation2008); the River Elbe in Germany (Ahrens et al. Citation2009a); the Danube River in Europe (Loos, Locoro, and Contini Citation2010); the Svitava River and Svratka River in the Czech Republic (Kovarova et al. Citation2012); the Llobregat River in Spain (Flores et al. Citation2013); the Kamo River and Uji River in Japan (Senthilkumar et al. Citation2007); the Chao Phraya River and Bangpakong River in Thailand (Kunacheva et al. Citation2009); the Yamuna River in India (Yeung et al. Citation2009); and the Pearl River and Yangtze River (So et al. Citation2007), Xiaoqing River (Beijing) (Zhao et al. Citation2007), Yellow River, Majia River, Tuhai River, Xiaoqing River (Shandong), and Mi River (Wang et al. Citation2014a) in China.
The studies about PFAAs in lake systems are relatively limited compared with those on rivers, and the majority of them are focused on several main lakes, such as the Great Lakes (Boulanger et al. Citation2004, De Silva et al. Citation2011), Lake Victoria in Kenya (Orata et al. Citation2009), Tokyo Bay (Taniyasu et al. Citation2003), Taihu Lake (Qiu, Jing, and Shi Citation2010, Yang, Zhu, and Liu Citation2011, Yu et al. Citation2013, Chen et al. Citation2015, Guo et al. Citation2015), Lake Chaohu (Gong et al. Citation2015), Tangxun Lake (Zhou et al. Citation2013), Nansi Lake in Shandong (Cao et al. Citation2015), and Dianchi Lake in Yunnan (Zhang et al. Citation2012).
The PFAAs in the water were mainly composed of PFOA and other short carbon chain PFCAs. The PFOS content in some samples, such as the Mississippi River in the United States, was higher, which was related to the point source emission of PFOS from the 3M factory. Due to the lack of water quality benchmarks, it is impossible to assess the degree of pollution of PFAAs in water quality, however, it can be seen from previous studies that point source emission influenced the contamination of PFAAs in the water directly. The PFAAs in the Mississippi River with point source emissions were approximately 369 ng/L. In Xiaoqinghe River, the PFAAs were detected up to 5069 ng/L near the fluorination plant, while up to 9850 ng/L of PFAAs were detected in the TangXun Lake, which was a sewage lake. In addition, PFAA levels in other natural waters were relatively lower.
The distribution of PFAAs in the sediments
Sediment is an important sink of neutral organic pollutants, where pollutants can be absorbed by suspended particles and then settle down into sediment or can also be desorbed into water through resuspension. However, due to both the hydrophobic and hydrophilic functional groups of PFAAs, their behaviors in the sediment are different from those of traditional persistent organic pollutants (POPs) (Nakata et al. Citation2006). By studying sediment, we can further understand the environmental behaviors of PFAAs in the aqueous phase.
A wide range of studies has been focused on the PFAAs in sediment worldwide, such as in the Ariake Sea in Japan (Nakata et al. Citation2006), the Kamo River, Uji River (Senthilkumar et al. Citation2007), and Main River in Germany (Becker, Gerstmann, and Frank Citation2008), the Savannah River in the United States (Kumar et al. Citation2009), pore water, surface sediment and columnar sediment in Tokyo Bay (Ahrens et al. Citation2009b, Citation2010), Lake Opabin and Lake Oesa in Canada (Benskin et al. Citation2011), surface and columnar sediment in Lake Ontario (Yeung et al. Citation2013), and sediment of various rivers and lakes in Korea (Lam et al. Citation2014). Compared to that from foreign countries, research on sediment in China started later, where the earliest report on PFAAs in sediment was carried out by Bao et al. by studying the Daliao River (Bao et al. Citation2009). Subsequent research has been carried out by studying several major rivers flowing through industrial factories, such as the part of Huangpu River and Pearl River located in Guangzhou (Bao et al. Citation2010), the Yangtze River (Pan and You Citation2010), and multiple rivers in Laizhou Bay (Zhao et al. Citation2013). The research on lake sediments has occurred only recently in China, focusing on Taihu Lake (Yang, Zhu, and Liu Citation2011, Chen et al. Citation2015), Dianchi Lake (Zhang et al. Citation2012), Tangxun Lake in Hubei (Zhou et al. Citation2013), and Nansi Lake in Shangdong (Cao et al. Citation2015). The short carbon chain PFCAs were still dominant in the sediments, while the relative content of PFOS and long carbon chain PFCAs, such as PFUnDA, were increased. Overall, the contamination levels in sediments were lower, except for those in the heavily polluted sediments, such as Tangxun Lake.
The distribution of PFAAs in organisms
Geisy and Kannan first reported the distribution of PFOS in organisms in 2001 (Giesy and Kannan Citation2001). PFOS has been detected in the tissues of fish, birds and marine mammals in polar areas far from cities and industrial factories, indicating that PFOS can bioaccumulate in food chains. After that, PFAAs in organisms have been studied extensively. Kannan et al. reported the concentrations of PFOS and PFOA in mink (liver), river otters, seals, and Atlantic salmon in various places in the United States (Kannan et al. Citation2002a, Citation2002b). Taniyasu et al. reported the concentrations of PFOS in edible eels and flounder in Japan (Taniyasu et al. Citation2003). Kumar et al. reported the concentration of PFAAs in various organisms in the United States. (Kumar et al. Citation2009). Bioaccumulation and biological magnification of PFAAs in both marine and freshwater ecosystems have been indicated (Houde et al. Citation2006b; Liu et al. Citation2018). Conder et al. summarized previous research, reporting that the bioaccumulation of PFAAs is related to the length of carbon chains and that PFSAs are more likely to bioaccumulate than PFCAs are (Conder et al. Citation2008). However, in contrast to the distribution of traditional hydrophobic and lipophilic organic pollutants in organisms, such as organochloride and polycyclic aromatic hydrocarbons, the concentrations of PFAAs are not significantly related to lipid content but to protein content, and lipids may even suppress the accumulation of PFOA and PFOS (Wen et al. Citation2016).
In previous studies, as a type of emerging contaminant, the PFAAs concentrations in organisms were higher than the concentrations of other POPs, such as organochlorine pesticides. The residual levels of PFAAs in the trout in Lake Ontario and Mongolian carp in Taihu Lake were up to 58.58 ng/g ww and 165 ng/g ww, respectively, higher than those in other freshwater, such as Pearl River and Lake Chaohu. In organisms, PFOS was the predominant contaminant, and long carbon chain PFCAs were bioaccumulated in organisms, and the proportion of PFCAs increased with time (Furdui et al. Citation2008). This might be due to the phase out of PFOS around 2002, while there are no restrictions on PFCAs. Long-term monitoring of PFAAs in organisms revealed that the concentration of PFAAs increased first and then decreased (Braune and Letcher Citation2013; Gewurtz et al. Citation2009), which also reflected the emission of PFAAs.
In addition to organisms, PFAAs at different concentrations have been detected widely in the blood, liver, urine, hair, breast milk, cord blood, and nails of both adults and children (Olsen et al. Citation2003, Kuklenyik et al. Citation2004, Karrman et al. Citation2006, So et al. Citation2006, Calafat et al. Citation2007a, Karstadt Citation2007, Olsen et al. Citation2007, Von Ehrenstein et al. Citation2008, Haug, Thomsen, and Bechert Citation2009, Li et al. Citation2013, Zhang et al. Citation2013b, Kim, Lee, and Oh Citation2014, Pinney et al. Citation2014, Zhang et al. Citation2015). Multiple pathways of human exposure to PFAAs have been reported, such as indoor and outdoor dust, drinking water, edible freshwater fish, and seafood (Taniyasu et al. Citation2003, Calafat et al. Citation2007b, Bjorklund, Thuresson, and De Wit Citation2009, Ericson et al. Citation2009), among which dietary exposure, especially the intake of aquatic products, is the main exposure source for humans (Haug et al. Citation2010, Domingo Citation2012). Thus, investigating the distribution of PFAAs in fish can provide more information for health risk assessment of PFAAs.
After the phase out of PFOS and PFOA by 3M, the contamination levels of PFOS were decreased in American and European environment media over last decades. However, in order to meet the industrial demands, the fluorine chemical manufacturers, such as 3M Corporate and Daikin Industries, established new production plants in China. The estimated emission of PFOS were increased from 30 tons per year in 2011 to 240 tons in 2011 (Xie et al. Citation2013), while the release of PFOA was from 17.5 tons in 2004 to 45.3 tons in 2012 (Li et al. Citation2015). In Xiaoqing River, the concentrations of PFAAs increased up to six-fold from 2011 to 2013. In addition, the detection rate and concentrations of PFBA and PFBS in different environmental media were increased. In the middle reach of Yangtze River, the PBFS became predominant contaminant (Tan et al. Citation2018). In some organisms, PFBS was found at higher level than PFOS in Lake Tana (Ahrens et al. Citation2016). This might be due to the production and use of the short carbon chain alternatives such as PFBA and PFBS, after the restrictions of PFOA and PFOS.
The main environmental behavior of PFAAs
The multi-media portioning of PFAAs
Due to the physicochemical characteristics of PFAAs, they are easy to dissociate in the aqueous phase. Although the vapor pressure is lower after dissociation, PFAAs can still be absorbed by particles in the atmosphere or water and can be transported through dry and wet deposition (). The typical partition of PFAAs includes air-solid, water-suspended solid, water-sediment, and suspended solid-sediment partition. The air-solid partition is considered to be one of the major factors in the long-distance transportation of the pollutants. A higher level of PFAAs has been reported in atmospheric particles than that in gas phase, indicating their facile adsorption by atmospheric particles (Barton et al. Citation2006, Jahnke et al. Citation2007, Kim and Kannan Citation2007), which can partially explain the long-distance transportation of PFAAs. The air-solid partition coefficient has been evaluated by Arp et al., indicating that the direct volatility from water to air is not the main source of PFAAs in the gas phase (Arp and Goss Citation2009). The PFAAs in atmosphere above wastewater treatment plants were analyzed and discovered that PFAAs are more likely to be adsorbed by particles compared to existing in the gas phase, and that the ratio of PFCAs in the solid phase is proportional to the length of carbon chains (Vierke et al. Citation2011). Ahrens et al. proved by experiments that the pH of water-soluble aerosols and the pKa of the compounds are the major factors of the air-solid partition coefficient (Ahrens et al. Citation2012). The partition of PFAAs in the water-suspended solid-sediment system is important to the multi-media fate of the compounds. The organic carbon normalized partition coefficient (Koc) has usually been used to quantify the partition of pollutants between the solid and aqueous phases. The adsorption of pollutants by sediment from water was studied as early as 1979 (Karickhoff, Brown, and Scott Citation1979). Ahrens et al. reported the partition of PFAAs between the pore water and sediment in Tokyo Bay, which discovered that PFOS is more likely to be adsorbed by sediment than PFCAs are and that the length of the carbon chain can influence the partition of PFAAs (Ahrens et al. Citation2009b). Furthermore, the organic carbon fraction (foc) of the sediment is also influence factor. When the foc is extremely low, the size of the sediment particle and the surface electrostatic interaction are the major factors in the partition (Johnson et al. Citation2007, Ahrens et al. Citation2011b).
Cross-interface exchange
The cross-interface exchanges in aquatic ecosystems mainly include water-air interface and water-sediment interface exchanges. The air-water partition coefficient (Kaw) is an important parameter affecting the environmental behaviors and fate of pollutants. The Kaw of PFOA was measured in laboratory, ranging from 7.65 × 10−4 to 1.39 × 10−3 (Li, Ellis, and Mackay Citation2007). It has also been reported that the oxygen anions could influence the partition of PFAAs at the air-water interface by studying different aerosols (Jing, Rodgers, and Amemiya Citation2009, Psillakis et al. Citation2009). Vierke et al. calculated the Kaw of PFAAs in wastewater treatment plants according to different pKa reported in previous literatures, and the results are similar to those by Li et al. (Vierke et al. Citation2013). The vapor pressure of water-soluble anions dissociated from PFAAs is very low, while that of neutral PFAAs is relatively high (Barton, Kaiser, and Russell Citation2007, Kaiser et al. Citation2010). The PFAAs accumulated in sediments with the increasing organic matter and the decreasing pH, according to the research on the water-sediment interface, and the length of the carbon chain could obviously influence the partition (Ahrens et al. Citation2009b). In addition, the salinity could also significantly influence the adsorption and desorption of PFAAs by sediment (Pan and You Citation2010).
Degradation
PFAAs have relatively long half-lives in water, and are difficult to degrade autonomously. They can be removed from atmosphere by oxidation, hydrolysis and photolysis after being adsorbed by particles (Hurley et al. Citation2004, McMurdo et al. Citation2008) as well as precipitation and deposition (Arp and Goss Citation2009). A high-frequency ultrasound has been reported to improve the degradation in the groundwater of landfills (Cheng et al. Citation2008) and the biodegradation (Liu and Avendano Citation2013, Weiner et al. Citation2013) of PFAAs. PFOS and short chain PFAAs were also hard to degrade by microbial under both anaerobic and aerobic conditions (Ochoa-Herrera et al. Citation2016). However, due to the stability of PFAAs, it is difficult to degrade them further in the natural environment, and they usually sink to the sediment by adsorbing to particles in the water (Prevedouros et al. Citation2006).
