ABSTRACT
Herbaceous species contribute to the largest proportion of the fodder. Despite the profound benefits obtained, anthropogenic disturbances are hindering its development. On the other hand, to ensure the sustainability of herbaceous species, communities and governments have been putting efforts in managing herbaceous species through the establishment of area exclosure (AE). Thus, the main importance of this research paper is to provide information about the role of AE on the restoration of herbaceous species. The objective of this study was to assess the effect of restoration on the herbaceous species following AE established on communal grazing land (CGL). A total of 124 and 73 quadrats of 1×1m2 size were laid down at 50 m intervals along parallel transects at AE and CGL, respectively, and data were analyzed by t-test unequal variances using R-software. AE displayed higher plant species richness and diversity than the CGL and showed a significant difference (p<0.001). Similarly, the AE had significantly (p< 0.001) higher forage biomass than the CGL. In general, herbaceous species diversity index and species richness were significantly (p< 0.001) higher in the AE compared to the CGL. The study concluded that effective AE has the potential to enhance the restoration of herbaceous species and hence forage productivity.
Introduction
Native herbaceous plant species contribute to the largest proportion of the livestock feed across all agro-ecological zones of Ethiopia (Getnet Citation2003). However, degradation of lands due to uncontrolled and excessive use of communal grazing land (CGL) of the undulated topography in the highlands and erratic rainfall in semi-arid areas have further reduced the availability of feed resources (Solomon and Teferi Citation2010). A common restoration practice in Ethiopia is the use of area exclosures (Tekle and Bekele Citation2000; Tekle Citation2001; Asefa et al. Citation2003; Mengistu et al. Citation2005). Restoration of plant species diversity is an important management strategy for rehabilitating landscapes, which have lost vegetation cover (Ormerod Citation2003). In the absence of restoration, the overall sustainability of ecological/ecosystem processes, including species diversity, will be further threatened (Martínez‐Garza and Howe Citation2003). The restoration of degraded lands should, therefore, begin with a clear appraisal of the effects of management on plant biodiversity (Ormerod Citation2003; Ruiz-Jaen and Aide Citation2005). Earlier studies (Oba, Vetaas, and Stensteth Citation2001; Asefa et al. Citation2003; Mengistu et al. Citation2005; Abebe et al. Citation2006) suggested that species diversity is higher in exclosures than continuously grazed areas.
Exclosures are (AE) areas closed from the human and domestic animals interferences with the goal of promoting natural regeneration of plants and reducing land degradation of formerly degraded communal grazing lands (Mekuria and Aynekulu Citation2013; Teketay et al. Citation2018). An exclosure refers to a specific land unit that is protected from the activities of a particular class of animals using appropriate barriers such as fencing to control the influence of animals (Young Citation1958). As the exclosures are not fenced, guards are hired by the local administration on a food-for-work basis (Yayneshet, Eik, and Moe Citation2009). In AE, it is generally believed that all the land resources will be protected from degradation (Tefera Citation2001). The effects of AE on the recovery of woody species diversity, population structure, and regeneration status, restoration of soils, and restoration of ecosystem carbon stocks have been well studied (e.g., Kindeya Citation2003; Aerts et al. Citation2004; Tefera et al. Citation2005; Descheemaeker et al. Citation2006; Muluberhan et al. Citation2006; Yayneshet, Eik, and Moe Citation2009; Wolde and Aynekulu Citation2011; Wolde et al. Citation2011b; Yayneshet Citation2011; Wolde and Mastewal Citation2013; Ombega et al. Citation2017; Eltalib, Lazim, and Dawelbait Citation2018; Tsegay et al. Citation2019; Tesfay, Anteneh, Tessema, Citation2019). In addition, in the Tigray region, Northern Ethiopia, some studies that were conducted earlier (Kindeya Citation2003; Aerts et al. Citation2004; Tefera et al. Citation2005; Muluberhan et al. Citation2006; Yayneshet, Eik, and Moe Citation2009; Yayneshet Citation2011; Wolde and Mastewal Citation2013; Tesfay et al. Citation2019) were specifically trying to estimate the role of AE in the recovery of vegetation diversity without considering specifying indigenous palatable herbaceous species. However, a replicated study on multiple sites that investigates the dynamics of vegetation restoration following the establishment of exclosures has not been conducted. Such information is, however, crucial for managing the existing exclosures in a sustainable manner, and for establishing new exclosures in the future (Mekuria and Veldkamp Citation2012). It is very important to have basic information about herbaceous plant species diversity and biomass production, as these may facilitate the efficient and effective use of rangeland resources as livestock feed.
Even though there are a lot of AE in the South zone of Tigray including forests in different agro-ecologies and land uses their roles regarding herbaceous species diversity and above ground, biomass has not been well studied and documented. So this study on the restoration of herbaceous species following exclosure establishment in communal grazing lands was conducted to fill the gap on this demand for basic information about the diversity restoration potential of the area with their variation across the land-use system. A change in species diversity is often used as an indicator of anthropogenic or natural disturbances in an ecosystem. Therefore, the characterization of biodiversity through inventories can be useful in the planning of operations that aim to conserve biodiversity (Kalema Citation2010). Based on the stated goals of restoration by AE, we hypothesize that: AE assisted enrichment planting would increase herbaceous species diversity and above ground biomass inference to the adjacent CGL in Northern Ethiopia. Therefore, this study was initiated to generate quantitative information and thereby evaluate whether AE has an impact on the biomass and diversity of herbaceous plant species in the South zone of Tigray, Northern Ethiopia.
Materials and methods
Description of the study area
The study was conducted in all districts of the Southern zone of Tigray. It is located at 680 km North of Addis Ababa and 180 km South of Mekele, the capital city of the Tigray regional state. The zone consists of five administrative districts, namely Raya Alamata, Alaje, Endamohoni, Ofla, and Raya Azebo . The Southern Tigray Zone is one of the seven zones of the region bordered to the south and west by Amhara Regional State, to the north with the southeastern zone of Tigray, and to the east with the Afar Regional State. Geographically, it is located between 12o15ʹand 13o41ʹ north latitude and 38o59ʹand 39o54ʹeast longitude with an altitudinal range of 1350-3925 m.a.s.l. Based on the traditional classification system, the Southern zone covers “Kola”, “Weynadega” and “Dega” agro-ecologies which support the growth of a variety of crops, livestock and tree species. Mixed farming is the major economic activity in the area.
The southern zone is characterized by a bimodal rainfall pattern and about 70–80% of the rain falls during the major rainy season that extends from June to September (Araya and Stroosnijder Citation2011). The mean annual rainfall of the zone is 600 mm (Tesfay et al. Citation2019). The mean minimum and maximum temperatures are 8 oC and 30 oC, respectively. Urban agriculture is a common practice in the southern zone (Ashebir, Pasquini, and Bihon Citation2007). Livestock production is a major component of the livelihood system and provides draught power, food, and income. In the study area, indigenous and multipurpose plant species like Acacia tortilis, Balanites aegyptiaca, Carissa spinarum, Grewia mollis, Olea europaea, Pittosporum viridiflorum, Tecleasimplicifolia, and Ziziphus spina-christi are found. The principal feed resources available for livestock in the Southern zone are crop-residue, grass hay, crop aftermaths, grazing lands, and weeds. Herbaceous plants also has a significantly important feed resource of livestock similar in the dry and wet season (Tesfay et al. Citation2018). Dominant soil types in the southern zone are Vertisol, Fluvisols, Luvisols and Cambisols (Tesfay et al. Citation2019). The major geological formations in the area can be broadly grouped into the trap volcanic and the alluvium. The geological structure to the east of the escarpment is complicated by north/south trending grabens separated by horsts (Hunting Citation1976). A number of large and small streams draining from the mountains and hills of southern Tigray flow to the low-lying flat to undulating plain. Most of the streams are intermittent and flow only during the rainy season (Amanuel, Girmay, and Atkilt Citation2015).
