ABSTRACT
Tropical seagrass meadows and coral reefs often function as interconnected marine habitats, but they are often studied and managed as homogenous units. As macrohabitats, seagrass meadows provide important benefits to adjacent reef ecosystems by acting as natural filters of sediments and nutrients, and by providing critical feeding, nursery, and refuge habitats for reef fishes and other fauna. Whilst the macrohabitat functions of seagrass meadows have been often acknowledged, their microhabitats functions have largely been neglected. The purpose of the study is to explore how seagrass meadows provide multiple benefits to adjacent coral reefs through various microhabitat functions. The paper reveals some of the diversity of microhabitats that seagrass meadows contain, such as macroalgal mats, rubble cavities, sand patches with sparse seagrass, anemone gardens, hard substratum, and sponges mixed with seagrass. We highlight the ways in which reef creatures have diversified and specialized in using these different microhabitats, and postulate that seagrass microhabitat diversity enhances the habitat function and faunal diversity of seagrass meadows.
Introduction
Seagrass meadows play a critical ecological role in complex coastal food webs that encompass adjacent and interconnected ecosystems such as mangroves and coral reefs (Unsworth et al. Citation2008; Nagelkerken Citation2009; Du et al. 2015). However, seagrass meadows are in decline globally, with an about 7% annual loss in surface area, on average, since 1990 (Cullen-Unsworth and Unsworth Citation2018). Although seagrass meadows are widely considered as important feeding, nursery, and refuge habitats for fishes, invertebrates, and other animals, they have mostly been considered as homogenous habitats (Nordlund et al. Citation2018; Unsworth, Nordlund, and Cullen-Unsworth Citation2018). Whilst the effects, and contexts, of the structure components of seagrass meadows on fish assemblages have been studied (e.g. canopy structure, seagrass density, presence of food, shade, and mangrove roots; Verweij et al. Citation2006; Zarco-Perello and Enríquez Citation2019), very few studies have evaluated the importance of microhabitats within seagrass meadows for marine fauna. In this study, we reveal the wide range of microhabitats that exists in seagrass meadows, and their function for animals that live as adults on coral reefs, using photos from seagrass microhabitats taken in the South China Sea and the Coral Triangle region. This provides a deeper understanding of the multiple scales at which connectivity exists between seagrass meadows and coral reefs, and the processes that underpin their exchange of fauna and their biodiversity.
Seagrass meadows are natural filters for adjacent, interconnected ecosystems
Seagrasses plants slow the flow of water as it moves across seagrass meadows (Koch and Gust Citation1999). This causes sediment particles to settle on the substratum, but suspended particles are also filtered out and settle on the seagrass leaves themselves (Photo 1.1–1.2). As such adjacent coral reefs as well as coral patches within seagrass meadows benefit from the improved water quality (Hemminga and Duarte Citation2000; Nagelkerken Citation2009).
Seagrass meadows provide microhabitats that act as nurseries for juveniles
Juvenile fishes and invertebrates are often associated in high abundances with structurally complex habitats like seagrasses (Heck, Hays, and Orth Citation2003). Seagrass meadows provide different types of microhabitats, such as macroalgal mats, rubble cavities, sand patches with sparse seagrass, anemone gardens, hard substratum, and sponges mixed with seagrass. Seagrass meadows provide important nursery areas for juveniles for about 20% of the world’s biggest fisheries (Cullen-Unsworth and Unsworth Citation2018). In the South China Sea and Coral Triangle, seagrass meadows with different canopy characteristics (e.g., shoot density, leaf area) support distinct fish communities (Pogoreutz et al. Citation2012), and around 64–93% of the fishes in seagrass meadows are juveniles (e.g., species belonging to the families Siganidae, Lethrinidae, Labridae, Apogonidae, Tetraodontidae, and Carangidae; Du et al. Citation2018, 2019; Xie et al. Citation2020). The juveniles of these species have adapted to the different seagrass microhabitats, and they show different behaviors from adults. For example, the juveniles of some species are light green to fit in with seagrass leaves, while the adults alter coloration once they move to coral reef (Photo 2.1, 2.2, 2.4). Moreover, juveniles of some fishes are solitary and can hide easily in dense seaweed-seagrass or rubble cavity microhabitats, while the adults become gregarious on coral reefs to reduce predation risk (Photo 2.3).
Photo 2.1 Juveniles of barhead spinefoot (Siganus virgatus) with green flat bodies can conceal themselves easily among the green seagrass leaves (2.1 a), while their adults alter their coloration once they move to coastal reef flats (2.1 b). Photo by Jianguo Du in Trat, Thailand.