Bioaccumulation and biological magnification
Bioaccumulation factors are usually used to describe the absorption of pollutants by organisms from the environment or food (van der Oost, Beyer, and Vermeulen Citation2003).The bioconcentration factor (BCF) is used to describe the accumulation of pollutants in aquatic organisms through only gill and skin absorption (Veith, Defoe, and Bergstedt Citation1979), and is usually evaluated and calculated based on lab experiments. In the natural environment, however, the accumulation of dissolved pollutants by aquatic organisms is usually described by the bioaccumulation factor (BAF). In addition, the biota-suspended solid accumulation factor (BSSAF) and biota-sediment accumulation factor (BSAF) are used to describe the accumulation of pollutants by organisms from suspended solids and sediment. Giesy and Kannan first reported the distribution of PFAAs in wildlife and proposed bioaccumulation and biomagnification with increasing trophic level (Giesy and Kannan Citation2001). Taniyasu et al. reported the bioaccumulation coefficient of PFOS in fish from different places in Japan, ranging from 274 to 41,600 (Taniyasu et al. Citation2003). Conder et al. summarized the previous research, proposing that the bioaccumulation and biomagnification of PFAAs are directly related to the length of the carbon chains and that PFSAs are easier to bioaccumulate than PFCAs (Conder et al. Citation2008). PFHxS has an exemption half-life time, which is longer than PFOS, however, a linear relationship between bioaccumulation factor (BAF) of PFSAs and carbon number was reported. The BAF value of PFHxS was lower than that of PFOS (Kwadijk, Korytár, and Koelmans Citation2010; Bhavsar et al. Citation2016). In addition, BAF value was also both related to the exposure concentrations of PFAAs, and its relationship with length of the carbon chains has also been proved by experiment (Liu et al. Citation2011). In addition to bioaccumulation, the biomagnification of PFAAs with increasing trophic level and along food chains has been indicated. The trophic magnification of PFAAs has been proved in the food web of trout in Lake Ontario and bottlenose dolphin in Sarasota Bay (Martin et al. Citation2004a, Houde et al. Citation2006a). The bioaccumulation and trophic magnification of PFAAs have been proved in freshwater and marine ecosystems from Taihu Lake and Laizhou Bay in China (Yang et al. Citation2012, Xu et al. Citation2014). Most of the long carbon chain PFCAs, PFBS, and PFOS were correlated with the trophic level significantly, which indicated that the concentrations of PFAAs in aquatic animals increased across trophic levels. The food web magnification factor values of long carbon chain PFCAs and all PFSAs were greater than 1.0, indicating obvious biomagnification for PFAAs in aquatic animals.
The toxicity of PFAAs
Since PFOS was detected in various wildlife in 2001 (Giesy and Kannan Citation2001), multiple studies have been carried out about the toxicity of PFAAs. shows the toxicities in different species. It has been reported that PFOS and most PFCAs can significantly suppress the population of algae such as Chlorella vulgaris (Desjardins et al. Citation2001, Boudreau et al. Citation2003, Sanderson et al. Citation2003). PFOS can suppress the growth of algae by increasing the permeability of the cell membrane and the membrane potential of mitochondria (Liu et al. Citation2008). A linear relationship between the length of carbon chains and the toxicity toward algae was proved, by exposing PFAAs to green algae, diatoms, and cyanobacteria, and the toxicity doubles with one more perfluoromethylene unit (Latala, Nedzi, and Stepnowski Citation2009). PFOA can also increase the fluorescent intensity of the membrane of Scenedesmus obliquus, resulting in dysfunction and physiological abnormalities of the algae cell membrane (Feng et al. Citation2010).
Table 1. The toxicity of PFAAs toward different species
According to the toxicity test of zooplankton, PFOA can significantly influence the population density of water flee (Sanderson et al. Citation2003). PFOS may have a greater impact on Chironomid larvae than PFOA does, suggesting that PFOS may be associated with the oxygen stress response (MacDonald et al. Citation2004) The EC50 and NOEC values of six PFCAs in Daphnia were calculated, and they were inversely proportional to the length of carbon chains of PFAAs (Ding et al. Citation2012). PFOS was approximately 2.5-fold more toxic to B. calyciflorus than PFOA and can affect the size of individuals and eggs and this toxicity is hereditary (Zhang et al. Citation2013a). PFCAs with long carbon chains, such as PFNA and PFDA, can significantly inhibit the p-gp transport proteins in mussels, but the inhibition is reversible as PFNA decreases (Stevenson et al. Citation2006). PFOS can cause oxidative damage to emerald mussels, and the hereditary damage is irreversible for long-term exposure (Wang et al. Citation2012). PFNA and PFDA also can weaken the internal defense system of green mussels, which shows the immunotoxicity (Liu and Gin Citation2018).
Toxicity tests of fish showed that PFOA can damage the activity of estrogen by inducing the estrogen response genes in the male Gobiocypris rarus (Wei et al. Citation2007). PFOS can alter the gene expression of the hypothalamic-pituitary-thyroid (HPT) axis of zebrafish and disrupt the synthesis and regulation of thyroid hormones (Shi et al. Citation2009). PFOS can also suppress the activity of efflux transport proteins in zebrafish as a chemosensitizer (Keiter et al. Citation2016).
In addition, PFAAs have been reported to be toxic and carcinogenic toward the liver as well as the development and immunity of mammals, and the compounds may also interfere with hormone levels in mammals. At different exposure doses, PFOA and PFOS can activate the peroxisome proliferator activated receptor-α (PPAR-α) to different extents (Takacs and Abbott Citation2007), while the activation of PPAR-α may induce liver tumors in rodents. Seacat et al. reported the subchronic toxicity of cynomolgus monkeys, discovering damage to the liver caused by long-term exposure to PFOS (Seacat et al. Citation2002). Exposing pregnant mice to PFOA can cause delayed development, including an increased time to open eyes and hair growth, and the effect can be even more significant if the exposure happened at the beginning of gestation (Wolf et al. Citation2007). The exposure to PFOA in mice can cause a decrease in the titer of the lgM antibody, which is related to their adaptive immune response (DeWitt et al. Citation2008). In addition, PFOA can suppress the genes responsible for the biosynthesis of thyroid hormones, significantly induce the estrogen responsive genes, and interfere with the endocrine system (Wei et al. Citation2008). PFOA may also decrease testosterone levels and increase estradiol levels (Lau et al. Citation2007). PFOA may also alter the production of steroid hormones or act indirectly through ovarian effects; it can interfere with the endocrine system and affect growth and sex hormones, including the activation of estrogen receptors (White, Fenton, and Hines Citation2011, Du et al. Citation2013), however, no evidence showed that the alternatives of PFOA and PFOS, including PFBA, PFHxA, PFBS, and PFHxS, could affect estrogen or androgen receptor signaling or steroid hormone biosynthesis at present exposure level (Behr et al. Citation2018).
So far, although the toxicity mechanism of the PFAAs on human is still not clear, epidemiological investigations of PFAAs have been reported widely, indicating that the exposure of PFAAs may be related to functional thyroid disease (Melzer et al. Citation2010, Winquist and Steenland Citation2014). The concentration of PFOA in plasma is an independent factor of increasing vascular disease risks (Shankar, Xiao, and Ducatman Citation2012).
Ecological and health risk assessment of PFAAs
Ecological risk of PFAAs
Ecological risk assessment includes evaluating the extent, scale, and the possibility of ecological harm after pollutants enter water bodies (Yin Citation1995). In 1998, the United States Environmental Protection Agency (USEPA) published the guidelines for ecological risk assessment (U.S. Environmental Protection Agency Citation1998), establishing the ecological risk assessment system. The risk assessment of aquatic ecosystems mainly focuses on the water and the sediment. The risk quotient (RQ) method and the species sensitivity distribution (SSD) method are two commonly used methods for ecological risk assessment.
The RQ method is the simplest and most widely used method. It measures the risks in water or sediment by calculating the ratio of pollutant concentrations and the environmental criteria. At present, studies focused on the ecological risk of PFAAs in water and sediment are limited, and the calculation of quotient value is the most widely used method (Lv et al. Citation2008, Mhadhbi et al. Citation2012, Gong et al. Citation2015). The ecological risks of PFAAs are predicted by the ratio of the measured environmental exposure and the predicted no effect concentration (PNEC) or no observed effect concentration (NOEC). When the ratio is less than 1, it is generally believed that the pollutants are relatively safe for the ecological environment; otherwise, the greater the ratio is, the higher the risk.
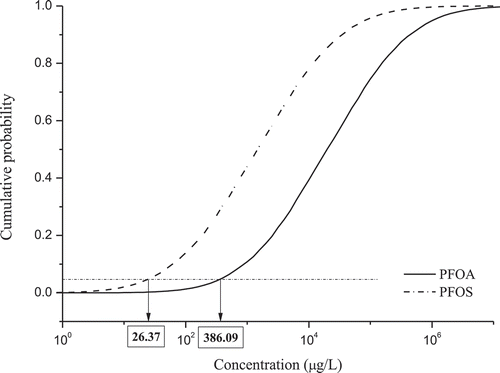
The SSD method was first proposed by USEPA in 1978 as part of the water quality criteria. The SSD curve is plotted according to the toxicity data of certain species, and the ecological risk is obtained according to the environmental exposure and the SSD curve. Researchers from Europe and the United States discussed their research results at the OECD conference in 1990, named the evaluation method SSD, and determined SSD to be an ecological modeling method (van Straalen and van Leeuwen Citation2002). SSD is widely used in the ecological risk assessment of persistent pollutants (Liu et al. Citation2012, He et al. Citation2013, Qin et al. Citation2013). However, the toxicity data of PFAAs except PFOA and PFOS were not sufficient, so only the risks of PFOA and PFOS were assessed. shows the comparison of SSD curves between PFOA and PFOS. According to the toxicity data of PFOA and PFOS provided by EPA Ecotox database (www.epa.gov/ecotox), the HC5 concentrations of PFOA and PFOS (hazardous concentration at 5% of species to protect 95% species) were 386.09 μg/L and 26.37 μg/L, respectively. It can be seen that the HC5 concentration of PFOS is lower than that of PFOA, indicating that the toxicity of PFOS is higher.
The SSD method was employed to discuss the ecological risks of PFOS and PFOA in different seasons and at different sampling points in Lake Chaohu and the influence of different distribution models on a SSD curve was compared (Liu et al. Citation2015c). In addition, SSD model was applied to predict the no-observed effect concentrations of PFOS and PFOA at levels of 35.16 µg/L and 2546 µg/L, respectively (Qi et al. Citation2011; Gredelj et al. Citation2018). The ecological risk of PFAAs was low at present exposure level, which were three orders of magnitude lower than HC5 or PNEC.
Health risk of PFAAs
Up to 12.8 mg/ml of PFOS were detected in the serum of workers at the factories producing fluorinated compounds in 1999 (Olsen et al. Citation1999). Subsequently, PFAAs at different concentrations were detected in the serum, urine, breast milk, hair, and nails of both adults and children (Olsen et al. Citation2003, Kuklenyik et al. Citation2004, Karrman et al. Citation2006, Calafat et al. Citation2007a, Karstadt Citation2007, Olsen et al. Citation2007, Von Ehrenstein et al. Citation2008, Haug, Thomsen, and Bechert Citation2009, Li et al. Citation2013, Zhang et al. Citation2013b, Kim, Lee, and Oh Citation2014, Pinney et al. Citation2014, Zhang et al. Citation2015). Human exposure to PFAAs may be derived from indoor and outdoor dust, drinking water, edible freshwater fish, and seafood (Taniyasu et al. Citation2003, Calafat et al. Citation2007b, Bjorklund, Thuresson, and De Wit Citation2009, Ericson et al. Citation2009), among which the consumption of aquatic products is the main source (Haug et al. Citation2010). In addition, PFOA and PFOS can be transferred in utero through the umbilical cord blood (Olsen, Butenhoff, and Zobel Citation2009). Breast milk is the main source of PFAAs in infants (So et al. Citation2006).
Currently, only the reference doses (RfD) of PFOA and PFOS have been reported. The lack of dose-response relationships of other PFAAs limits research on their health risks. The present studies about the health risks of PFAAs have been focused on the consumption of edible aquatic products (fish) by calculating the ratio of exposure levels (i.e., the acceptable daily intake, ADI, or total daily intake, TDI) and RfD (So et al. Citation2006, Wang et al. Citation2010, Zhang et al. Citation2010).