Sampling and data collection methods of herbaceous species
Site selection and sampling procedure
A reconnaissance survey was undertaken in 5 districts of the Zone to identify the presence of AE and adjacent CGL in consultation with agricultural experts and user groups. The priority areas were identified by the local agricultural experts and user groups who agreed to strictly protect them from any form of grazing, manual harvesting of grass, and tree cutting (Yayneshet, Eik, and Moe Citation2009). Accordingly, five CGL and five AE (5-10 km apart and established in the same year) were systematically selected for sampling across the Southern zone of Tigray. To minimize the effects of spatial variability, an AE was included only when its location relative to others was not farther than 10 km (Yayneshet Citation2011). These rangeland sites were selected based on management practices, and the similarity in slope and soil type of the sites. In the absence of undisturbed reference sites (Ruiz-Jaen and Aide Citation2005), CGL was chosen as a benchmark against which the rehabilitation success was compared. The AE ages ranged from 5 to 7 years. The AE and CGL covered a total area of 50-150 and 25-70 ha, respectively (Southern Zone bureau of agriculture and rural development (BOARD) Citation2018). Adjacent to the AE in a similar scenario, there is CGL used by the local community with no restriction to access resources that were used as a control for each exclosed area. Therefore, this study was conducted in these two land-use systems. It is ensuring that the AE and CGL were homogenous in biophysical factors before the AE are restricted for rehabilitation and are similar in topographic and climatic characteristics.
A systematic line transect sampling technique was used in this study following the vegetation type and distributional pattern. Thirty parallel transect lines land-use systems x 5 locations x 3 transect lines) were established each 50 m apart from the other in order to assess vegetation in the two land-use systems. Along the transect lines, a total of 197 quadrats (124 for AE and 73 for CGL) was used for sampling herbaceous vegetation attributes (biomass, diversity, richness, and evenness) from the two land-use systems. At each site/location, 24-39 quadrats were designated and an average 8 quadrats/transect was used. The ratio/proportion of the number of species identified in the two areas (1.5) is almost comparable to the ratio of the number of quadrats in the two areas (~1.7). The proportion of the area covered in the two areas (~2-2.14). The number of quadrats per site was established based on the vegetation density, spatial heterogeneity of vegetation and areas of the land-use system (Mengistu et al. Citation2005). Indigenous grass and legumes species assessment was conducted after the main rainy season in the month of October when most plants were at over 50% flowering, which makes the identification of the plants easy.
Comprehensive data on grasses and legumes were collected from the quadrat with 1 m x 1 m. To study the composition and diversity of species identity, and the number of individual grasses and legume species were counted (Brady et al. Citation1995). Herbaceous aboveground biomass production was estimated using the destructive method (t’Mannetje and Jones Citation2000). Forb and grass materials rooted within the quadrat (1 m2) were clipped 2cm above the ground level (clipping at grazing height to give a more applicable measure of forage biomass). The various plant species clipped were then sorted into their relevant functional groups (perennial grasses, forbs, and annual grasses). Their fresh biomass was immediately weighed to determine their aboveground fresh biomass and later oven-dried to a constant weight at 70 °C for 48 h, after which aboveground biomass production was then determined and expressed in tons DM/ha (Verdoodt, Mureithi, and Van Ranst Citation2010). The dry matter (on air-dry matter basis) per quadrate was obtained by dividing the total weight of all quadrants by their numbers. Herbaceous species were classified based on the succession theory described by Dyksterhuis et al. (Citation1949) and on the ecological information (Vorster Citation1982). Accordingly, these species were grouped into (i) highly palatable species: those which occur in rangeland in good condition and decrease with over-grazing and (ii) less palatable species: those which occur in rangeland in good condition. In addition, species were grouped into annuals and perennials as well as by their abundance (widely distributed and most widely distributed) (Solomon, Snyman, and Smit Citation2007).
Data analysis
For identification of species in the field, vernacular names from key informants were used and the specimens were identified by CitationEdwards et al. 000) and CitationHedberg, Ensermu Kelbessa, and Demissew 006). The herbaceous species were classified into grasses, legumes and non-leguminous forbs (Hannaway et al. Citation1999) within each quadrat to determine the contribution of each group in the quadrat (ILCA (International Livestock Center for Africa) Citation1990). The Shannon diversity indices and evenness were used to look at the level of species diversity and evenness of species distribution (Kent and Coker Citation1992). Species diversity in AE and adjacent CGL was determined using species richness, Shannon index of diversity (H’) and Shannon equitability or evenness (E) (Magurran Citation1988). The similarity in the herbaceous species composition of the two land-use systems was computed using Sørensen’s similarity coefficient (Magurran Citation1988, Citation2004). Prior to further statistical analysis, normality and equality variance of the data was checked using Kolmogorov- Smirnov and Levene’s test, respectively (Mekuria et al. Citation2015). T-test with unequal variances was used to determine if there were significant differences between means of the various herbaceous characteristics with respect to different land management practices using R-software version 3.3.3 (The R Core Team Citation2018). Significant differences were declared at p< 0.05. For data that did not require analysis, simple descriptive statistics were employed where appropriate.
Results
Specificities and anomalies in herbaceous species composition and functional groups
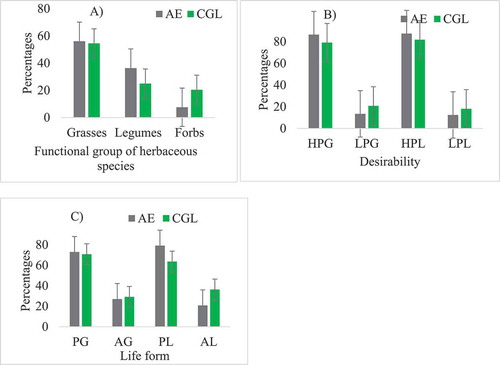
The total number of herbaceous species identified was 66 in the AE and 44 in the CGL of the study area. ) shows the life forms, desirability and functional grouping of herbaceous species in the study areas. The life form distribution of herbaceous species is 56.06% grass, 36.36% legumes and 7.58% forbs, at AE. On the other hand, the life form distribution of herbaceous plant species is 54.55% grass, 25.00% legumes and 20.45% forbs in the CGL . AE (79.17%) had a higher percentage of perennial grasses and CGL (63.64%) the lower . The proportion of highly and less palatable grass species were different between the land-use systems. AE had a higher proportion of highly palatable (86.49%) species and CGL had the lower (79.17%). Less palatable species were greater in the AE than in the CGL areas . The estimated SØrensen similarity index between land uses system was 0.37%; which means that there was 0.63 (63%) difference in herbaceous species composition between the two land-use systems.