Photo 2.2 A juvenile bell’s sea urchin (Salmacis belli) has a light green body and short spines, typically banded with green or brown colors, to fit in with the seagrass leaves. Adults, in contrast, have white body with brown rectangular clusters of short spines on coral reefs. Photo by Jianguo Du in Trat, Thailand.

Seagrass meadows provide microhabitats that act as foraging areas for reef creatures
Seagrass meadows are important foraging habitats for many animals, especially for regular visitors (Hemminga and Duarte Citation2000; Igulu et al. Citation2013). Half of the 55 faunal species in South Sulawesi, Indonesia feed on seagrass material directly or indirectly (Vonk et al. Citation2008), and more than 82% of the fish species mainly feed on seagrass and its epiphytes in North Sulawesi, Indonesia and Wenchang, China (Du et al. Citation2016, Citation2019). Reef species employ different feeding modes in the various seagrass microhabitats. For example, some species graze on invertebrates on seagrass leaves (copperband butterflyfish Chelmon rostratus) (Photo 3.1), whilst others feed on zoobenthos that live hidden in the sandy bottom of seagrass meadows (freckled goatfish Upeneus tragula) (Photo 3.2), and some feed directly on both living and decomposing seagrass leaves (Photo 3.3). Moreover, in seagrass-coral reef mosaics, various fish species hide on coral reefs during the day, and feed in adjacent seagrass meadows at night (e.g., species belonging to the Haemulidae, Apogonidae, Lutjanidae; Nagelkerken et al. Citation2008; Ogden, Nagelkerken, and McIvor Citation2015). As such, seagrass meadows provide a trophic subsidy for adjacent habitats through diel and tidal movements by fishes (Heck et al. Citation2008; Dorenbosch et al. Citation2004; Du et al. Citation2020) and benthic invertebrates like urchins (Tertschnig Citation1989; Ceccherelli et al. Citation2009).
Photo 3.1 A copperband butterflyfish (Chelmon rostratus) with a flat body is hunting invertebrates on seagrass leaves during twilight (3.1 a), whilst adults on coral reefs feed on worms during day time (3.1 b). Photo by Jianguo Du in Trat, Thailand.

Photo 3.2 A freckled goatfish (Upeneus tragula) with a spindle body is foraging on infaunal prey in the sandy bottom of seagrass meadows at night (3.2 a), and shelters on coral reef areas during day time (3.2 b). Photo by Jianguo Du in Trat, Thailand.

Photo 3.3 A striped sea urchin (Tripneustes gratilla) covered and camouflaged with seagrass leaves in the seagrass-macroalgae microhabitat, feeding on both living and decomposing seagrass leaves (3.3 a). The same species covered with coral and skeletal debris on coral reefs, feeding on microbial and microalgal films (3.3 b). Photo by Jianguo Du in Xisha Islands, China.