The estimated daily intake (EDI) of adults through diet were various from different areas. Su et al., found that the EDI value of PFAAs (including PFBA, PFOA, and PFOS) was up to 310 ng/kg bw/day only through eating homemade eggs ranged around a fluorochemical industrial park in China (Su et al. Citation2017), while at a low exposure scenario, the EDI was 479 ng/kg bw/day through fifteen subgroups of food (including meat, egg, vegetables, milk rice, etc.) (Wang, Zhang, and Zhang Citation2017). The EDI of PFOA and PFOS through fish consumption ranged from 0.02 to 5.05 ng/kg bw/day and 0.04 to 17 ng/kg bw/day in European countries (Hölzer et al. Citation2011, Noorlander et al. Citation2011; Domingo et al. Citation2012; Munschy et al. Citation2013; Rivière et al. Citation2014; Vestergren et al. Citation2012; Johansson et al. Citation2014; Squadrone et al. Citation2015). The study of health risk of PFAAs through respiratory exposure is limited. Zhang et al. reported the estimated daily intake (EDI) of indoor dust in Beijing and Shanghai, China (Zhang et al. Citation2010). The health risks of PFOA and PFOS by respiratory exposure in Shenzhen and Lake Chaohu were reported (Liu et al. Citation2015a, Citation2018). Overall, the health risk of PFAAs exposed to general population (non-occupational exposure) was lower than the acceptable level recommended by USEPA.
Multimedia fate modeling
The multi-media fate model is a mathematical model developed in the 1980s and emphasizes the environmental conditions as well as the properties of the pollutants, which determine the distribution, mobility, and transformation of pollutants in various environmental media. The concept of fugacity was proposed by Lewis in 1901 to describe the balance distribution of substances in various phases (Mackay and Paterson Citation1981). Mackay first applied the concept of fugacity to a multi-media model for predicting the distribution of organic compounds in the environment of an ecosystem (Mackay Citation1979), and a quantitative water-air-sediment-interaction model (QWASI) was proposed to describe the fate of chemicals in Lake Ontario (Mackay, Joy, and Paterson Citation1983). After that, many studies on the migration, transformation, and dynamic distribution of typical POPs such as polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides (OCPs) in various environmental media model base on modified QWASI framework were reported. The framework is shown in . It contains water, sediment, suspended solids, and air. The environmental process such as advection, evaporation, sedimentation, resuspension and occurrence of sediments, dry and wet deposition of air, and degradation of chemicals are considered. The relative rates of each process are compared and the mathematical equations for establishing the model in steady state and non-steady state are given.
Figure 5. The framework of QWASI model
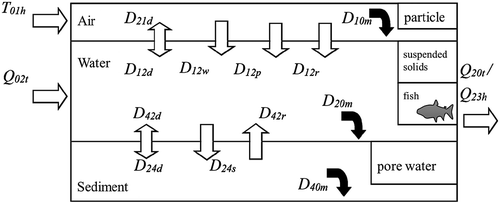
Multilevel fugacity models are widely used. Wania and Mackay established a global distribution model based on fugacity for POPs, such as hexachlorobenzene (HCB), gamma-hexachlorocyclohexane (γ-HCH) and dichlorodiphenyltrichloroethane (DDT), were simulated and validated (Wania and Mackay Citation1995). The distribution of γ-HCH in Liaohe Basin from 1952 to 2001 was simulated using a level IV model (Liu, Quan, and Yang Citation2007). Ao et al., and Dong et al., studied the large-scale temporal variations of HCH isomers in the Yellow River Basin and Lanzhou using similar methods, respectively (Ao et al. Citation2009, Dong et al. Citation2009). The level IV multimedia fate model was used to successfully simulate the spatial and seasonal variations of PAHs in Haihe River Plain within one year (Wang et al. Citation2011a). At present, there is a lack of studies on the migration and transformation of PFAAs using the multimedia fugacity model: Liu et al. simulated the migration of benzo(a)pyrene and PFOS in Bohai Sea region on the regional scale using the spatial multimedia migration model (Liu et al. Citation2015b); Cui et al. used a level III multimedia fugacity model to simulate the migration of PFOS in Shenzhen (Cui et al. Citation2016); Qin et al. applied the IV-level model to simulate the temporal trend and fate of PAHs in Lake Chaohu (Qin Citation2013); and Kong et al. first evaluated the fate, transport, and transformation of PFOS and PFOA in Lake Chaohu using fugacity-based multimedia fate model (Kong et al. Citation2018). However, the model is constructed based on the physicochemical properties of the chemical and the environmental meteorological conditions of the study area. Since PFAAs are structurally hydrophobic and oleophobic, and have surface activity. The physical and chemical parameters such as Kow (octanol-water partition coefficient), Koa (octanol-air partition coefficient), PL (vapor pressure), and Henry’s constant are not easily determined by experiments. If the method can be improved to obtain the measured physical and chemical parameters, it can be reduced the uncertainty due to model calculation parameters. In addition, it would be better if the protein content in the organism and the mineral composition in the sediments, were considered when analyzing the distribution balance of PFAAs in water-organisms and water-environmental media.
Conclusions and perspectives
This review summarizes the studies on the occurrence, partition, toxicity, and risks of PFAAs. PFAAs enter the environment through the direct emission of fluorine industry parks or the use of related production, and spread widely through their environmental behavior such as degradation, partition, or bioaccumulation, and eventually sink in sediments or animals. The application of multimedia fate model on the simulation of PFAAs was reviewed. Due to high toxicity and bioaccumulation of PFAAs, their ecological and health risks are concerned. Moreover, with the increasing use of the perfluorinated alternatives, such as PFBA and PFBS, the toxicities of short carbon chain PFAAs, should be studied.
Based on the previous studies on PFAAs, experiment methods for the properties of PFAAs, such as Kow, vapor pressure, and Henry constant, should be further developed. These parameters are important for the simulation of transport, transformation and fate of PFAAs using multimedia fate model, and they can reduce the uncertainty of the model. Otherwise, the toxicity data of PFAAs are still rare. The individual exposure to PFAAs was reported; however, due to the lack of dose-response data of PFAAs, the carcinogenic risks of PFAAs have not evaluated yet. Therefore, the toxicity of PFAAs should be concerned for further study.
Supplemental Material
Download MS Word (20.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- 3M. 2010. What is 3M Doing? February 14. http://solutions.3m.com/wps/portal/3M/en_US/PFOS/PFOA/Information/Action/
- Ahrens, L., H. Gashaw, M. Sjöholm, S. Gebrehiwot, A. Getahun, E. Derbe, K. Bishop, and S. Åkerblom. 2016. “Poly- and Perfluoroalkylated Substances (Pfass) in Water, Sediment and Fish Muscle Tissue from Lake Tana, Ethiopia and Implications for Human Exposure.” Chemosphere 165: 352–357. doi:10.1016/j.chemosphere.2016.09.007.
- Ahrens, L., L. W. Y. Yeung, S. Taniyasu, P. K. S. Lam, and N. Yamashita. 2011b. “Partitioning of Perfluorooctanoate (PFOA), Perfluorooctane Sulfonate (PFOS) and Perfluorooctane Sulfonamide (PFOSA) between Water and Sediment.” Chemosphere 85: 731–737. doi:10.1016/j.chemosphere.2011.06.046.
- Ahrens, L., M. Shoeib, S. Del Vento, G. Codling, and C. Halsall. 2011a. “Polyfluoroalkyl Compounds in the Canadian Arctic Atmosphere.” Environmental Chemistry 8: 399–406. doi:10.1071/EN10131.
- Ahrens, L., N. Yamashita, L. W. Y. Yeung, S. Taniyasu, Y. Horii, P. K. S. Lam, and R. Ebinghaus. 2009b. “Partitioning Behavior of Per- and Polyfluoroalkyl Compounds between Pore Water and Sediment in Two Sediment Cores from Tokyo Bay, Japan.” Environmental Science & Technology 43: 6969–6975. doi:10.1021/es901213s.
- Ahrens, L., S. Felizeter, R. Sturm, Z. Y. Xie, and R. Ebinghaus. 2009a. “Polyfluorinated Compounds in Waste Water Treatment Plant Effluents and Surface Waters along the River Elbe, Germany.” Marine Pollution Bulletin 58: 1326–1333. doi:10.1016/j.marpolbul.2009.04.028.
- Ahrens, L., S. Taniyasu, L. W. Y. Yeung, N. Yamashita, P. K. S. Lam, and R. Ebinghaus. 2010. “Distribution of Polyfluoroalkyl Compounds in Water, Suspended Particulate Matter and Sediment from Tokyo Bay, Japan.” Chemosphere 79: 266–272. doi:10.1016/j.chemosphere.2010.01.045.
- Ahrens, L., T. Harner, M. Shoeib, D. A. Lane, and J. G. Murphy. 2012. “Improved Characterization of Gas-Particle Partitioning for Per- and Polyfluoroalkyl Substances in the Atmosphere Using Annular Diffusion Denuder Samplers.” Environmental Science & Technology 46: 7199–7206. doi:10.1021/es300898s.
- UNEP. 2015. Proposal to list pentadecafluorooctanoic acid (CAS No: 335-67-1, PFOA, perfluorooctanoic acid), its salts and PFOA-related compounds in Annexes A, B and/or C to the Stockholm Convention on Persistent Organic Pollutants. http://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-POPRC.11-5.English.pdf
- UNEP. 2017. Proposal to list perfluorohexane sulfonic acid (CAS No: 355-46-4, PFHxS), its salts and PFHxS-related compounds in Annexes A, B and/or C to the Stockholm Convention on Persistent Organic Pollutants. http://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-POPRC.13-4.English.pdf
- Ao, J., J. Chen, F. Tian, and X. Cai. 2009. “Application of a Level IV Fugacity Model to Simulate the Long-Term Fate of Hexachlorocyclohexane Isomers in the Lower Reach of Yellow River Basin, China.” Chemosphere 74: 370–376. doi:10.1016/j.chemosphere.2008.09.085.
- Arp, H. P. H., and K. U. Goss. 2009. “Gas/Particle Partitioning Behavior of Perfluorocarboxylic Acids with Terrestrial Aerosols.” Environmental Science & Technology 43: 8542–8547. doi:10.1021/es901864s.
- Asahi 1982. Cleaning compositions; glass cleaner.JP57119999. Japanese Patent, Asahi Glass Co. Ltd.
- Austin, M. E., B. S. Kasturi, M. Barber, K. Kannan, P. S. MohanKumar, and S. M. J. MohanKumar. 2003. “Neuroendocrine Effects of Perfluorooctane Sulfonate in Rats.” Environmental Health Perspectives 111: 1485–1489. doi:10.1289/ehp.6128.
- Bao, J., W. Liu, L. Liu, Y. H. Jin, X. R. Ran, and Z. X. Zhang. 2010. “Perfluorinated Compounds in Urban River Sediments from Guangzhou and Shanghai of China.” Chemosphere 80: 123–130. doi:10.1016/j.chemosphere.2010.04.008.
- Bao, J., Y. H. Jin, W. Liu, X. R. Ran, and Z. X. Zhang. 2009. “Perfluorinated Compounds in Sediments from the Daliao River System of Northeast China.” Chemosphere 77: 652–657. doi:10.1016/j.chemosphere.2009.08.018.
- Barber, J. L., U. Berger, C. Chaemfa, S. Huber, A. Jahnke, C. Temme, and K. C. Jones. 2007. “Analysis of Per- and Polyfluorinated Alkyl Substances in Air Samples from Northwest Europe.” Journal of Environmental Monitoring 9: 530–541. doi:10.1039/b701417a.
- Barton, C. A., L. E. Butler, C. J. Zarzecki, J. Flaherty, and M. Kaiser. 2006. “Characterizing Perfluorooctanoate in Ambient Air near the Fence Line of a Manufacturing Facility: Comparing Modeled and Monitored Values.” Journal of the Air & Waste Management Association 56: 48–55. doi:10.1080/10473289.2006.10464429.
- Barton, C. A., M. A. Kaiser, and M. H. Russell. 2007. “Partitioning and Removal of Perfluorooctanoate during Rain Events: The Importance of Physical-Chemical Properties.” Journal of Environmental Monitoring 9: 839–846. doi:10.1039/b703510a.
- Becker, A. M., S. Gerstmann, and H. Frank. 2008. “Perfluorooctanoic Acid and Perfluorooctane Sulfonate in the Sediment of the Roter Main River, Bayreuth, Germany.” Environmental Pollution 156: 818–820. doi:10.1016/j.envpol.2008.05.024.
- Begley, T. H., K. White, P. Honigfort, M. L. Twaroski, R. Neches, and R. A. Walker. 2005. “Perfluorochemicals: Potential Sources of and Migration from Food Packaging.” Food Additives & Contaminants 22: 1023–1031. doi:10.1080/02652030500183474.
- Behr, A. C., D. Lichtenstein, A. Braeuning, A. Lampen, and T. Buhrke. 2018. “Perfluoroalkylated Substances (PFAS) Affect neither Estrogen and Androgen Receptor Activity nor Steroidogenesis in Human Cells in Vitro.” Toxicology Letters 291: 51–60. doi:10.1016/j.toxlet.2018.03.029.