Figure 2. The functional group (A), desirability (B) and life forms (C) of herbaceous species in the two land-use systems (HPG= high palatable grasses, LPG= less palatable grasses, HPL= high palatable legumes, LPL= less palatable legumes, PG= Perennial grass, AG= annual grass, PL= Perennial legume, AL= annual legume)
Herbaceous species diversity
Palatable herbaceous species diversity, evenness, and species richness in the study area are presented in . Palatable herbaceous species diversity in the AE had significantly higher than the CGL (t =4.34, df =135, p<0.001). Higher species richness was observed in the AE (4.98), while the lower was recorded in CGL (3.14) and the difference is statistically significant (t=5.00, df =171, p<0.001 . The number of individuals herbaceous species was statistically significant between the two land-use systems (t = 3.26, df = 182, p = 0.001).
Table 1. Mean values of vegetation characteristics in exclosures and adjacent grazing lands (AE=Area Exclosure, CGL= Communal Grazing land)
Aboveground herbaceous biomass
There was a highly significant difference (p < 0.001) in the biomass of herbaceous plants between AE and CGL plots. Aboveground biomass of perennial grasses was significantly (t = 5.37, df = 40.32, p-value < 0.001) higher in the AE than in the CGL . Statistically, management practice shows significant effect on the aboveground biomass of annual grasses (t = −3.45, df = 37.90, p-value = 0.001) . Forbs aboveground biomass was significantly (p<0.001) difference between the land use system (AE and CGL) and higher results were obtained in CGL practice.
Table 2. Aboveground herbaceous biomass (tons/ha) in AE and CGL of the study area (AE=Area Exclosure, CGL= Communal Grazing land)
Dry-matter yield of herbaceous species
Dry matter yields were significantly (p<0.001) different among the land-use system (AE and CGL) and higher results were obtained from the AE practice . Dry matter yields of grass species were higher in the AE than CGL (4.03 and 2.20 ton/ha, respectively). The total dry matter yields measured in AE was 42.17% higher than that of the adjacent CGL.
Table 3. Dry matter yield (tons/ha) (Mean) of herbaceous species in AE and CGL of the study area (AE=Area Exclosure, CGL= Communal Grazing land)
Discussions
Specificities and anomalies in herbaceous species composition and functional groups
The number of individual perennial grasses and legumes were higher at the AE than CGL in the study area. This finding could be an indication that perennial grasses were replaced with annual grasses on the CGL which was subjected to higher grazing pressure. Similar reports have been frequently recorded in heavily grazed arid and semi-arid savannas of Africa (Gemedo, Maass, and Isselstein Citation2006; Mphinyane and Rethman Citation2006; Solomon, Snyman, and Smit Citation2007). The higher composition of the perennial grasses may imply the potential productive nature of the rangeland for livestock production (O’connor, Pickett, and Pickett Citation1992; Amaha Citation2006; Mapako Citation2011). Repeated grazing might lead to a reduction in herbaceous species composition, which might accelerate the decline in rangeland conditions. Similarly, CitationVan Der Westhuizen et al. 001) argued that in arid and semiarid rangelands, herbaceous species composition is greatly influenced by the effects of grazing pressure. As reported by CitationAngassa et al. 010), heavy grazing pressure may reduce plant species composition. Abundance and dominance of forbs and annual grasses is an indication of the poor range condition due to mismanagement or changes in plant species composition in the ecosystem (Camp Citation1997). CitationAnderson and Hoffman 007) noted that poorly managed CGL had a lower proportion of perennial grasses compared to forbs and annual grasses. The increased number of perennial grasses compared to forbs and annuals in the AE could also be an indication of reduced runoff, a fact attributable to improved ground cover (De Groot, Field-Juma, and Hall Citation1992). Similar results were reported by CitationDe-Val and Crawley 005) indicating that in AE highly desirable perennial grasses were found to be abundant. Livestock herbivory can cause shifts in plant species composition by replacing highly palatable grasses with unpalatable species (Rutherford, Powrie, and Husted Citation2012). The dominance of annuals in the CGL could be attributed to poor land management as a result of overgrazing (Solomon, Snyman, and Smit Citation2007). Rangelands are ecologically favored when perennials dominate the ecosystem. In semiarid savannahs, perennial grasses give a better indication of the health status of rangelands than annuals. Perennials yield higher dry matter and provide better soil protection than annuals (Van Wyk and Van Oudtshoorn Citation1999).
The present result suggests that the main reason for a lower number of grass species in communal grazing land is the high grazing intensity throughout the year. Hence, heavy grazing tends to reduce the presence of palatable species and consequently become dominated by other herbaceous plants or bushes (DeHaan, Steinfeld, and Blackburn Citation1997). The same result was reported in eastern Tigray in CGL and AE by CitationEmiru 002); CitationGebrewahd 014) reported that there was an increment of herbaceous species in the AE due to the absence of grazing animals. Ayana (Citation1999) reported that species composition could depend on grazing management and livestock population. CitationTessema et al. 011) reported that the rapid disappearance of the perennial grass community and their subsequent replacement by annual herbs is due to heavy grazing. Selective grazing of palatable herbaceous plants by livestock enhances the growth of annuals and unpalatable herbaceous plants (Skarpe Citation1992) resulting in the decline of palatable species (Fensham et al. Citation2010). On the contrary, annual grasses were higher within the CGL compared to the AE (Ombega et al. Citation2017).
The forbs proportion in the CGL was higher than in the AE. The CGL was highly dominated by forbs, this is in line with prior studies (e.g., Sternberg et al. Citation2000; Mphinyane et al. Citation2008; Kgosikoma Citation2011) reporting that herbaceous plants are highly responsive to grazing pressure. The dominance of forbs in the CGL could be attributed to poor land management as a result of overgrazing (Solomon, Snyman, and Smit Citation2007; Ombega et al. Citation2017). Overgrazing affects the botanical composition by depressing the vigor and presence of dominant species, which then enables colonization by less competitive, but, grazing -tolerant plant species (Sternberg et al. Citation2000; Ayana and Oba Citation2007; Angassa and Oba Citation2010). The unpalatable species might increase the total species pool, thereby indicating higher plant diversity than often expected (Oba et al. Citation2003). Nonetheless, disturbances such as overgrazing favor the establishment of invasive species, survival, and the dominance of short-lived, unflavored annual plant species rather than the palatable perennial species (Byers Citation2002).
Herbaceous species diversity
The overall diversity of herbaceous plants is much higher in AE than CGL, which may be a consequence of the high species richness in AE. The findings of this study are consistent with those of CitationEmiru 002), CitationAngassa et al. 010), CitationYayneshet 011), CitationGebrewahd 014), CitationMureithi et al. 016), and CitationOmbega et al. 017) who reported a higher species richness and diversity in areas under AE than in CGL. The present result suggests that the main reasons for low palatable herbaceous species richness in CGL are increased grazing pressure (Sisay and Baars Citation2002; Desalew Citation2008; Angassa et al. Citation2010); and heavy grazing, trampling and inappropriate management interventions (Amaha Citation2006), might lead to a reduction in herbaceous species diversity. In addition, continuous grazing affects the amount of plant litter at the soil surface and exerts indirect pressures on the germination and seedling establishment patterns (Lishan Citation2007; Desalew Citation2008). The high diversity measured in the AE might be explained by increased litter accumulation, improved soil organic matter and other nutrients inside the AE that eventually lead to increased species richness (Hiernaux Citation1998). However, the results contrast with other studies that reported species richness to be considerably increased under moderate grazing compared to no or heavy grazing treatments (Loeser, Sisk, and Crews Citation2007; Dorrough et al. Citation2007).