Seagrass meadows provide microhabitats that act as refuge for marine fauna
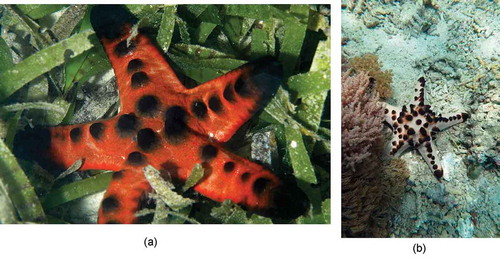
Seagrass meadows not only provide a dense network of seagrass roots and shoots that reduces the risk of predation (Verweij et al. Citation2006; Ooi et al. Citation2011; Nordlund et al. Citation2018), but also a variety of microhabitats, such as coral patches, sand patches with sparse seagrass, cavities, algal mats, sponge gardens, and rubble patches. Many seagrass fauna have diversified and specialized their use of these habitats to reduce predation risk, by adapting their behaviors and body coloration to fit in with the background of the microhabitat. There are three different types of adaptive behaviors we observed. First, some burrowing fishes that hide inside cavities or commensal shrimp that are associated with anemone hosts, venture further from their hide-outs in seagrass meadows than on coral reefs due to the protective seagrass microhabitat (Photo 4.1–4.2). Second, species like longfin grouper, cylindrical sandperch and horned sea star can adjust their body coloration to match the background (Photo 4.3–4.5). Third, some species can take advantage of their size and body shape to mimic seagrass leaves, such as wolf fangbelly (Petroscirtes lupus) (Photo 4.6).
Photo 4.1 A yellow prawn-goby (Cryptocentrus cinctus) strays from its cavity under the protection of sparse seagrass leaves (4.1 a), but is more cautious when its cavity is solely surrounded by bare sand patches (4.1 b). Photo by Jianguo Du in Johor, Malaysia.

Photo 4.2 A glass anemone shrimp (Periclimenes brevicarpalis) wanders from its commensal anemone using the adjoining seagrass-leaf microhabitat as protective cover (4.2 a), but rarely leaves its host anemone on the coral reef (4.2 b). Photo by Jianguo Du in Johor, Malaysia.

Photo 4.3 A longfin grouper (Epinephelus quoyanus) has adjusted its body coloration and patterning to match that of the hard substratum microhabitat within lush seagrass meadows (4.3 a), a behavior which is also employed on coral reefs (4.3 b). Photo by Jianguo Du in Trat, Thailand.

Photo 4.4 A cylindrical sandperch (Parapercis cylindrica) has adjusted its body coloration and patterning of blotches and stripes to that of the microhabitat background (mixture of seagrass, fleshy algae, sponge and sand patches) in seagrass meadows (4.4 a) and coral reefs (4.4 b). Photo by Jianguo Du in Xisha Islands, China.