- Benskin, J. P., A. O. De Silva, and J. W. Martin. 2010. “Isomer Profiling of Perfluorinated Substances as A Tool for Source Tracking: A Review of Early Findings and Future Applications.” Reviews of Environmental Contamination and Toxicology: Perfluorinated Alkylated Substances 208: 111–160. D. M. Whitacre and P. DeVoogt. New York, Springer.
- Benskin, J. P., V. Phillips, V. L. St Louis, and J. W. Martin. 2011. “Source Elucidation of Perfluorinated Carboxylic Acids in Remote Alpine Lake Sediment Cores.” Environmental Science & Technology 45: 7188–7194. doi:10.1021/es2011176.
- Bhavsar, S. P., C. Fowler, S. Day, S. Petro, N. Gandhi, S. B. Gewurtz, C. H. Hao, X. M. Zhao, K. G. Drouillard, and D. Morse. 2016. “High Levels, Partitioning and Fish Consumption Based Water Guidelines of Perfluoroalkyl Acids Downstream of a Former Firefighting Training Facility in Canada.” Environment International 94: 415–423. doi:10.1016/j.envint.2016.05.023.
- Bjorklund, J. A., K. Thuresson, and C. A. De Wit. 2009. “Perfluoroalkyl Compounds (Pfcs) in Indoor Dust: Concentrations, Human Exposure Estimates, and Sources.” Environmental Science & Technology 43: 2276–2281. doi:10.1021/es803201a.
- Boudreau, T. M., P. K. Sibley, S. A. Mabury, D. G. C. Muir, and K. R. Solomon. 2003. “Laboratory Evaluation of the Toxicity of Perfluorooctane Sulfonate (PFOS) on Selenastrum Capricornutum, Chlorella Vulgaris, Lemna Gibba, Daphnia Magna, and Daphnia Pulicaria.” Archives of Environmental Contamination and Toxicology 44: 307–313. doi:10.1007/s00244-002-2102-6.
- Boulanger, B., J. Vargo, J. L. Schnoor, and K. C. Hornbuckle. 2004. “Detection of Perfluorooctane Surfactants in Great Lakes Water.” Environmental Science & Technology 38: 4064–4070. doi:10.1021/es0496975.
- Braune, B., and R. Letcher. 2013. “Perfluorinated Sulfonate and Carboxylate Compounds in Eggs of Seabirds Breeding in the Canadian Arctic: Temporal Trends (1975–2011) and Interspecies Comparison.” Environmental Science & Technology 47 (1): 616–624. doi:10.1021/es303733d.
- Bultman, D. A., and M. T. Pike. 1981. “The Use of Fluorochemical Surfactants in Floor Polish.” Chemical Times and Trends 4: 41–44.
- Cai, M. H., Z. Y. Xie, A. Moller, Z. G. Yin, P. Huang, M. G. Cai, H. Z. Yang, R. Sturm, J. F. He, and R. Ebinghaus. 2012. “Polyfluorinated Compounds in the Atmosphere along a Cruise Pathway from the Japan Sea to the Arctic Ocean.” Chemosphere 87: 989–997. doi:10.1016/j.chemosphere.2011.11.010.
- Calafat, A. M., L. Y. Wong, Z. Kuklenyik, J. A. Reidy, and L. L. Needham. 2007b. “Polyfluoroalkyl Chemicals in the US Population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and Comparisons with NHANES 1999–2000.” Environmental Health Perspectives 115: 1596–1602. doi:10.1289/ehp.10598.
- Calafat, A. M., Z. Kuklenyik, J. A. Reidy, S. P. Caudill, J. S. Tully, and L. L. Needham. 2007a. “Serum Concentrations of 11 Polyfluoroalkyl Compounds in the US Population: Data from the National Health and Nutrition Examination Survey (NHANES) 1999–2000.” Environmental Science & Technology 41: 2237–2242. doi:10.1021/es062686m.
- Cao, Y. X., X. Z. Cao, H. Wang, Y. Wan, and S. L. Wang. 2015. “Assessment on the Distribution and Partitioning of Perfluorinated Compounds in the Water and Sediment of Nansi Lake, China.” Environmental Monitoring and Assessment 187: 611. doi:10.1007/s10661-015-4831-9.
- Cella, J. A., R. A. Lukey, A. E. Fiebig Jr., and F. J. Pum 1976. Perfluorinated compounds in hair treatment compositions.United States Patent 3993745, Alberto Culver Company.
- Chen, C. L., T. Y. Wang, Y. L. Lv, W. Luo, and J. Geng. 2011. “Estimation of Perfluorinated Compounds Emissions from Major Rivers and Wastewater Treatment Plants in China.” Environmental Science 32: 1073–1080.
- Chen, X. W., L. Y. Zhu, X. Y. Pan, S. H. Fang, Y. F. Zhang, and L. P. Yang. 2015. “Isomeric Specific Partitioning Behaviors of Perfluoroalkyl Substances in Water Dissolved Phase, Suspended Particulate Matters and Sediments in Liao River Basin and Taihu Lake, China.” Water Research 80: 235–244. doi:10.1016/j.watres.2015.04.032.
- Cheng, J., C. D. Vecitis, H. Park, B. T. Mader, and M. R. Hoffmann. 2008. “Sonochemical Degradation of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoate (PFOA) in Landfill Groundwater: Environmental Matrix Effects.” Environmental Science & Technology 42: 8057–8063. doi:10.1021/es8013858.
- Chiappero, M. S., F. E. Malanca, G. A. Arguello, S. T. Wooldridge, M. D. Hurley, J. C. Ball, T. J. Wallington, R. L. Waterland, and R. C. Buck. 2006. “Atmospheric Chemistry of Perfluoroaldehydes (Cxf2x+1cho) and Fluorotelomer Aldehydes (Cxf2x+1ch2cho): Quantification of the Important Role of Photolysis.” Journal of Physical Chemistry A 110: 11944–11953. doi:10.1021/jp064262k.
- Clarke, B. O., and S. R. Smith. 2011. “Review of 'Emerging' Organic Contaminants in Biosolids and Assessment of International Research Priorities for the Agricultural use of Biosolids.” Environment International 37 (1): 226–247. doi:10.1016/j.envint.2010.06.004.
- Commission Regulation (EU). 2017. COMMISSION REGULATION (EU) 2017/1000 of 13 June 2017 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Perfluorooctanoic Acid (PFOA), Its Salts and PFOA-related Substances.
- Conder, J. M., R. A. Hoke, W. De Wolf, M. H. Russell, and R. C. Buck. 2008. “Are PFCAs Bioaccumulative? A Critical Review and Comparison with Regulatory Lipophilic Compounds.” Environmental Science & Technology 42: 995–1003. doi:10.1021/es070895g.
- Cui, X. Y., H. Zhang, J. Luo, and R. B. Zhang. 2016. “Simulation of Multimedia Transfer and Fate of Perfluorooctane Sulfonate (PFOS) in Shenzhen Region.” Environmental Science 37 (8): 3001–3006.
- D’eon, J. C., M. D. Hurley, T. J. Wallington, and S. A. Mabu-ry. 2006. “Atmospheric Chemistry of N-Methyl Perfluorobutane Sulfonamidoethanol, C4F9SO2N(CH3)CH2CH2OH: Kinetics and Mechanism of Reaction with OH.” Environmental Science & Technology 40: 1862–1868. doi:10.1021/es0520767.
- De Silva, A. O., C. Spencer, B. F. Scott, S. Backus, and D. C. G. Muir. 2011. “Detection of a Cyclic Perfluorinated Acid, Perfluoroethylcyclohexane Sulfonate, in the Great Lakes of North America.” Environmental Science & Technology 45: 8060–8066. doi:10.1021/es200135c.
- Desjardins, D., C. Sutherland, R. VanHoven, and H. Krueger. 2001. PFOS: A 7-D Toxicity Test with Duckweed (Lemna Gibba G3). Easton, MD: Wildlife International, Ltd. Project. EPA Docket number: AR226-1030a054.
- DeWitt, J. C., C. B. Copeland, M. J. Strynar, and R. W. Luebke. 2008. “Perfluorooctanoic Acid-Induced Immunomodulation in Adult C57BL/6J or C57BL/6N Female Mice.” Environmental Health Perspectives 116: 644–650. doi:10.1289/ehp.10896.
- Ding, G. H., T. Fromel, E. J. van Den Brandhof, R. Baerselman, and W. J. G. M. Peijnenburg. 2012. “Acute Toxicity of Poly- and Perfluorinated Compounds to Two Cladocerans, Daphnia Magna and Chydorus Sphaericus.” Environmental Toxicology and Chemistry 31: 605–610. doi:10.1002/etc.1713.
- Domingo, J. L. 2012. “Health Risks of Dietary Exposure to Perfluorinated Compounds.” Environment International 40: 187–195. doi:10.1016/j.envint.2011.08.001.
- Domingo, J. L., I. E. Jogsten, U. Eriksson, I. Martorell, G. Perelló, M. Nadal, and B. V. Bavel. 2012. “Human Dietary Exposure to Perfluoroalkyl Substances in Catalonia, Spain.” Temporal Trend Food Chemistry 135: 1575–1582.
- Dong, J., H. Gao, S. Wang, H. Yao, and M. Ma. 2009. “Simulation of the Transfer and Fate of HCHs since the 1950s in Lanzhou, China.” Ecotoxicology and Environmental Safety 72: 1950–1956. doi:10.1016/j.ecoenv.2009.04.009.
- Dreyer, A., M. Shoeib, S. Fiedler, J. Barber, T. Harner, K. W. Schramm, K. C. Jones, and R. Ebinghaus. 2010. “Field Intercomparison on the Determination of Volatile and Semivolatile Polyfluorinated Compounds in Air.” Environmental Chemistry 7: 350–358. doi:10.1071/EN10053.
- Dreyer, A., V. Matthias, C. Temme, and R. Ebinghaus. 2009. “Annual Time Series of Air Concentrations of Polyfluorinated Compounds.” Environmental Science & Technology 43: 4029–4036. doi:10.1021/es900257w.
- Du, G. Z., H. Y. Huang, J. L. Hu, Y. F. Qin, D. Wu, L. Song, Y. K. Xia, and X. R. Wang. 2013. “Endocrine-Related Effects of Perfluorooctanoic Acid (PFOA) in Zebrafish, H295R Steroidogenesis and Receptor Reporter Gene Assays.” Chemosphere 91: 1099–1106. doi:10.1016/j.chemosphere.2013.01.012.
- Ellis, D. A., J. W. Martin, A. O. De Silva, S. A. Mabury, M. D. Hurley, M. P. S. Andersen, and T. J. Wallington. 2004. “Degradation of Fluorotelomer Alcohols: A Likely Atmospheric Source of Perfluorinated Carboxylic Acids.” Environmental Science & Technology 38: 3316–3321. doi:10.1021/es049860w.
- Enders, H. 1961. “Polymers of Fluorocarbons in Textile Finishing.” Textil-Rundschau 16: 531–539.
- Ericson, I., J. L. Domingo, M. Nadal, E. Bigas, X. Llebaria, B. van Bavel, and G. Lindstrom. 2009. “Levels of Perfluorinated Chemicals in Municipal Drinking Water from Catalonia, Spain: Public Health Implications.” Archives of Environmental Contamination and Toxicology 57: 631–638. doi:10.1007/s00244-009-9375-y.
- Feng, Z., X. M. Zheng, H. L. Liu, Y. Gong, Y. Yu, and H. X. Yu. 2010. “Effect of Perfluorooctane Acid on Cell Membrane Properties of Scenedesmus Obliquus.” Asian Journal of Ecotoxicology 5 (4): 537–542.
- Flores, C., F. Ventura, J. Martin-Alonso, and J. Caixach. 2013. “Occurrence of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoate (PFOA) in NE Spanish Surface Waters and Their Removal in a Drinking Water Treatment Plant that Combines Conventional and Advanced Treatments in Parallel Lines.” Science of the Total Environment 461: 618–626. doi:10.1016/j.scitotenv.2013.05.026.
- Fraser, A. J., T. F. Webster, D. J. Watkins, M. J. Strynar, K. Kato, A. M. Calafat, V. Vieira, and M. D. M.,McClean. 2013. “Polyfluorinated Compounds in Dust from Homes, Offices, and Vehicles as Predictors of Concentrations in Office Workers’ Serum.” Environment International 60: 128–136. doi:10.1016/j.envint.2013.08.012.
- Furdui, V., P. Helm, P. Crozier, C. Lucaciu, E. Reiner, C. Marvin, D. Whittle, S. Mabury, and G. Tomy 2008. "Temporal Trends of Perfluoroalkyl Compounds with Isomer Analysis in Lake Trout from Lake Ontario (1979−2004)". Environmental Science & Technology 42(13): 4739–4744.