There is a global perception that heavy livestock grazing reduces biodiversity and that biodiversity is maximized in primary vegetation (Alkemade et al. Citation2000) . A well-known contemporary grazing-diversity model indicates a decline in diversity with heavy grazing intensity, at least outside areas of high resources (Cingolani, Noy-Meir, and Díaz Citation2005). The decline in the proportion of herbaceous species abundance due to the effect of grazing pressure is consistent with other studies (Angassa and Oba Citation2010; Sisay and Baars Citation2002; Terefe, Ebro, and Tessema Citation2010). Under continuous and increased grazing pressure, palatable plants (decreasers) would die and with the death of decreasers less palatable plants (increasers) become dominant (Mengistu, Angassa, and Abebe Citation2015). This may be related to the presence of high animal grazing pressures due to the presence of a high number of livestock and human activities which is to the damage of palatable herbaceous species. Hence, the palatable herbaceous species dominate the AE and this result agrees with the research findings of CitationTeshome 006), CitationLishan 007), CitationKetama 007), and CitationTesfaye 008). The major reason for the low number of palatable herbaceous species in the CGL is the lack of adequate protection from disturbance by livestock and other related human activities. The “extinction” of most valuable herbaceous species and their replacement by less important annuals was also reported on the work of CitationAbdulatife 009) and CitationIbrahim 016).
Herbaceous species similarity
The estimated SØrensen similarity index of herbaceous species in terms of species richness of the two land-use systems was about 0.37%; which means there was 0.63 (63%) difference in herbaceous species composition between two land-use systems. This indicated a higher dissimilarity of herbaceous species between the two land use systems. Again, the difference in species composition is due to the reappearance of species that were lost from the adjacent communal grazing land over time. This dissimilarity difference to some extent might have resulted from the management role provided by AE in the restoration of fast-growing plants in degraded grazing lands. The extent of species similarity and difference between the land uses might be related to the level of disturbance in the composition of species between the sites (Melkamu and Abdella Citation2019).
Herbaceous biomass production
Aboveground biomass of herbaceous plant species is significantly higher (P ≤ 0.05) in the AE than in the CGL areas. The results are in line with CitationLazim 009), CitationAngassa et al. 010), CitationLazim, Babo, and Elsheikh 012), CitationEltalib, Lazim, and Dawelbait 018), and CitationOmbega et al. 017) who reported that protection, gradually increased plant forage biomass of herbaceous plants. Higher biomass production of herbaceous plant species in the AE could be as a result of improved land management due to the establishment of soil and water conservation (Ruto Citation2015). Proper grazing management through livestock exclusion has been found to enhance above ground biomass in areas that are severely degraded (Wasonga, Nyariki, and Ngugi Citation2011). Moreover, lower biomass production of perennial grasses in the CGL could be a result of year-round grazing which could not allow quick vegetation recovery in the study area (Verdoodt, Mureithi, and Van Ranst Citation2010). These findings corroborate with those of CitationSingh et al. 011) and CitationMekuria and Aynekulu 013) who reported higher biomass production in AE. Grazing throughout the year has consistently reduced the herbaceous aboveground biomass production capability of the grazed areas (Yayneshet, Eik, and Moe Citation2009). Improved above ground biomass in the rehabilitated area could be due to reduced grazing pressure in the rehabilitated sites (Ombega et al. Citation2017).
The biomass production in the AE better than the CGL areas this might be due to better rangeland management practices in the AE, but the CGL areas have deteriorated through continuous overgrazing and the mismanagement system of the community (Ahmed Citation2006; Ibrahim Citation2016). On the other hand, the highest scores for biomass were recorded at enclosed sites reflecting the benefits of reduced disturbance such as the effects of heavy grazing, trampling, and inappropriate management interventions (Ahmed Citation2006). Similarly, CitationAngassa and Oba 010) show that the biomass of herbaceous species was significantly greater in exclosures than in the free grazed areas. CitationVan Der Westhuizen et al. 001) argued that in arid and semiarid rangelands, biomass production of herbaceous is greatly influenced by the effects of grazing pressure. Heavy grazing leads to excessive defoliation of herbaceous vegetation, reducing standing biomass, often triggered by a decline in net primary productivity, as the intensity of grazing increases (Cingolani et al. Citation2003; Friedel et al. Citation2003; Bilotta, Brazier, and Haygarth Citation2007; Stephen et al. Citation2014).
The dry matter yield of grass species was higher in AE than in the CGL. The impact of management factors may be the main reason for the significant difference in terms of herbaceous dry matter yield between the AE and CGL (Mengistu, Angassa, and Abebe Citation2015). The low dry matter yields of forage in the CGL as compared to AE corresponded with the reports of CitationAmsalu 000); CitationAmaha 006); CitationGemedo, Maass, and Isselstein 006); CitationTeklu, Negessa, and Angassa 010); CitationShenkute et al. 011); Teshome et al. Citation2012). The dry matter yield of forb species in the CGL site was found to be higher than in the AE site. The increase in forbs in pasture lands threatens livestock production because encroaching forbs species suppress palatable grasses and herbs through competition for soil moisture and nutrients (Scholes and Archer Citation1997). The increase in the dry matter yield of forbs in the CGL might be evidence for poor range conditions (Gemedo, Maass, and Isselstein Citation2006). This might point out that such low dry matter yield herbaceous in the CGL could directly affect livestock production and sustainability of the rangeland over time (Ahmed Citation2006). Area exclosures are effective in restoring dry matter yield of herbaceous species than the CGL areas. The results of the current study suggest that with continuous grazing pressure on communal lands, the above ground biomass grass production is low, both because of heavy utilization and destruction of grassroots by trampling livestock (Quinfeng, Phillip, and David Citation1999). Consequently, the production capacity of grasses and their ultimate contribution to the total dry matter yield were reduced (Mapako Citation2011).
Conclusions and recommendation
This study showed that the establishment of area exclosures on degraded free grazing lands in the Southern zone of Tigray is a viable option to restore herbaceous vegetation composition, richness, diversity, and aboveground biomass. This is particularly important to consider when planning activities aimed at the responsible management, sustainable utilization, and conservation of the herbaceous plant species at the study sites. Moreover, the study concluded that AE is the potential option for future herbaceous palatable plant species improvement and conservation of key forage species. Area exclosure from a land rehabilitation point of view is expressed through increasing biomass production, species diversity and composition of herbaceous species in the study area. Therefore, based on the present results, the authors recommend the following points: AE is an advisable strategy of herbaceous species rehabilitation, the need for further studies on temporal and spatial herbaceous species biomass should be thoroughly examined under various regimes of grazing exclusions. Although the current study will contribute towards the thoughtful of AE on the herbaceous plant species restoration, further studies that aim to integrate feeds that have higher nutritive values within the feeding system are needed to further appraise feed intake, digestibility, animal’s responses, and anti-nutritional factors for sustainable animal production.