Conclusions
Seagrass meadows play an essential role in tropical coastal ecosystems by maintaining ecosystem integrity and connectivity. Seagrass meadows are usually studied from a macro habitat perspective, including at seascape levels and in terms of exchange of materials. However, an understanding of how this connectivity is supported from a micro habitat perspective is limited and has largely been neglected. We reveal that seagrass meadows provide a diversified habitat role for adjacent coral reef ecosystems by providing microhabitats for a range of juvenile and adult coral reef creatures. We therefore suggest that the study of habitat and nursery function of seagrass meadows and other coastal ecosystems should be broadened to include that of microhabitats. This could include assessing the role of microhabitats as part of ecological niches, through field observations and laboratory experiments that manipulate microhabitats. Moreover, the important role of microhabitats should be considered in marine conservation and management, by not only protecting whole ecosystems, but including variants of the same ecosystem (e.g., seagrass meadows) that occur with a different diversity of microhabitats.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ceccherelli, G., A. Pais, S. Pinna, and S. Serra. 2009. “On the Movement of the Sea Urchin Paracentrotus Lividus Towards Posidonia Oceanica Seagrass Patches.” Journal of Shellfish Research 28 (2): 397–8. doi:10.2983/035.028.0224.
- Cullen-Unsworth, L. C., and R. Unsworth. 2018. “A Call for Seagrass Protection.” Science 361 (6401): 446–448. doi:10.1126/science.aat7318.
- Dorenbosch, M., M. C. Verweij, I. Nagelkerken, N. Jiddawi, and G. van der Velde. 2004. “Homing and Daytime Tidal Movements of Juvenile Snappers (Lutjanidae) between Shallow-water Nursery Habitats in Zanzibar, Western Indian Ocean.” Environmental Biology of Fishes 70: 203–209. doi:10.1023/B:EBFI.0000033336.10737.f5.
- Du, J. G., G. Q. Ye, Q. L. Zhou, B. Chen, W. J. Hu, and X. Q. Zheng. 2015. “Progress and Prospects of Coastal Ecological Connectivity Studies.” Acta Ecologica Sinica 35 (21): 6923–6933. (In Chinese with English Abstract).
- Du, J. G., M. L. Xie, Y. Y. Wang, Z. H. Chen, W. H. Liu, J. L. Liao, and B. Chen. 2020. “Connectivity of Fish Assemblages along the Mangrove-seagrass-coral Reef Continuum in Wenchang, China.” Acta Oceanologica Sinica. doi:10.1007/s13131-019-1490-7.
- Du, J. G., X. Q. Zheng, T. Peristiwady, J. J. Liao, P. C. Makatipu, X. J. Yin, W. J. Hu, W. Koagouw, and B. Chen. 2016. “Food Sources and Trophic Structure of Fishes and Benthic Macroinvertebrates in a Tropical Seagrass Meadow Revealed by Stable Isotope Analysis.” Marine Biology Research 12 (7): 748–757. doi:10.1080/17451000.2016.1183791.
- Du, J. G., Y. G. Wang, T. Peristiwady, J. J. Liao, P. C. Makatipu, R. Huwae, P. L. Ju, K. H. Loh, and B. Chen. 2018. “Temporal and Spatial Variation of Fish Community and Their Nursery in a Tropical Seagrass Meadow.” Acta Oceanologica Sinica 37 (12): 63–72. doi:10.1007/s13131-018-1288-z.
- Du, J. G., Z. H. Chen, M. L. Xie, M. R. Chen, X. Q. Zheng, J. J. Liao, and B. Chen. 2019. “Analysis of Organic Carbon Sources in Tropical Seagrass Fish: A Case Study of the East Coast of Hainan Province.” Marine Biology Research 15 (8–9): 513–522. doi:10.1080/17451000.2019.1673896.
- Heck, K. L., G. C. Hays, and R. J. Orth. 2003. “Critical Evaluation of the Nursery Role Hypothesis for Seagrass Meadows.” Marine Ecology Progress Series 253: 123–136. doi:10.3354/meps253123.
- Heck, K. L., T. J. B. Carruthers, C. M. Duarte, A. R. Hughes, G. Kendrick, R. J. Orth, and S. W. Williams. 2008. “Trophic Transfers from Seagrass Meadows Subsidize Diverse Marine and Terrestrial Consumers.” Ecosystems 11: 1198–1210. doi:10.1007/s10021-008-9155-y.