- Gewurtz, S. B., S. P. Bhavsar, P. W. Crozier, M. L. Diamond, P. A. Helm, C. H. Marvin, and E. J. Reiner. 2009. “Perfluoroalkyl Contaminants in Window Film: Indoor/Outdoor, Urban/ Rural, and Winter/Summer Contamination and Assessment of Carpet as a Possible Source.” Environmental Science & Technology 43: 7317–7323. doi:10.1021/es9002718.
- Giesy, J. P., and K. Kannan. 2001. “Global Distribution of Perfluorooctane Sulfonate in Wildlife.” Environmental Science & Technology 35: 1339–1342. doi:10.1021/es001834k.
- Giesy, J. P., and K. Kannan. 2002. “Peer Reviewed: Perfluorochemical Surfactants in the Environment.” Environmental Science & Technology 36: 146A–152A.
- Gong, X. X., B. Li, Y. Y. Liu, R. X. Liu, and Y. H. Song. 2015. “Pollution Levels and Ecological Risk Assessment of Typical Perfluorinated Compounds in Riverine Water.” Acta Scientiae Circumstantiae 35 (7): 2177–2184.
- Gredelj, A., A. Barausse, L. Grechi, and L. Palmeri. 2018. “Deriving Predicted No-Effect Concentrations (Pnecs) for Emerging Contaminants in the River Po, Italy, Using Three Approaches: Assessment Factor, Species Sensitivity Distribution and AQUATOX Ecosystem Modelling.” Environment International 119: 66–78. doi:10.1016/j.envint.2018.06.017.
- Guo, C., Y. Zhang, X. Zhao, P. Du, S. Liu, J. Lv, F. Xu, W. Meng, and J. Xu. 2015. “Distribution, Source Characterization and Inventory of Perfluoroalkyl Substances in Taihu Lake, China.” Chemosphere 127: 201–207. doi:10.1016/j.chemosphere.2015.01.053.
- Hansen, K. J., H. O. Johnson, J. S. Eldridge, J. L. Butenhoff, and L. A. Dick. 2002. “Quantitative Characterization of Trace Levels of PFOS and PFOA in the Tennessee River.” Environmental Science & Technology 36: 1681–1685. doi:10.1021/es010780r.
- Hart, K., V. A. Gill, and K. Kannan. 2009. “Temporal Trends (1992–2007) of Perfluorinated Chemicals in Northern Sea Otters (Enhydra Lutris Kenyoni) from South-Central Alaska.” Archives of Environmental Contamination and Toxicology 56: 607–614. doi:10.1007/s00244-008-9242-2.
- Haug, L. S., C. Thomsen, A. L. Brantsaeter, H. E. Kvalem, M. Haugen, G. Becher, J. Alexander, H. M. Meltzer, and H. K. Knutsen. 2010. “Diet and Particularly Seafood are Major Sources of Perfluorinated Compounds in Humans.” Environment International 36: 772–778. doi:10.1016/j.envint.2010.05.016.
- Haug, L. S., C. Thomsen, and G. Bechert. 2009. “Time Trends and the Influence of Age and Gender on Serum Concentrations of Perfluorinated Compounds in Archived Human Samples.” Environmental Science & Technology 43: 2131–2136. doi:10.1021/es802827u.
- Haug, L. S., S. Huber, M. Schabach, G. Becher, and C. Thomsen. 2011. “Investigation on Per- and Polyfluorinated Compounds in Paired Samples of House Dust and Indoor Air from Norwegian Homes.” Environmental Science & Technology 45 (19): 7991–7998. doi:10.1021/es103456h.
- He, W., N. Qin, X. Z. Kong, W. X. Liu, Q. S. He, H. L. Ouyang, C. Yang, et al. 2013. “Spatio-Temporal Distributions and the Ecological and Health Risks of Phthalate Esters (Paes) in the Surface Water of a Large, Shallow Chinese Lake.” Science of the Total Environment 461: 672–680. doi:10.1016/j.scitotenv.2013.05.049.
- Hickey, N. J., D. Crump, S. P. Jones, and S. W. Kennedy. 2009. “Effects of 18 Perfluoroalkyl Compounds on Mrna Expression in Chicken Embryo Hepatocyte Cultures.” Toxicological Sciences 111: 311–320. doi:10.1093/toxsci/kfp160.
- Hölzer, J., T. Göen, P. Just, R. Reupert, K. Rauchfuss, M. Kraft, J. Müller, and M. Wilhelm. 2011. “Perfluorinated Compounds in Fish and Blood of Anglers at Lake Möhne, Sauerland Area, Germany.” Environmental Science and Technology 45: 8046–8052. doi:10.1021/es104391z.
- Houde, M., J. W. Martin, R. J. Letcher, K. R. Solomon, and D. C. G. Muir. 2006b. “Biological Monitoring of Polyfluoroalkyl Substances: A Review.” Environmental Science & Technology 40: 3463–3473. doi:10.1021/es052580b.
- Houde, M., T. A. D. Bujas, J. Small, R. S. Wells, P. A. Fair, G. D. Bossart, K. R. Solomon, and D. C. G. Muir. 2006a. “Biomagnification of Perfluoroalkyl Compounds in the Bottlenose Dolphin (Tursiops Truncatus) Food Web.” Environmental Science & Technology 40: 4138–4144. doi:10.1021/es060233b.
- Hurley, M. D., M. P. S. Andersen, T. J. Wallington, D. A. Ellis, J. W. Martin, and S. A. Mabury. 2004. “Atmospheric Chemistry of Perfluorinated Carboxylic Acids: Reaction with OH Radicals and Atmospheric Lifetimes.” Journal of Physical Chemistry A 108: 615–620. doi:10.1021/jp036343b.
- Jahnke, A., U. Berger, R. Ebinghaus, and C. Temme. 2007. “Latitudinal Gradient of Airborne Polyfluorinated Alkyl Substances in the Marine Atmosphere between Germany and South Africa (53 Degrees N-33 Degrees S).” Environmental Science & Technology 41: 3055–3061. doi:10.1021/es062389h.
- Jing, P., P. J. Rodgers, and S. Amemiya. 2009. “High Lipophilicity of Perfluoroalkyl Carboxylate and Sulfonate: Implications for Their Membrane Permeability.” Journal of the American Chemical Society 131: 2290–2296. doi:10.1021/ja807961s.
- Johansson, J. H., U. Berger, R. Vestergren, I. T. Cousins, A. Bignert, A. Glynn, and P. O. Darnerud. 2014. “Temporal Trends (1999−2010) of Perfluoroalkyl Acids in Commonly Consumed Food Items.” Environmental Pollution (Barking, Essex : 1987) 188: 102–108. doi:10.1016/j.envpol.2014.01.026.
- Johnson, R. L., A. J. Anschutz, J. M. Smolen, M. F. Simcik, and R. L. Penn. 2007. “The Adsorption of Perfluorooctane Sulfonate onto Sand, Clay, and Iron Oxide Surfaces.” Journal of Chemical and Engineering Data 52: 1165–1170. doi:10.1021/je060285g.
- Kaiser, M. A., B. J. Dawson, C. A. Barton, and M. A. Botelho. 2010. “Understanding Potential Exposure Sources of Perfluorinated Carboxylic Acids in the Workplace.” Annals of Occupational Hygiene 54: 915–922. doi:10.1093/annhyg/meq066.
- Kannan, K., J. Newsted, R. S. Halbrook, and J. P. Giesy. 2002b. “Perfluorooctanesulfonate and Related Fluorinated Hydrocarbons in Mink and River Otters from the United States.” Environmental Science & Technology 36: 2566–2571. doi:10.1021/es0205028.
- Kannan, K., S. Corsolini, J. Falandysz, G. Oehme, S. Focardi, and J. P. Giesy. 2002a. “Perfluorooctanesulfonate and Related Fluorinated Hydrocarbons in Marine Mammals, Fishes, and Birds from Coasts of the Baltic and the Mediterranean Seas.” Environmental Science & Technology 36: 3210–3216. doi:10.1021/es020519q.
- Karickhoff, S. W., D. S. Brown, and T. A. Scott. 1979. “Sorption of Hydrophobic Pollutants on Natural Sediments.” Water Research 13: 241–248. doi:10.1016/0043-1354(79)90201-X.
- Karrman, A., J. F. Mueller, B. Van Bavel, F. Harden, L. M. L. Toms, and G. Lindstrom. 2006. “Levels of 12 Perfluorinated Chemicals in Pooled Australian Serum, Collected 2002–2003, in Relation to Age, Gender, and Region.” Environmental Science & Technology 40: 3742–3748. doi:10.1021/es060301u.
- Karstadt, M. L. 2007. “Serum PFOA Levels in Residents of Communities near a Teflon-Production Facility.” Environmental Health Perspectives 115: A486–A487. doi:10.1289/ehp.10468.
- Keiter, S., K. Burkhardt-Medicke, P. Wellner, B. Kais, H. Farber, D. Skutlarek, M. Engwall, T. Braunbeck, S. H. Keiter, and T. Luckenbach. 2016. “Does Perfluorooctane Sulfonate (PFOS) Act as Chemosensitizer in Zebrafish Embryos?” Science of the Total Environment 548: 317–324. doi:10.1016/j.scitotenv.2015.12.089.
- Kim, D. H., M. Y. Lee, and J. E. Oh. 2014. “Perfluorinated Compounds in Serum and Urine Samples from Children Aged 5–13 Years in South Korea.” Environmental Pollution 192: 171–178. doi:10.1016/j.envpol.2014.05.024.
- Kim, S.-K., and K. Kannan. 2007. “Perfluorinated Acids in Air, Rain, Snow, Surface Runoff, and Lakes: Relative Importance of Pathways to Contamination of Urban Lakes.” Environmental Science & Technology 41: 8328–8334. doi:10.1021/es072107t.
- Kong, X. Z., W. X. Liu, W. He, F. L. Xu, A. A. Koelmans, and W. M. Mooij. 2018. “Multimedia Fate Modeling of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulphonate (PFOS) in the Shallow Lake Chaohu, China.” Environmental Pollution 237: 339–348. doi:10.1016/j.envpol.2018.02.026.
- Kovarova, J., P. Marsalek, J. Blahova, J. Jurcikova, B. Kasikova, and Z. Svobodova. 2012. “Occurrence of Perfluoroalkyl Substances in Fish and Water from the Svitava and Svratka Rivers, Czech Republic.” Bulletin of Environmental Contamination and Toxicology 88: 456–460. doi:10.1007/s00128-011-0484-8.
- Krusic, P. J., A. A. Marchione, F. Davidson, M. A. Kaiser, C. P. C. Kao, R. E. Richardson, M. Botelho, R. L. Waterland, and R. C. Buck. 2005. “Vapor Pressure and Intramolecular Hydrogen Bonding in Fluorotelomer Alcohols.” Journal of Physical Chemistry A 109: 6232–6241. doi:10.1021/jp0502961.
- Kuklenyik, Z., J. A. Reich, J. S. Tully, L. L. Needham, and A. M. Calafat. 2004. “Automated Solid-Phase Extraction and Measurement of Perfluorinated Organic Acids and Amides in Human Serum and Milk.” Environmental Science & Technology 38: 3698–3704. doi:10.1021/es040332u.
- Kumar, K. S., Y. Zushi, S. Masunaga, M. Gilligan, C. Pride, and K. S. Sajwan. 2009. “Perfluorinated Organic Contaminants in Sediment and Aquatic Wildlife, Including Sharks, from Georgia, USA.” Marine Pollution Bulletin 58: 621–629. doi:10.1016/j.marpolbul.2008.12.006.
- Kunacheva, C., S. K. Boontanon, S. Fujii, S. Tanaka, C. Musirat, C. Artsalee, and T. Wongwattana. 2009. “Contamination of Perfluorinated Compounds (Pfcs) in Chao Phraya River and Bangpakong River, Thailand.” Water Science and Technology 60: 975–982. doi:10.2166/wst.2009.462.
- Kwadijk, C. J. A. F., P. Korytár, and A. A Koelmans. 2010. “"Distribution of Perfluorinated Compounds in Aquatic Systems in the Netherlands".” Environmental Science & Technology 44: 3746–3751. doi:10.1021/es100485e.
- Lai, S. C., J. W. Song, T. L. Song, Z. J. Huang, Y. Y. Zhang, Y. Zhao, G. C. Liu, et al. 2016. “Neutral Polyfluoroalkyl Substances in the Atmosphere over the Northern South China Sea.” Environmental Pollution 214: 449–455. doi:10.1016/j.envpol.2016.04.047.
- Lam, N. H., C. R. Cho, J. S. Lee, H. Y. Soh, B. C. Lee, J. A. Lee, N. Tatarozako, et al. 2014. “Perfluorinated Alkyl Substances in Water, Sediment, Plankton and Fish from Korean Rivers and Lakes: A Nationwide Survey.” Science of the Total Environment 491: 154–162. doi:10.1016/j.scitotenv.2014.01.045.