List of abbreviations
AE= area exclosure; CGL= communal grazing land
Authors’ contributions
All authors contributed to the development of the concept and implementation of the study. TA, NG, TGe, and TGi carried out field data collection and data analysis and drafted the manuscript.
Competing interests
We declare that we do not have competing interests.
Acknowledgments
We would like to acknowledge the local community of the study area. The Authors are also very grateful for the reviewers and Yonglong Lu (Ph.D.), Editor in Chief, for their constructive comment on the early version of this article.
Reference
- Abdulatife, M. 2009. “Assessment of pastoral perceptions, range condition, and chemical composition of major feed resources in Chifra district of Afar Regional State.” An MSc Thesis, Haramaya University.
- Abebe, M. H., G. Oba, A. Angassa, and R. B. Weladji. 2006. “The role of area enclosures and fallow age in the restoration of plant diversity in northern Ethiopia.” African journal of ecology 44 (4): 507–11.
- Aerts, R., T. Wagendrop, E. November, B. Mintesinot, J. Deckers, and B. Muys. 2004. “Ecosystem thermal buffer capacity as an indicator of the restoration status of protected areas in the Northern Ethiopian Highlands.” Restoration Ecology 12 (4): 586–596. doi:10.1111/j.1061-2971.2004.00324.x.
- Ahmed, H. 2006. “Assessment and evaluation of utilization practices of natural pastures and other forages in Basona Worana wereda of North Shoa Zone.” School of Graduate Studies of Alemaya University, Dire Dewa, p 174.
- Alkemade, R., M. Van Oorschot, L. Miles, C. Nellemann, M. Bakkenes, and B. Ten Brink. 2000. “GLOBIO3: a framework to investigate options for reducing global terrestrial biodiversity loss.” Ecosystems 12 (3): 374–390. doi:10.1007/s10021-009-9229-5.
- Amaha, K. 2006. “Characterization of Rangeland resources and dynamics of the pastoral production systems in the Somali region of Eastern Ethiopia.” A PhD thesis Presented to the University of the Free State, Bloemfontein, South Africa. 54–232p.
- Amanuel, Z., G. Girmay, and G. Atkilt 2015. “Characterisation of Agricultural Soils in Cascape Intervention Woredas in Southern Tigray, Ethiopia.”
- Amsalu, S. 2000. “Herbaceous species composition, dry matter and condition of the major grazing areas in the middle rift valley, Ethiopia.” M.Sc Thesis. Alemaya University, Dire Dewa, Ethiopia. 159 pp.
- Anderson, P., and M. Hoffman. 2007. “The impacts of sustained heavy grazing on plant diversity and composition in lowland and upland habitats across the Kamiesberg mountain range in the Succulent Karoo, South Africa.” Journal of Arid Environments 70 (4): 686–700. doi:10.1016/j.jaridenv.2006.05.017.
- Angassa, A., and G. Oba. 2010. “Effects of grazing pressure, age of enclosures and seasonality on bush cover dynamics and vegetation composition in southern Ethiopia.” Journal of arid environments 74 (1): 111–120. doi:10.1016/j.jaridenv.2009.07.015.
- Angassa, A., G. Oba, A. C. Treydte, and R. B. Weladji. 2010. “Role of traditional enclosures on the diversity of herbaceous vegetation in a semi-arid rangeland, southern Ethiopia.” Livestock Research for Rural Development 22 (9): 1–8.
- Angassa, A., & Oba, G (2007) Relating long-term rainfall variability to cattle population dynamics in communal rangelands and a government ranch in southern Ethiopia. Agricultural systems, 94(3), 715–725 doi:10.1016/j.agsy.2007.02.012
- Araya, A., and L. Stroosnijder. 2011. “Assessing drought risk and irrigation need in northern Ethiopia.” Agricultural and Forest Meteorology 151 (4): 425–436. doi:10.1016/j.agrformet.2010.11.014.
- Asefa, D. T., G. Oba, R. B. Weladji, and J. E. Coleman. 2003. “An assessment of restoration of biodiversity in degraded High Mountain grazing lands in northern Ethiopia.” Land Degradation & Development 14 (1): 25–38. doi:10.1002/ldr.505.
- Ashebir, D., M. Pasquini, and W. Bihon. 2007. “Urban agriculture in Mekelle, Tigray state, Ethiopia: Principal characteristics, opportunities and constraints for further research and development.” Cities 24 (3): 218–228. doi:10.1016/j.cities.2007.01.008.
- Ayana, A. 1999. “Range Condition and Traditional Grazing Management in Borona.” An MSc. Thesis Presented to the School of Graduate Studies of Alemaya University, Alemaya Ethiopia.73p.
- Bilotta, G. S., R. E. Brazier, and P. M. Haygarth. 2007. “The impacts of grazing animals on the quality of soils, vegetation, and surface waters in intensively managed grasslands.” Advances in Agronomy 94: 237–250.
- Brady, W. W., J. E. Mitchell, C. D. Bonham, and J. W. Cook. 1995. “Assessing the power of the point-line transect to monitor changes in plant basal cover.” Journal of Range Management 48 (2): 187. doi:10.2307/4002808.
- Byers, J. E. 2002. “Impact of non-indigenous species on natives enhanced by anthropogenic alteration of selection regimes.” Oikos 97 (3): 449–458. doi:10.1034/j.1600-0706.2002.970316.x.
- Camp, K. G. T. 1997. The bioresource groups of KwaZulu-Natal. Pietermaritzburg: KwaZulu Natal Department of Agriculture.
- Cingolani, A. M., I. Noy-Meir, and S. Díaz. 2005. “Grazing effects on rangeland diversity: a synthesis of contemporary models.” Ecological applications 15 (2): 757–773. doi:10.1890/03-5272.
- Cingolani, A. M., M. R. Cabido, D. Renison, and V. Solís Neffa. 2003. “Combined effects of environment and grazing on vegetation structure in Argentine granite grasslands.” Journal of Vegetation Science 14 (2): 223–232. doi:10.1111/j.1654-1103.2003.tb02147.x.
- De Groot, P., A. Field-Juma, and D. O. Hall. 1992. Reclaiming the land: revegetation in semi-arid Kenya. Nairobi, Kenya: African Center for Technology Studies (ACTS) Press.
- DeHaan, C., H. Steinfeld, and H. Blackburn. 1997. Livestock and The Environment. Finding a Lance. Suffolk, UK: A Study Sponsored by European Commission, FAO, World Bank, and Others.
- Desalew, T. 2008. “Assessment of feed resources and rangeland condition in Metema district of north Gondar zone, Ethiopia.” Doctoral dissertation, Haramaya University.
- Descheemaeker, K., J. Nyssen, J. Rossi, J. Poesen, M. Haile, D. Raes, and S. Deckers. 2006. “Sediment deposition and pedogenesis in exclosures in the Tigray Highlands.” Ethiopia Geoderma 132 (3–4): 291–314. doi:10.1016/j.geoderma.2005.04.027.
- De-Val, E., and M. J. Crawley. 2005. “Are grazing increaser species better tolerators than decreasers? An experimental assessment of defoliation tolerance in eight British grassland species.” Journal of Ecology 93 (5): 1005–1016. doi:10.1111/j.1365-2745.2005.01011.x.