- Hemminga, M. A., and C. M. Duarte. 2000. Seagrass Ecology. Cambridge: Cambridge University Press.
- Igulu, M. M., I. Nagelkerken, G. van der Velde, and Y. D. Mgaya. 2013. “Mangrove Fish Production Is Largely Fuelled by External Food Sources: A Stable Isotope Analysis of Fishes at the Individual, Species, and Community Levels from across the Globe.” Ecosystems 16: 1336–1352. doi:10.1007/s10021-013-9687-7.
- Koch, E. W., and G. Gust. 1999. “Water Flow in Tide- and Wave-dominated Beds of the Seagrass Thalassia Testudinum.” Marine Ecology Progress Series 184: 63–72.
- Nagelkerken, I. 2009. “Evaluation of Nursery Function of Mangroves and Seagrass Beds for Tropical Decapods and Reef Fishes: Patterns and Underlying Mechanisms.” In Ecological Connectivity among Tropical Coastal Ecosystems, edited by I. Nagelkerken, 357–399. Dordrecht, the Netherlands: Springer Science and Business Media.
- Nagelkerken, I., J. Bothwell, R. S. Nemeth, J. M. Pitt, and G. van der Velde. 2008. “Interlinkage between Caribbean Coral Reefs and Seagrass Beds through Feeding Migrations by Grunts (Haemulidae) Depends on Habitat Accessibility.” Marine Ecology Progress Series 368: 155–164. doi:10.3354/meps07528.
- Nordlund, L. M., R. K. F. Unsworth, M. Gullström, and L. C. Cullen-Unsworth. 2018. “Global Significance of Seagrass Fishery Activity.” Fish and Fisheries 19: 399–412. doi:10.1111/faf.12259.
- Ogden, J. C., I. Nagelkerken, and C. C. McIvor. 2015. “Connectivity in the Tropical Coastal Seascape: Implications for Marine Spatial Planning and Resources Management.” In Interrelationships between Coral Reefs and Fisheries, edited by S. A. Bortone, 253–273. Florida: CRC Marine Biology Series, Taylor & Francis Group.
- Ooi, J. L. S., G. A. Kendrick, K. P. V. Niel, and Y. A. Affendi. 2011. “Knowledge Gaps in Tropical Southeast Asian Seagrass Systems.” Estuarine, Coastal and Shelf Science 92 (1): 118–131.
- Pogoreutz, C., D. Kneer, M. Litaay, H. Asmus, and H. Ahnelt. 2012. “The Influence of Canopy Structure and Tidal Level on Fish Assemblages in Tropical Southeast Asian Seagrass Meadows.” Estuarine, Coastal and Shelf Science 107: 58–68. doi:10.1016/j.ecss.2012.04.022.
- Tertschnig, W. P. 1989. “Diel Activity Patterns and Foraging Dynamics of the Sea Urchin Tripneustes Ventricosus in a Tropical Seagrass Community and a Reef Environment (Virgin Islands).” Marine Ecology 10 (1): 3–21.
- Unsworth, R. K. F., L. M. Nordlund, and L. C. Cullen-Unsworth. 2018. “Seagrass Meadows Support Global Fisheries Production.” Conservation Letters e12566. doi:10.1111/conl.12566.
- Unsworth, R. K. F., P. S. De León, S. L. Garrard, J. Jompa, D. J. Smith, and J. J. Bell. 2008. “High Connectivity of Indo-Pacific Seagrass Fish Assemblages with Mangrove and Coral Reef Habitats.” Marine Ecology Progress Series 353: 213–224. doi:10.3354/meps07199.
- Verweij, M. C., I. Nagelkerken, D. de Graaff, M. Peeters, E. J. Bakker, and G. van der Velde. 2006. “Structure, Food and Shade Attract Juvenile Coral Reef Fish to Mangrove and Seagrass Habitats: A Field Experiment.” Marine Ecology Progress Series 306: 257–268. doi:10.3354/meps306257.
- Vonk, J. A., M. J. A. Christianen, and J.Stapel. 2008. “Redefining the Trophic Importance of Seagrasses for Fauna in Tropical Indo-Pacific Meadows.” Estuarine, Coastal and Shelf Science 79: 653–60. doi:10.1016/j.ecss.2008.06.002
- Xie, M. L., K. H. Loh, Z. H. Chen, J. J. Liao, B. Chen, W. H. Liu, and J. G. Du. 2020. “Length-weight Relationships of Eight Fish from Seagrass Meadows in Wenchang, China.” Journal of Applied Ichthyology 36 (3): 361–363. doi:10.1111/jai.14023.
- Zarco-Perello, S., and S. Enríquez. 2019. “Remote Underwater Video Reveals Higher Fish Diversity and Abundance in Seagrass Meadows, and Habitat Differences in Trophic Interactions.” Scientific Reports 9: 6596. doi:10.1038/s41598-019-43037-5.