- Lange, C.C., 2000. The aerobic biodegradation of N-EtFOSE alcohol by the microbial activity present in municipal wastewater treatment sludge. Biodegradation Study Report; 3M Project ID: LIMSE00-2252; 3M Company: St. Paul, MN
- Latala, A., M. Nedzi, and P. Stepnowski. 2009. “Acute Toxicity Assessment of Perfluorinated Carboxylic Acids Towards the Baltic Microalgae.” Environmental Toxicology and Pharmacology 28: 167–171. doi:10.1016/j.etap.2009.03.010.
- Lau, C., K. Anitole, C. Hodes, D. Lai, A. Pfahles-Hutchens, and J. Seed. 2007. “Perfluoroalkyl Acids: A Review of Monitoring and Toxicological Findings.” Toxicological Sciences 99: 366–394. doi:10.1093/toxsci/kfm128.
- Li, H. X., D. Ellis, and D. Mackay. 2007. “Measurement of Low Air-Water Partition Coefficients of Organic Acids by Evaporation from a Water Surface.” Journal of Chemical and Engineering Data 52: 1580–1584. doi:10.1021/je600556d.
- Li, J. G., F. F. Guo, Y. X. Wang, J. L. Zhang, Y. X. Zhong, Y. F. Zhao, and Y. N. Wu. 2013. “Can Nail, Hair and Urine Be Used for Biomonitoring of Human Exposure to Perfluorooctane Sulfonate and Perfluorooctanoic Acid?.” Environment International 53: 47–52. doi:10.1016/j.envint.2012.12.002.
- Li, L., Z. Zhai, J. Liu, and J. Hu. 2015. “Estimating Industrial and Domestic Environmental Releases of Perfluorooctanoic Acid and Its Salts in China from 2004 to 2012.” Chemosphere 129: 100–109. doi:10.1016/j.chemosphere.2014.11.049.
- Lion-Corporation 1988. Surfactants for stable air fresheners.Japanese Patent JP63222767, Lion Corporation. doi: 10.3168/jds.S0022-0302(88)79586-7.
- Liu, B., H. Zhang, D. Yao, J. Li, L. Xie, X. Wang, Y. Wang, G. Liu, and B. Yang. 2015a. “Perfluorinated Compounds (Pfcs) in the Atmosphere of Shenzhen, China: Spatial Distribution, Sources and Health Risk Assessment.” Chemosphere 138: 511–518. doi:10.1016/j.chemosphere.2015.07.012.
- Liu, C., and K. Y. H. Gin. 2018. “Immunotoxicity in Green Mussels under Perfluoroalkyl Substance (PFAS) Exposure: Reversible Response and Response Model Development.” Environmental Toxicology and Chemistry 27: 1138–1145. doi:10.1002/etc.4060.
- Liu, C. H., K. Y. H. Gin, V. W. C. Chang, B. P. L. Goh, and M. Reinhard. 2011. “Novel Perspectives on the Bioaccumulation of PFCs - the Concentration Dependency.” Environmental Science & Technology 45: 9758–9764. doi:10.1021/es202078n.
- Liu, J. X., and S. M. Avendano. 2013. “Microbial Degradation of Polyfluoroalkyl Chemicals in the Environment: A Review.” Environment International 61: 98–114. doi:10.1016/j.envint.2013.08.022.
- Liu, S., Y. Lu, S. Xie, T. Wang, K. C. Jones, and A. J. Sweetman. 2015b. “Exploring the Fate, Transport and Risk of Perfluorooctane Sulfonate (PFOS) in a Coastal Region of China Using a Multimedia Model.” Environment International 85: 15–26. doi:10.1016/j.envint.2015.08.007.
- Liu, W., S. Chen, X. Quan, and Y. H. Jin. 2008. “Toxic Effect of Serial Perfluorosulfonic and Perfluorocarboxylic Acids on the Membrane System of a Freshwater Alga Measured by Flow Cytometry.” Environmental Toxicology and Chemistry 27: 1597–1604. doi:10.1897/07-459.
- Liu, W. X., W. He, J. Y. Wu, N. Qin, Q. S. He, and F. L. Xu. 2018. “Residues, Bioaccumulations and Biomagnification of Perfluoroalkyl Acids (Pfaas) in Aquatic Animals from Lake Chaohu, China.” Environmental Pollution 240: 607–614. doi:10.1016/j.envpol.2018.05.001.
- Liu, W. X., W. He, N. Qin, X. Z. Kong, Q. S. He, B. Yang, C. Yang, S. E. Jorgensen, and F. L. Xu. 2015c. “Temporal-Spatial Distributions and Ecological Risks of Perfluoroalkyl Acids (Pfaas) in the Surface Water from the Fifth-Largest Freshwater Lake in China (Lake Chaohu).” Environmental Pollution 200: 24–34. doi:10.1016/j.envpol.2015.01.028.
- Liu, W. X., W. He, N. Qin, X. Z. Kong, Q. S. He, H. L. Ouyang, B. Yang, et al. 2012. “Residues, Distributions, Sources, and Ecological Risks of OCPs in the Water from Lake Chaohu, China.” Scientific World Journal 2012: 1–16. doi:10.1100/2012/897697.
- Liu, Z., X. Quan, and F. Yang. 2007. “Long-Term Fate of Three Hexachlorocyclohexanes in the Lower Reach of Liao River Basin: Dynamic Mass Budgets and Pathways.” Chemosphere 69: 1159–1165. doi:10.1016/j.chemosphere.2007.03.072.
- Loos, R., G. Locoro, and S. Contini. 2010. “Occurrence of Polar Organic Contaminants in the Dissolved Water Phase of the Danube River and Its Major Tributaries Using SPE-LC-MS2 Analysis.” Water Research 44: 2325–2335. doi:10.1016/j.watres.2009.12.035.
- Loos, R., G. Locoro, T. Huber, J. Wollgast, E. H. Christoph, A. De Jager, B. M. Gawlik, G. Hanke, G. Umlauf, and J. M. Zaldivar. 2008. “Analysis of Perfluorooctanoate (PFOA) and Other Perfluorinated Compounds (Pfcs) in the River Po Watershed in N-Italy.” Chemosphere 71: 306–313. doi:10.1016/j.chemosphere.2007.09.022.
- Lv, G., L. B. Wang, J. Liu, and S. F. Li. 2008. “The Aquatic Ecological Risk and Health Risk Assessment of Perfluorooctane Sulfonyl Compounds.” Resources and Environment in the Yangtze Basin 17 (6): 904–908.
- MacDonald, M. M., A. L. Warne, N. L. Stock, S. A. Mabury, K. R. Solomon, and P. K. Sibley. 2004. “Toxicity of Perfluorooctane Sulfonic Acid and Perfluorooctanoic Acid to Chironomus Tentans.” Environmental Toxicology and Chemistry 23: 2116–2123.
- Mackay, D. 1979. “Finding Fugacity Feasible.” Environmental Science & Technology 13: 1218–1223. doi:10.1021/es60158a003.
- Mackay, D., M. Joy, and S. Paterson. 1983. “A Quatittative Water, Air Sediment Interaction (Qwasi) Fugacity Model for Describong the Fate of Chemicals in Lakes.” Chemosphere 12: 981–997. doi:10.1016/0045-6535(83)90251-5.
- Mackay, D., and S. Paterson. 1981. “Calculating Fugacity.” Environmental Science & Technology 15: 1006–1014. doi:10.1021/es00091a001.
- Martin, J. W., D. M. Whittle, D. C. G. Muir, and S. A. Mabury. 2004a. “Perfluoroalkyl Contaminants in a Food Web from Lake Ontario.” Environmental Science & Technology 38: 5379–5385. doi:10.1021/es049331s.
- Martin, J. W., M. M. Smithwick, B. M. Braune, P. F. Hoekstra, D. C. G. Muir, and S. A. Mabury. 2004b. “Identification of Long-chain Perfluorinated Acids in Biotafrom The Canadian Arctic.” Environmental Science & Technology 38: 373–380. doi:10.1021/es034727+.
- McMurdo, C. J., D. A. Ellis, E. Webster, J. Butler, R. D. Christensen, and L. K. Reid. 2008. “Aerosol Enrichment of the Surfactant PFO and Mediation of the Water - Air Transport of Gaseous PFOA.” Environmental Science & Technology 42: 3969–3974. doi:10.1021/es7032026.
- Melzer, D., N. Rice, M. H. Depledge, W. E. Henley, and T. S. Galloway. 2010. “Association between Serum Perfluorooctanoic Acid (PFOA) and Thyroid Disease in the US National Health and Nutrition Examination Survey.” Environmental Health Perspectives 118: 686–692. doi:10.1289/ehp.0901584.
- Mhadhbi, L., D. Rial, S. Perez, and R. Beiras. 2012. “Ecological Risk Assessment of Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS) in Marine Environment Using Isochrysis Galbana, Paracentrotus Lividus, Siriella Armata and Psetta Maxima.” Journal of Environmental Monitoring 14: 1375–1382. doi:10.1039/c2em30037k.
- Mollenhauer, M. A. M., B. J. Carter, M. M. Peden-Adams, G. D. Bossart, and P. A. Fair. 2009. “Gene Expression Changes in Bottlenose Dolphin, Tursiops Truncatus, Skin Cells Following Exposure to Methylmercury (Mehg) or Perfluorooctane Sulfonate (Pfos).” Aquatic Toxicology 91: 10–18. doi:10.1016/j.aquatox.2008.09.013.
- Moriwaki, H., Y. Takata, and R. Arakawa. 2003. “Concentrations of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) in Vacuum Cleaner Dust Collected in Japanese Homes.” Journal of Environmental Monitoring 5: 753–757.
- Muller, C. E., A. C. Gerecke, C. Bogdal, Z. Y. Wang, M. Scheringer, and K. Hungerbuhler. 2012. “Atmospheric Fate of Poly- and Perfluorinated Alkyl Substances (Pfass): I. Day-Night Patterns of Air Concentrations in Summer in Zurich, Switzerland.” Environmental Pollution 169: 196–203. doi:10.1016/j.envpol.2012.04.010.
- Munschy, C., P. Marchand, A. Venisseau, B. Veyrand, and Z. Zendong. 2013. “Levels and Trends of the Emerging Contaminants HBCDs (Hexabromocyclododecanes) and PFCs (Perfluorinated Compounds) in Marine Shellfish along French Coasts.” Chemospher 91: 233–240. doi:10.1016/j.chemosphere.2012.12.063.
- Murakami, M., and H. Takada. 2008. “Perfluorinated Surfactants (Pfss) in Size-Fractionated Street Dust in Tokyo.” Chemosphere 73: 1172–1177. doi:10.1016/j.chemosphere.2008.07.063.
- Murakami, M., K. Kuroda, N. Sato, T. Fukushi, S. Takizawa, and H. Takada. 2009. “Groundwater Pollution by Perfluorinated Surfactants in Tokyo.” Environmental Science & Technology 43 (10): 3480–3486. doi:10.1021/es803556w.
- Nakata, H., K. Kannan, T. Nasu, H. S. Cho, E. Sinclair, and A. Takemura. 2006. “Perfluorinated Contaminants in Sediments and Aquatic Organisms Collected from Shallow Water and Tidal Flat Areas of the Ariake Sea, Japan: Environmental Fate of Perfluorooctane Sulfonate in Aquatic Ecosystems.” Environmental Science & Technology 40: 4916–4921. doi:10.1021/es0603195.
- Noorlander, C. W., S. P. J. Van Leeuwen, J. D. Te Biesebeek, M. J. B. Mengelers, and M. J. Zeilmaker. 2011. “Levels of Perfluorinated Compounds in Food and Dietary Intake of PFOS and PFOA in the Netherlands.” Journal of Agricultural and Food Chemistry 59: 7496–7505. doi:10.1021/jf104943p.
- O’Hagan, D. 2008. “Understanding organofluorine chemistry. An Introduction to the C-F Bond.” Chemical Society Reviews 37: 308–319.
- Ochoa-Herrera, V., J. A. Field, A. Luna-Velasco, and R. Sierra-Alvarez. 2016. “Microbial Toxicity and Biodegradability of Perfluorooctane Sulfonate (PFOS) and Shorter Chain Perfluoroalkyl and Polyfluoroalkyl Substances (Pfass).” Environmental Science: Processes & Impacts 18: 1236–1246.
- Olsen, G. W., J. L. Butenhoff, and L. R. Zobel. 2009. “Perfluoroalkyl Chemicals and Human Fetal Development: An Epidemiologic Review with Clinical and Toxicological Perspectives.” Reproductive Toxicology 27: 212–230. doi:10.1016/j.reprotox.2009.02.001.