- Dorrough, J., J. Ash, S. Bruce, and S. McIntyre. 2007. “From plant neighborhood to landscape scales: how grazing modifies native and exotic plant species richness in grassland.” Plant Ecology 191 (2): 185–198. doi:10.1007/s11258-006-9236-y.
- Dyksterhuis, E. J., D. T. Asefa, G. Oba, R. B. Weladji, and J. E. Colman. 1949. “Condition and management of rangeland based on quantitative ecology.” Journal of Range Management 2 (3): 104–115. doi:10.2307/3893680.
- Edwards, S., T. Mesfin, D. Sebsebe, and I. Hedberg, eds.. 2000. Flora of Ethiopia and Eritrea, Vol. 2. Part 1. Uppsal: The National Herbarium, Addis Ababa University, Addis Ababa and Department of Systematic Botany, Uppsala University.
- Eltalib, M. A. M., A. M. M. Lazim, and E. M. Dawelbait. 2018. “The Influence of Enclosures on Vegetation Attributes of Degraded Rangelands of North Darfur, Sudan.” Sudan Journal Design Research 7 (1): 13–25.
- Emiru, B. 2002. “Actual and potential contributions of enclosure to enhance biodiversity in dry lands of Eastern Tigray, with particular emphasis on woody plants.” MSc Thesis, Swedish University, Sweden.
- Fensham, R. J., R. J. Fairfax, J. M. Dwyer, and J. E. Byers. 2010. “Vegetation responses to the first 20 years of cattle grazing in an Australian desert.” Ecology 91 (3): 681–692. doi:10.1890/08-2356.1.
- Friedel, M. H., A. D. Sparrow, J. E. Kinloch, and D. J. Tongway. 2003. “Degradation and recovery processes in arid grazing lands of central Australia. Part 2: vegetation.” Journal of arid environments 55: 327–348.
- Gebrewahd, A. 2014. “Herbaceous vegetation restoration potential and soil physical condition in a mountain grazing land of Eastern Tigray, Ethiopia.” JAEID 2014 108 (1): 81–106.
- Gemedo, D. T., B. L. Maass, and J. Isselstein. 2006. “Rangeland condition and trend in the semi-arid Borana lowlands, southern Oromia, Ethiopia.” African Journal of Range and Forage Science 23 (1): 49–58. doi:10.2989/10220110609485886.
- Getnet, A. 2003. “Feed Resource Development and Utilization: Possible Options and Recommendations under Ethiopian Condition.” Training Handout Prepared For Agricultural Subject Matter Specialists (SMS), Holeta Agricultural Research Centre, Addis Ababa, Ethiopia, pp:1–37.
- Hannaway, K. J., D. B. Hannaway, P. E. Shuler, M. L. Niess, S. Griffith, G. W. Fick, and V. G. Allen. 1999. “World Wide Web Curriculum Design Using National Collaboration.” Journal of Natural Resources and Life Sciences Education 28 (1): 59–62. doi:10.2134/jnrlse.1999.0059.
- Hedberg, I., E. S. Ensermu Kelbessa, and S. Demissew, eds.. 2006. Flora of Ethiopia and Eritrea, Vol. 5. Gentianaceae to Cyclocheilaceae. Sweden: The National Herbarium, Addis Ababa University and Uppsala University Department of Systematic Botany, Uppsala University.
- Hiernaux, P. 1998. “Effects of grazing on plant species composition and spatial distribution in rangelands of the Sahel.” Plant Ecology 138 (2): 191–202. doi:10.1023/A:1009752606688.
- Hunting 1976. “Central Tigray Development Study, Working papers”, Hunting Technical Service Limited, London, England.
- Ibrahim, M. A. 2016. “Impact of Enclosure on Plant Species Composition and Biomass Production in Ewa Woreda of Afar Region State, Ethiopia.” Journal Biodivers Endanger Species 4: 157.
- ILCA (International Livestock Center for Africa) 1990. “Livestock Systems Research Manual.” Working Paper 1, Vol. 1. ILCA, Addis Ababa, Ethiopia. 287p.
- Kalema, V. N. 2010. “Diversity, use, and resilience of woody plants in a multiple land-use equatorial African savanna, Uganda.” Ph.D. Thesis, Johannesbur: University of the Witwatersrand
- Kent, M., and P. Coker. 1992. Vegetation Description and Analysis: a Practical approach. New York: Bent Haven Press.
- Ketama 2007. “Biomass production, utilization practices and range condition in the nuer zone of Gambella, Ethiopia.” An MSc thesis, Presented to the School of Graduate Studies of Alemaya University, Dirdewa Ethiopia. 125p.
- Kgosikoma, O. E. 2011. “Understanding the savanna dynamics in relation to rangeland management systems and Environmental conditions in semi-arid Botswana.” PhD thesis. University of Edinburgh.
- Kindeya, G. 2003. “Ecology and management of Boswellia papyearifera (Del.) Hochst. Dry forests in Tigray, Northern Ethiopia.” Doctoral Dissertation. Georg-August University of Göttingen. Germany. pp. 182.
- Lazim, A. M. 2009. “Response of Natural Range Vegetation to Four Management Practices at Tillow Area (South Kordofan State).” A Ph.D. Thesis, Sudan Academy of Science. Khartoum, Sudan.
- Lazim, A. M., M. F. Babo, and S. E. Elsheikh. 2012. “A note on impact of management practices on herbaceous forage productivity and carrying capacity in the Rangelands of South Kordofan.” Sudan. Sudan Journal Design Research 4 (1): 135–142.
- Lishan, T. 2007. “Woody and herbaceous species composition and the condition of the rangelands in Shinile zone of Somali Regional State, Ethiopia.” M.Sc. Thesis, School of Graduate Studies. Haramaya University.
- Loeser, M. R., T. D. Sisk, and T. E. Crews. 2007. “Impact of grazing intensity during drought in an Arizona Grassland.” Conservation Biology 21 (1): 87–97. doi:10.1111/j.1523-1739.2006.00606.x.
- Magurran, A. 2004. Measuring biological diversity. Oxford, UK: Blackwell Publishing.
- Magurran, A. E. (1988) Ecological diversity and its measurement. Princeton, NJ: Princeton University Press; p. 93.
- Mapako, L. 2011. “Assessment of vegetation diversity and rangeland condition in the Highveld communal grazing lands of Swaziland (Doctoral dissertation).”
- Martínez‐Garza, C., and H. F. Howe. 2003. “Restoring tropical diversity: beating the time tax on species loss.” Journal of Applied Ecology 40 (3): 423–429. doi:10.1046/j.1365-2664.2003.00819.x.
- Mekuria, W., and E. Aynekulu. 2013. “Exclosure land management for restoration of the soils in degraded communal grazing lands in northern Ethiopia.” Land Degradation & Development 24 (6): 528–538. doi:10.1002/ldr.1146.
- Mekuria, W., and E. Veldkamp. 2012. “Restoration of native vegetation following exclosure establishment on communal grazing lands in Tigray, Ethiopia.” Applied Vegetation Science 15 (1): 71–83. doi:10.1111/j.1654-109X.2011.01145.x.
- Mekuria, W., S. Langan, R. Johnston, B. Belay, D. Amare, T. Gashaw, G. Desta, A. Noble, and A. Wale. 2015. “Restoring aboveground carbon and biodiversity: a case study from the Nile basin, Ethiopia.” Forest Science and Technology 11 (2): 86–96. doi:10.1080/21580103.2014.966862.
- Melkamu, T., and G. Abdella. 2019. “Effect of exclosure on woody species diversity and population structure in comparison with adjacent open grazing land: the case of Jabi Tehnan district north western Ethiopia.” Ecosystem Health and Sustainability 5 (1): 98–109. doi:10.1080/20964129.2019.1593794.
- Mengistu, A., A. Angassa, and A. Abebe. 2015. “The Effects of Area Enclosures on Rangeland Condition, Herbaceous Biomass and Nutritional Quality in Southeast Ethiopia.” Science, Technology and Arts Research Journal 4 (2): 79–88.
- Mengistu, T., D. Teketay, H. Hulten, and Y. Yemshaw. 2005. “The role of enclosures in the recovery of woody vegetation in degraded dry land hillsides of central and northern Ethiopia.” Journal of arid environments 60 (2): 259–281.
- Mphinyane, W. N., G. Tacheba, S. Mangope, and J. Makore. 2008. “Influence of stocking rate on herbage production, steers livemass gain and carcass price on semi-arid sweet bushveld in Southern Botswana.” African Journal Agricultural Research 3: 084–090.
- Mphinyane, W. N., and N. F. G. Rethman. 2006. “Livestock utilization of grass species at different distances from water on both traditional cattle post and ranch management systems in Botswana.” African Journal of Range and Forage Science 23 (2): 147–151. doi:10.2989/10220110609485897.
- Muluberhan, H., O. Gufu, A. Angassa, and R. B. Weladji. 2006. “The role of area enclosures and fallow age in the restoration of plant diversity in northern Ethiopia.” African Journal of Ecology 44 (4): 507–514.
- Mureithi, S. M., A. Verdoodt, J. T. Njoka, C. K. Gachene, F. Warinwa, and E. Van Ranst. 2016. “Impact of community conservation management on herbaceous layer and soil nutrients in a Kenyan semi-arid savannah.” Land Degradation and Development 27 (8): 1820–1830. doi:10.1002/ldr.2315.
- O’Connor, T. G., and G. A. Pickett. 1992. “The influence of grazing on seed production and seed banks of some African Savanna grasslands.” Journal of Applied Ecology 29 (1): 247–260. doi:10.2307/2404367.
- Oba, G., O. R. Vetaas, and N. C. Stensteth. 2001. “Relationships between biomass and plant species richness in arid-zone grazing lands.” Journal of Applied Ecology 38 (4): 836–846. doi:10.1046/j.1365-2664.2001.00638.x.
- Oba, G., R. B. Weladji, W. J. Lusigi, and N. C. Stenseth. 2003. “Scale dependent effects of grazing on rangeland degradation in northern Kenya: a test of equilibrium and non-equilibrium hypothesis.” Land Degradation & Development 14 (1): 83–94. doi:10.1002/ldr.524.
- Ombega, N. J., S. M. Mureithi, O. K. Koech, A. N. Karuma, and C. K. K. Gachene. 2017. “Effect of rangeland rehabilitation on the herbaceous species composition and diversity in Suswa catchment, Narok County, Kenya.” Ecological Processes 6 (1): 41. doi:10.1186/s13717-017-0109-1.
- Ormerod, S. J. 2003. “Restoration in applied ecology: editor’s introduction.” Journal of Applied Ecology 40 (1): 44–50. doi:10.1046/j.1365-2664.2003.00799.x.
- Quinfeng, G., W. R. Phillip, and W. G. David. 1999. “Structure of seed banks: comparisons across four North American desert sites.” Journal of arid environments 42 (1): 1–14. doi:10.1006/jare.1999.0502.
- Ruiz-Jaen, M. C., and T. M. Aide. 2005. “Restoration success: how is it being measured?.” Restoration Ecology 13 (3): 569–577. doi:10.1111/j.1526-100X.2005.00072.x.
- Rutherford, M. C., L. W. Powrie, and L. B. Husted. 2012. “Herbivore-driven land degradation: consequences for plant diversity and soil in arid subtropical thicket in southeastern Africa.” Land Degradation & Development 25 (6): 541–553. doi:10.1002/ldr.2181.
- Ruto, A. C. 2015. “Optimizing moisture and nutrient variability under different cropping patterns in terraced farms for improved crop performance.” In: Narok County, Kenya Doctoral dissertation, Department of Land Resources Management and Agricultural Technology, Faculty of Agriculture, University of Nairobi
- Scholes, R. J., and S. R. Archer. 1997. “Tree-grass interactions in savannas.” Annual review of ecology and systematics 28 (1): 517–544. doi:10.1146/annurev.ecolsys.28.1.517.
- Shenkute, B., H. Abubeker, E. Abule, A. Tadese, and A. Nura. 2011. “Identification of potential untapped herbaceous flora in the mid rift valley of Ethiopia and their nutritive value.” African Journal of Agricultural research 6 (17): 4153–4158.
- Singh, G., G. R. Choadhary, B. Ram, and N. K. Limba. 2011. “Effects of rainwater harvesting on herbage diversity and productivity in degraded Aravalli hills in western India.” Journal of Forestry Research 22 (3): 329. doi:10.1007/s11676-011-0177-5.
- Sisay, A., and R. M. T. Baars. 2002. “Grass composition and rangeland condition of the major grazing areas in the mid Rift Valley, Ethiopia.” African Journal of Range and Forage Science 19 (3): 161–166. doi:10.2989/10220110209485789.
- Skarpe, C. 1992. “Dynamics of savanna ecosystems.” Journal of Vegetation Science 3 (3): 293–300. doi:10.2307/3235754.
- Solomon, M., and A. Teferi. 2010. “Chemical composition, in vitro dry matter digestibility and in Sacco degradability of selected browse species used as animal feeds under semi-arid conditions in Northern Ethiopia.” Agro Forest System 80: 173–184.
- Solomon, T. B., H. A. Snyman, and G. N. Smit. 2007. “Rangeland dynamics in southern Ethiopia: (1) Botanical composition of grasses and soil characteristics in relation to land-use and distance from water in semi-arid Borana rangelands.” Journal of environmental management 85: 429–442.
- Southern Zone bureau of agriculture and rural development (BOARD) 2018. “Natural resources core process, annual report. Southern Zone of Office of Agriculture and rural Development.” Unpublished, Tigrigna version.
- Stephen, M., A. V. Mureithi, T. Jesse, C. K. K. G. Njoka, W. Fiesta, and E. Van Ranst. 2014. Impact of Community Conservation Management on Herbaceous Layer and Soil Nutrients in a Kenyan Semi-Arid Savannah. Land Degrad: Develop. doi:10.1002/ldr.2315.
- Sternberg, M., M. Gutman, A. Perevolotsky, E. D. Ungar, and J. Kigel. 2000. “Vegetation response to grazing management in a Mediterranean herbaceous community: a functional group approach.” Journal of Applied Ecology 37 (2): 224–237. doi:10.1046/j.1365-2664.2000.00491.x.
- t’Mannetje, L., and R. M. Jones. 2000. Field and laboratory methods for grassland and animal production research. UK, Wallingford: CABI.