- Olsen, G. W., J. M. Burris, D. J. Ehresman, J. W. Froehlich, A. M. Seacat, J. L. Butenhoff, and L. R. Zobel. 2007. “Half-Life of Serum Elimination of Perfluorooctanesulfonate, Perfluorohexanesulfonate, and Perfluorooctanoate in Retired Fluorochemical Production Workers.” Environmental Health Perspectives 115: 1298–1305. doi:10.1289/ehp.10009.
- Olsen, G. W., J. M. Burris, J. H. Mandel, and L. R. Zobel. 1999. “Serum Perfluorooctane Sulfonate and Hepatic and Lipid Clinical Chemistry Tests in Fluorochemical Production Employees.” Journal of Occupational and Environmental Medicine 41: 799–806.
- Olsen, G. W., T. R. Church, J. P. Miller, J. M. Burris, K. J. Hansen, J. K. Lundberg, J. B. Armitage, et al. 2003. “Perfluorooctanesulfonate and Other Fluorochemicals in the Serum of American Red Cross Adult Blood Donors.” Environmental Health Perspectives 111: 1892–1901. doi:10.1289/ehp.6316.
- Orata, F., N. Quinete, F. Werres, and R. D. Wilken. 2009. “Determination of Perfluorooctanoic Acid and Perfluorooctane Sulfonate in Lake Victoria Gulf Water.” Bulletin of Environmental Contamination and Toxicology 82: 218–222. doi:10.1007/s00128-008-9543-1.
- Pan, G., and C. You. 2010. “Sediment-Water Distribution of Perfluorooctane Sulfonate (PFOS) in Yangtze River Estuary.” Environmental Pollution 158: 1363–1367. doi:10.1016/j.envpol.2010.01.011.
- Pinney, S. M., F. M. Biro, G. C. Windham, R. L. Herrick, L. Yaghjyan, A. M. Calafat, P. Succop, et al. 2014. “Serum Biomarkers of Polyfluoroalkyl Compound Exposure in Young Girls in Greater Cincinnati and the San Francisco Bay Area, USA.” Environmental Pollution 184: 327–334. doi:10.1016/j.envpol.2013.09.008.
- Prevedouros, K., I. T. Cousins, R. C. Buck, and S. H. Korzeniowski. 2006. “Sources, Fate and Transport of Perfluorocarboxylates.” Environmental Science & Technology 40: 32–44. doi:10.1021/es0512475.
- Psillakis, E., J. Cheng, M. R. Hoffmann, and A. J. Colussi. 2009. “Enrichment Factors of Perfluoroalkyl Oxoanions at the Air/Water Interface.” Journal of Physical Chemistry A 113: 8826–8829. doi:10.1021/jp902795m.
- Qi, P., Y. Wang, J. Mu, and J. Wang. 2011. “Aquatic Predicted No‐Effect‐Concentration Derivation for Perfluorooctane Sulfonic Acid.” Environmental Toxicology and Chemistry 30: 836–842. doi:10.1002/etc.460.
- Qin, N. 2013. Multimedia Distribution, Fate and Risk Assessment of PAHs in Lake Chaohu Ecosystem. Beijing: Peking University.
- Qin, N., W. He, X. Z. Kong, W. X. Liu, Q. S. He, B. Yang, H. L. Ouyang, Q. M. Wang, and F. L. Xu. 2013. “Ecological Risk Assessment of Polycyclic Aromatic Hydrocarbons (Pahs) in the Water from a Large Chinese Lake Based on Multiple Indicators.” Ecological Indicators 24: 599–608. doi:10.1016/j.ecolind.2012.08.019.
- Qiu, Y., H. Jing, and H. Shi. 2010. “Perfluorocarboxylic Acids (Pfcas) and Perfluoroalkyl Sulfonates (Pfass) in Surface and Tap Water around Lake Taihu in China.” Frontiers of Environmental Science & Engineering in China 4: 301–310. doi:10.1007/s11783-010-0236-8.
- Rhoads, K. R., E. M. L. Janssen, R. G. Luthy, and C. S. Criddle. 2008. “Aerobic Biotransformation and Fate of N-Ethyl Perfluorooctane Sulfonamidoethanol (N-Etfose) in Activated Sludge.” Environmental Science & Technology 42: 2873–2878. doi:10.1021/es702866c.
- Rivière, G., V. Sirot, A. Tard, J. Jean, P. Marchand, B. Veyrand, B. Le Bizec, and J. C. Leblanc. 2014. “Food Risk Assessment for Perfluoroalkyl Acids and Brominated Flame Retardants in the French Population: Results from the Second French Total Diet Study.” Science of the Total Environment 491–492: 176–183. doi:10.1016/j.scitotenv.2014.01.104.
- Sanderson, H., T. M. Boudreau, S. A. Mabury, and K. R. Solomon. 2003. “Impact of Perfluorooctanoic Acid on the Structure of the Zooplankton Community in Indoor Microcosms.” Aquatic Toxicology 62: 227–234.
- Schultz, R. K., and S. N. Quessy 1992. Use of soluble fluorosurfactants for the preparation of metered-dose aerosol formulations.World Patent WO9114422, Google Patents.
- Seacat, A. M., P. J. Thomford, K. J. Hansen, G. W. Olsen, M. T. Case, and J. L. Butenhoff. 2002. “Subchronic Toxicity Studies on Perfluorooctanesulfonate Potassium Salt in Cynomolgus Monkeys.” Toxicological Sciences 68: 249–264.
- Senthilkumar, K., E. Ohi, K. Sajwan, T. Takasuga, and K. Kannan. 2007. “Perfluorinated Compounds in River Water, River Sediment, Market Fish, and Wildlife Samples from Japan.” Bulletin of Environmental Contamination and Toxicology 79: 427–431. doi:10.1007/s00128-007-9243-2.
- Shankar, A., J. Xiao, and A. Ducatman. 2012. “Perfluorooctanoic Acid and Cardiovascular Disease in US Adults.” Archives of Internal Medicine 172: 1397–1403. doi:10.1001/archinternmed.2012.3393.
- Shi, X. J., C. S. Liu, G. Q. Wu, and B. S. Zhou. 2009. “Waterborne Exposure to PFOS Causes Disruption of the Hypothalamus-Pituitary-Thyroid Axis in Zebrafish Larvae.” Chemosphere 77: 1010–1018. doi:10.1016/j.chemosphere.2009.07.074.
- Shoeib, M., P. Vlahos, T. Hamer, A. Peters, M. Graustein, and J. Narayan. 2010. “Survey of Polyfluorinated Chemicals (Pfcs) in the Atmosphere over the Northeast Atlantic Ocean.” Atmospheric Environment 44: 2887–2893. doi:10.1016/j.atmosenv.2010.04.056.
- Slotkin, T. A., E. A. MacKillop, R. L. Melnick, K. A. Thayer, and F. J. Seidler. 2008. “Developmental Neurotoxicity of Perfluorinated Chemicals Modeled in Vitro.” Environmental Health Perspectives 116: 716–722. doi:10.1289/ehp.11253.
- So, M. K., N. Yamashita, S. Taniyasu, Q. T. Jiang, J. P. Giesy, K. Chen, and P. K. S. Lam. 2006. “Health Risks in Infants Associated with Exposure to Perfluorinated Compounds in Human Breast Milk from Zhoushan, China.” Environmental Science & Technology 40: 2924–2929. doi:10.1021/es060031f.
- So, M. K., Y. Miyake, W. Y. Yeung, Y. M. Ho, S. Taniyasu, P. Rostkowski, N. Yamashita, et al. 2007. “Perfluorinated Compounds in the Pearl River and Yangtze River of China.” Chemosphere 68: 2085–2095. doi:10.1016/j.chemosphere.2007.02.008.
- Squadrone, S., V. Ciccotelli, M. Prearo, L. Favaro, T. Scanzio, C. Foglini, and M. C. Abete. 2015. “Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA): Emerging Contaminants of Increasing Concern in Fish from Lake Varese, Italy, China.” Environmental Monitoring and Assessment 187: 438. doi:10.1007/s10661-015-4686-0.
- Stevenson, C. N., L. A. MacManus-Spencer, T. Luckenbach, R. G. Luthy, and D. Epel. 2006. “New Perspectives on Perfluorochemical Ecotoxicology: Inhibition and Induction of an Efflux Transporter in the Marine Mussel, Mytilus Californianus.” Environmental Science & Technology 40: 5580–5585. doi:10.1021/es0602593.
- Strynar, M. J., and A. B. Lindstrom. 2008. “Perfluorinated Compounds in House Dust from Ohio and North Carolina, USA.” Environmental Science & Technology 42: 3751–3756. doi:10.1021/es7032058.
- Su, H., Y. Shi, Y. Lu, P. Wang, M. Zhang, and A. Sweetman. 2017. “Home Produced Eggs: An Important Pathway of Human Exposure to Perfluorobutanoic Acid (PFBA) and Perfluorooctanoic Acid (PFOA) around a Fluorochemical Industrial Park in China.” Environment International 101: 1–6. doi:10.1016/j.envint.2017.01.016.
- Sun, H. W., F. S. Li, T. Zhang, X. Z. Zhang, N. He, Q. Song, L. J. Zhao, L. N. Sun, and T. H. Sun. 2011. “Perfluorinated Compounds in Surface Waters and Wwtps in Shenyang, China: Mass Flows and Source Analysis.” Water Research 45: 4483–4490. doi:10.1016/j.watres.2011.05.036.
- Takacs, M. L., and B. D. Abbott. 2007. “Activation of Mouse and Human Peroxisome Proliferator-Activated Receptors (Alpha, Beta/ Delta, Gamma) by Perfluorooctanoic Acid and Perfluorooctane Sulfonate.” Toxicological Sciences 95: 108–117. doi:10.1093/toxsci/kfl135.
- Tan, K., G. Lu, X. Yuan, Y. Zheng, P. Shao, J. Cai, Y. Zhao, X. Zhu, and Y. Yang. 2018. “Perfluoroalkyl Substances in Water from the Yangtze River and Its Tributaries at the Dividing Point between the Middle and Lower Reaches.” Bulletin of Environmental Contamination and Toxicology. doi:10.1007/s00128-018-2444-z.
- Taniyasu, S., K. Kannan, Y. Horii, N. Hanari, and N. Yamashita. 2003. “A Survey of Perfluorooctane Sulfonate and Related Perfluorinated Organic Compounds in Water, Fish, Birds, and Humans from Japan.” Environmental Science & Technology 37: 2634–2639. doi:10.1021/es0303440.
- U.S. Environmental Protection Agency. 1998. Guidelines for Ecological Risk Assessment. Washington, DC: U.S. Environmental Protection Agency.
- UNEP. 2009. Listing of perfluorooctane sulfonic acid, its salts and perfluorooctane sulfonyl fluoride. http://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-COP.4-SC-4-17.English.pdf
- van der Oost, R., J. Beyer, and N. P. E. Vermeulen. 2003. “Fish Bioaccumulation and Biomarkers in Environmental Risk Assessment: A Review.” Environmental Toxicology and Pharmacology 13 (2): 57–149. doi:10.1016/S1382-6689(02)00126-6.
- van Straalen, N. M., and C. J. van Leeuwen. 2002. "European History of Species Sensitivity Distributions" In Species Sensitivity Distributions in Ecotoxicology, edited by Posthuma, L., Suter II, G., and Traas, T. Boca Raton: RC Press.
- Veith, G. D., D. L. Defoe, and B. V. Bergstedt. 1979. “Measuring and Estimating the Bioconcentration Factor of Chemicals in Fish.” Journal of the Fisheries Research Board f Canada 36 (9): 1040–1048. doi:10.1139/f79-146.
- Vestergren, R., U. Berger, A. Glynn, and I. T. Cousins. 2012. “Dietary Exposure to Perfluoroalkyl Acids for the Swedish Population in 1999, 2005 and 2010.” Environment International 49: 120–127. doi:10.1016/j.envint.2012.08.016.
- Vierke, L., L. Ahrens, M. Shoeib, E. J. Reiner, R. Guo, W. U. Palm, R. Ebinghaus, and T. Harner. 2011. “Air Concentrations and Particle-Gas Partitioning of Polyfluoroalkyl Compounds at a Wastewater Treatment Plant.” Environmental Chemistry 8: 363–371. doi:10.1071/EN10133.
- Vierke, L., L. Ahrens, M. Shoeib, W. U. Palm, E. M. Webster, D. A. Ellis, R. Ebinghaus, and T. Harner. 2013. “In Situ Air-Water and Particle-Water Partitioning of Perfluorocarboxylic Acids, Perfluorosulfonic Acids and Perfluorooctyl Sulfonamide at a Wastewater Treatm-ent Plant.” Chemosphere 92: 941–948. doi:10.1016/j.chemosphere.2013.02.067.
- Von Ehrenstein, O. S., E. P. Hines, K. Kato, Z. Kuklenyik, A. M. Calafat, and S. E. Fenton. 2008. “Perfluoroalkyl Acids in the Serum and Milk of Breastfeeding North Carolina Women.” Epidemiology 19: S338–S339.
- Wang, H. W., S. W. Ma, Z. Zhang, H. G. Chen, Z. F. Huang, X. Y. Gong, W. G. Cai, and X. P. Jia. 2012. “Effects of Perfluorooctane Sulfonate (PFOS) Exposure on Antioxidant Enzymes of Perna Viridis.” Asian Journal of Ecotoxicology 7 (5): 508–516.
- Wang, J., Y. Shi, Y. Pan, and Y. Cai. 2010. “Perfluorooctane Sulfonate (PFOS) and Other Fluorochemicals in Viscera and Muscle of Farmed Pigs and Chickens in Beijing, China.” Chinese Science Bulletin 55: 3550–3555. doi:10.1007/s11434-010-4098-y.
- Wang, P., Y. Lu, T. Wang, Y. Fu, Z. Zhu, S. Liu, S. Xie, Y. Xiao, and J. P. Giesy. 2014a. “Occurrence and Transport of 17 Perfluoroalkyl Acids in 12 Coastal Rivers in South Bohai Coastal Region of China with Concentrated Fluoropolymer Facilities.” Environmental Pollution 190: 115–122. doi:10.1016/j.envpol.2014.03.030.
- Wang, R., H. Cao, W. Li, W. Wang, W. Wang, L. Zhang, J. Liu, H. Ouyang, and S. Tao. 2011a. “Spatial and Seasonal Variations of Polycyclic Aromatic Hydrocarbons in Haihe Plain, China.” Environmental Pollution 159: 1413–1418. doi:10.1016/j.envpol.2010.12.030.
- Wang, T., P. Wang, J. Meng, S. Liu, Y. Lu, J. S. Khim, and J. P. Giesy. 2015. “A Review of Sources, Multimedia Distribution and Health Risks of Perfluoroalkyl Acids (Pfaas) in China.” Chemosphere 129: 87–99. doi:10.1016/j.chemosphere.2014.09.021.
- Wang, X., R. Zhang, and H. Zhang. 2017. “The Occurrence, Exposure and Risk Assessment of Perfluoroalkyl Acids in Food from Mainland, China. Food Additives and Contaminants.” Part A 34: 1990–1998.
- Wang, Y. W., J. J. Fu, T. Wang, Y. Liang, Y. Y. Pan, Y. Q. Cai, and G. B. Jiang. 2010. “Distribution of Perfluorooctane Sulfonate and other Perfluorochemicals in the Ambient Environment around a Manufacturing Facility in China.” Environmental Science & Technology 44 (21): 8062–8067. doi:10.1021/es101810h.
- Wang, Z., Z. Y. Xie, A. Moller, W. Y. Mi, H. Wolschke, and R. Ebinghaus. 2014. “Atmospheric Concentrations and Gas/Particle Partitioning of Neutral Poly- and Perfluoroalkyl Substances in Northern German Coast.” Atmospheric Environment 95: 207–213. doi:10.1016/j.atmosenv.2014.06.036.
- Wania, F., and D. Mackay. 1995. “A Global Distribution Model for Persistent Organic-Chemicals.” Science of the Total Environment 160–61: 211–232. doi:10.1016/0048-9697(95)04358-8.
- Washington, J. W., W. M. Henderson, J. J. Ellington, T. M. Jenkins, and J. J. Evans. 2008. “Analysis of Perfluorinated Carboxylic Acids in Soils Ii: Optimization of Chromatography and Extraction.” Journal of Chromatography A 1181 (1–2): 21–32. doi:10.1016/j.chroma.2007.12.042.
- Webster, E. M., and D. A. Ellis. 2012. “Understanding The Atmospheric Measurement and Behavior of Perfluorooctanoic Acid.” Environmental Toxicology and Chemistry 31 (9): 2041–2046. doi:10.1002/etc.1932.
- Wei, Y. H., J. Y. Dai, M. Liu, J. S. Wang, M. Q. Xu, J. M. Zha, and Z. J. Wang. 2007. “Estrogen-Like Properties of Perfluorooctanoic Acid as Revealed by Expressing Hepatic Estrogen-Responsive Genes in Rare Minnows (Gobiocypris Rarus).” Environmental Toxicology and Chemistry 26: 2440–2447. doi:10.1897/07-008R1.1.
- Wei, Y. H., Y. Liu, H. S. Wang, Y. Tao, and J. Y. Dai. 2008. “Toxicogenomic Analysis of the Hepatic Effects of Perfluorooctanoic Acid on Rare Minnows (Gobiocypris Rarus).” Toxicology and Applied Pharmacology 226: 285–297. doi:10.1016/j.taap.2007.09.023.
- Weiner, B., L. W. Y. Yeung, E. B. Marchington, L. A. D’Agostino, and S. A. Mabury. 2013. “Organic Fluorine Content in Aqueous Film Forming Foams (Afffs) and Biodegradation of the Foam Component 6: 2 Fluorotelomermercaptoalkylamido Sulfonate (6: 2 FTSAS).” Environmental Chemistry 10: 486–493. doi:10.1071/EN13128.
- Wen, B., Y. Wu, H. Zhang, Y. Liu, X. Hu, H. Huang, and S. Zhang. 2016. “The Roles of Protein and Lipid in the Accumulation and Distribution of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoate (PFOA) in Plants Grown in Biosolids-Amended Soils.” Environmental Pollution 216: 682–688. doi:10.1016/j.envpol.2016.06.032.
- White, S. S., S. E. Fenton, and E. P. Hines. 2011. “Endocrine Disrupting Properties of Perfluorooctanoic Acid.” Journal of Steroid Biochemistry and Molecular Biology 127: 16–26. doi:10.1016/j.jsbmb.2011.03.011.
- Winquist, A., and K. Steenland. 2014. “Perfluorooctanoic Acid Exposure and Thyroid Disease in Community and Worker Cohorts.” Epidemiology 25: 255–264. doi:10.1097/EDE.0000000000000040.
- Wolf, C. J., S. E. Fenton, J. E. Schmid, A. M. Calafat, Z. Kuklenyik, X. A. Bryant, J. Thibodeaux, et al. 2007. “Developmental Toxicity of Perfluorooctanoic Acid in the CD-1 Mouse after Cross-Foster and Restricted Gestational Exposures.” Toxicological Sciences 95: 462–473. doi:10.1093/toxsci/kfl159.
- Xiao, F., M. F. Simcik, and J. S. Gulliver. 2013. “Mechanisms for Removal of Perfluorooctane Sulfonate (Pfos) and Perfluorooctanoate (Pfoa) from Drinking Water by Conventional and Enhanced Coagulation.” Water Research 47: 49–56. doi:10.1016/j.watres.2012.09.024.
- Xie, S., T. Wang, S. Liu, K. C. Jones, A. J. Sweetman, and Y. Lu. 2013. “Industrial Source Identification and Emission Estimation of Perfluorooctane Sulfonate N Hina.” Environment International 52: 1–8. doi:10.1016/j.envint.2012.11.004.
- Xu, J., C. S. Guo, Y. Zhang, and W. Meng. 2014. “Bioaccumulation and Trophic Transfer of Perfluorinated Compounds in a Eutrophic Freshwater Food Web.” Environmental Pollution 184: 254–261. doi:10.1016/j.envpol.2013.09.011.
- Yang, L., S. Tian, L. Zhu, Z. Liu, and Y. Zhang. 2012. “Bioaccumulation and Distribution of Perfloroalkyl Acids in Seafood Products from Bohai Bay, China.” Environmental Toxicology and Chemistry 31: 1972–1979. doi:10.1002/etc.1917.
- Yang, L. P., L. Y. Zhu, and Z. T. Liu. 2011. “Occurrence and Partition of Perfluorinated Compounds in Water and Sediment from Liao River and Taihu Lake, China.” Chemosphere 83: 806–814. doi:10.1016/j.chemosphere.2011.02.075.
- Yeung, L. W. Y., A. O. De Silva, E. I. H. Loi, C. H. Marvin, S. Taniyasu, N. Yamashita, S. A. Mabury, D. C. G. Muir, and P. K. S. Lam. 2013. “Perfluoroalkyl Substances and Extractable Organic Fluorine in Surface Sediments and Cores from Lake Ontario.” Environment International 59: 389–397. doi:10.1016/j.envint.2013.06.026.
- Yeung, L. W. Y., N. Yamashita, S. Taniyasu, P. K. S. Lam, R. K. Sinha, D. V. Borole, and K. Kannan. 2009. “A Survey of Perfluorinated Compounds in Surface Water and Biota Including Dolphins from the Ganges River and in Other Waterbodies in India.” Chemosphere 76: 55–62. doi:10.1016/j.chemosphere.2009.02.055.
- Yin, H. W. 1995. “Procedure of Ecological Risk Assessment for Water Environment.” Shanghai Environmental Sciences 11: 11–14.
- Yu, N. Y., W. Shi, B. B. Zhang, G. Y. Su, J. F. Feng, X. W. Zhang, S. Wei, and H. X. Yu. 2013. “Occurrence of Perfluoroalkyl Acids Including Perfluorooctane Sulfonate Isomers in Huai River Basin and Taihu Lake in Jiangsu Province, China.” Environmental Science & Technology 47: 710–717. doi:10.1021/es3037803.
- Zareitalabad, P., J. Siemens, M. Hamer, and W. Amelung. 2013. “Perfluorooctanoic Acid (PFOA) and Perfluorooctanesulfonic Acid (PFOS) in Surface Waters, Sediments, Soils and Wastewater - A Review on Concentrations and Distribution Coeffici-ents.” Chemosphere 91: 725–732. doi:10.1016/j.chemosphere.2013.02.024.
- Zhang, L. L., J. F. Niu, Y. Li, Y. J. Wang, and D. Sun. 2013a. “Evaluating the Sub-Lethal Toxicity of PFOS and PFOA Using Rotifer Brachionus Calyciflorus.” Environmental Pollution 180: 34–40. doi:10.1016/j.envpol.2013.04.031.
- Zhang, T., H. W. Sun, Q. Wu, X. Z. Zhang, S. H. Yun, and K. Kannan. 2010. “Perfluorochemicals in Meat, Eggs and Indoor Dust in China: Assessment of Sources and Pathways of Human Exposure to Perfluorochemicals.” Environmental Science & Technology 44: 3572–3579. doi:10.1021/es1000159.
- Zhang, T., X. J. Chen, D. Wang, R. D. Li, Y. F. Ma, W. W. Mo, H. W. Sun, and K. Kannan. 2015. “Perchlorate in Indoor Dust and Human Urine in China: Contribution of Indoor Dust to Total Daily Intake.” Environmental Science & Technology 49: 2443–2450. doi:10.1021/es504444e.
- Zhang, Y., S. Beesoon, L. Zhu, and J. W. Martin. 2013b. “Biomonitoring of Perfluoroalkyl Acids in Human Urine and Estimates of Biological Half-Life.” Environmental Science & Technology 47: 10619–10627. doi:10.1021/es401905e.
- Zhang, Y., W. Meng, C. Guo, J. Xu, T. Yu, W. Fan, and L. Li. 2012. “Determination and Partitioning Behav-ior of Perfluoroalkyl Carboxylic Acids and Perfluorooctanesulfonate in Water and Sediment from Dianchi Lake, China.” Chemosphere 88: 1292–1299. doi:10.1016/j.chemosphere.2012.03.103.
- Zhao, X. L., J. D. Li, Y. L. Shi, Y. Q. Cai, S. F. Mou, and G. B. Jiang. 2007. “Determination of Perfluorinated Compounds in Wastewater and River Water Samples by Mixed Hemimicelle-Based Solid-Phase Extraction before Liquid Chromatography-Electrospray Tandem Mass Spectrometry Detection.” Journal of Chromatography A 1154: 52–59. doi:10.1016/j.chroma.2007.03.093.
- Zhao, Z., J. Tang, Z. Xie, Y. Chen, X. Pan, G. Zhong, R. Sturm, G. Zhang, and R. Ebinghaus. 2013. “Perfluoroalkyl Acids (Pfaas) in Riverine and Coastal Sediments of Laizhou Bay, North China.” Science of the Total Environment 447: 415–423. doi:10.1016/j.scitotenv.2012.12.095.
- Zhou, Z., Y. Liang, Y. L. Shi, L. Xu, and Y. Q. Cai. 2013. “Occurrence and Transport of Perfluoroalkyl Acids (Pfaas), Including Short-Chain PFAAs in Tangxun Lake, China.” Environmental Science & Technology 47: 9249–9257. doi:10.1021/es402120y.
- Zhou, Z., Y. L. Shi, W. H. Li, L. Xu, and Y. Q. Cai. 2012. “Perfluorinated Compounds in Surface Water and Organisms from Baiyangdian Lake in North China: Source Profiles, Bioaccumulation and Potential Risk.” Bulletin of Environmental Contamination and Toxicology 89: 519–524. doi:10.1007/s00128-012-0745-1.
- Zoellner-Braue 1995. Metal effect drawing inks.German Patent DE4327982.