- Tefera, M. 2001. “The role of enclosures in the restoration of woody species in degraded hillsides of Biyo and Tiya, Central and Northern Ethiopia.” Doctoral dissertation, MSc thesis, Swedish University of Agricultural Sciences, Skinnskatteberg, Sweden.
- Tefera, M., T. Demel, H. Hulten, and Y. Yonas. 2005. “The role of enclosure in the recovery of woody vegetation in degraded dryland hillsides of central and northern Ethiopia.” Journal of arid environments 60 (2): 259–281.
- Teketay, D., K. Kashe, J. Madome, M. Kabelo, J. Neelo, M. Mmusi, and W. Masamba. 2018. “Enhancement Of Diversity, Stand Structure and Regeneration of Woody Species Through Area Exclosure: The case of a Mopane Woodland in Northern Botswana.” Ecological Processes 7 (1): 299. doi:10.1186/s13717-018-0116-x.
- Tekle, K. 2001. “Natural regeneration of degraded hill slopes in Southern Wello, Ethiopia: a study based on permanent plots.” Applied Geography 21 (3): 275–300. doi:10.1016/S0143-6228(01)00006-6.
- Tekle, K., and T. Bekele. 2000. “The role of soil seed banks in the rehabilitation of degraded hill slopes in southern Wello, Ethiopia.” Biotropica 32 (1): 23–32. doi:10.1111/j.1744-7429.2000.tb00444.x.
- Teklu, B., T. Negessa, and A. Angassa. 2010. “Effect of farming system on floristic composition, yieldand nutritional content of forages at the natural pasture of Assosa Zone.” Tropical and Subtropical Agroecosystem 12: 583–592.
- Terefe, A., A. Ebro, and Z. K. Tessema. 2010. “Rangeland dynamics in South Omo Zone of Southern Ethiopia: Assessment of rangeland condition in relation to altitude and Grazing types.” Livestock Research for Rural Development 22 (10).
- Tesfay, A., B. Anteneh, and Z. Tessema. 2019. “Woody species diversity, population structure, and regeneration status in the Gra-Kahsu natural vegetation, southern Tigray of Ethiopia.” Heliyon 5 (1): e01120. doi:10.1016/j.heliyon.2019.
- Tesfay, A., W. Solomon, G. Nguse, G. Tesfay, and G. Tsgehiwet 2018. “Utilization of indigenous trees and shrub species as animal feed resources, in the case of south Tigray, north Ethiopia.” The implication for livestock feed resource. Tigray Agricultural Research Institute, Alamata Agriculture Research Center (unpublished).
- Tesfay, B., G. Tsegay, K. Haftu, A. Teferi, and H. Bereket, (eds.) 2019. “Participatory Agricultural Production Constraints Appraisal: Implication for Research and Development Interventions in Southern, North Western and Western Zones of Tigray. Tigray Agricultural Research Institute and Agricultural Growth Program-II.” Proceedings of the Workshop held 09-15 November 2018, Capital Hotel, Wukro, Tigray, Ethiopia and Working paper no. 1.
- Tesfaye, D. 2008. “Assessment of feed resources and rangeland conditions in metema district of north Gondar zone, Ethiopia.” MSc thesis, Presented to the School of Graduate Studies of Alemaya University, Dirdewa Ethiopia. 135p.
- Teshome, A. 2006. “Traditional utilization practices and condition assessment of the rangelands in Rayitu district of Bale Zone, Ethiopia.” MSc thesis, Presented to the School of Graduate Studies of Alemaya University, Dirdewa Ethiopia. 125p.
- Teshome, A., E. Abule, and N. Lisanework. 2012. “Evaluation of rangeland in arid and semi-arid grazing land of southeast Ethiopia.” International Journal of Agricultural Science 2 (7): 221–234.
- Tessema, Z., B. W. De, R. Baars, and H. Prins. 2011. “Changes in vegetation structure, herbaceous biomass and soil nutrients in response to grazing in semi‐arid savannas in Ethiopia.” Journal of Arid Environments 75 (7): 662–670. doi:10.1016/j.jaridenv.2011.02.004.
- The R Core Team. 2018. R: A Language and Environment for Statistical Computing, Version. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
- Tsegay, G., Z. K. Tessema, S. Negasi, and B. Emiru. 2019. “Carbon sequestration and soil restoration potential of grazing lands under exclosure management in a semi-arid environment of northern Ethiopia.” Ecology and evolution 9: 6468–6479.
- Van Der Westhuizen, H., H. Snyman, W. Van Rensburg, and J. Potgieter. 2001. “The quantification of grazing capacity from grazing and production values for species in the semi-arid grassland biome of southern Africa.” African Journal of Range & Forage Science 18 (1): 43–52. doi:10.2989/10220110109485754.
- Van Wyk, E., and F. Van Oudtshoorn. 1999. Guide to grasses of Southern Africa. First ed. Pretoria, South Africa: Briza Publications.
- Verdoodt, A., S. M. Mureithi, and E. Van Ranst. 2010. “Impacts of management and enclosure age on the recovery of herbaceous rangeland vegetation in semiarid Kenya.” Journal of Arid Environments 74 (9): 1066–1073. doi:10.1016/j.jaridenv.2010.03.007.
- Vorster, M. 1982. “The development of the ecological index method for assessing veld condition in the Karoo.” Proceedings of the Annual Congresses of the Grassland Society of Southern Africa 17 (1): 84–89. doi:10.1080/00725560.1982.9648962.
- Wasonga, V. O., D. M. Nyariki, and R. K. Ngugi. 2011. “Assessing Socio-Ecological Change Dynamics Using Local Knowledge in the Semi-Arid Lowlands of Baringo District, Kenya.” Environmental Research Journal 5 (1): 11–17. doi:10.3923/erj.2011.11.17.
- Wolde, M., and E. Aynekulu. 2011. “Enclosure land management for restoration of the soils in degraded communal grazing lands in northern Ethiopia.” Land Degradation and Development 24: 528–538.
- Wolde, M., E. Veldkamp, M. D. Corre, and M. Haile. 2011b. “Restoration of ecosystem carbon stocks following enclosure establishment in communal grazing lands in Tigray, Ethiopia.” Soil Science Society of America Journal 75 (1): 246–256. doi:10.2136/sssaj2010.0176.
- Wolde, M., and Y. Mastewal. 2013. “Changes in woody species composition following establishing exclosures on grazing lands in the lowlands of Northern Ethiopia.” African Journal of Environmental Science and Technology 7 (1): 30–40.
- Wondie, M., M. Eyayu, and G. Temesgen. 2014. “A comparative study of woody plant species diversity at Adey Amba enclosed forest and nearby open site in West Belessa district, northwestern Ethiopia.” JBAH 4: 15.
- Yayneshet, T. 2011. “Restoration of degraded semi-arid communal grazing land vegetation using the enclosure model.Mekelle University, Mekelle, Ethiopia.” International Journal Water Resources Arid Environmental 1 (5): 382–386.
- Yayneshet, T., L. O. Eik, and S. R. Moe. 2009. “The effects of exclosures in restoring degraded semi-arid vegetation in communal grazing lands in northern Ethiopia.” Journal of Arid Environments 73 (4–5): 542–549. doi:10.1016/j.jaridenv.2008.12.002.
- Young, S. 1958. “Exclosures in big game management in Utah.” Journal of Range Management 11 (4): 187–190. doi:10.2307/3893669.