The biodiversity of microfungi associated with decomposing leaf litter of Anacardium occidentale and the pattern of fungal colonization was investigated. Fungi colonizing decomposing litter were induced to sporulate by a moist chamber incubation technique. A total of 77 species belonging to 57 genera were recorded. Depending on the first appearance of the fungi and their persistence, three main groups of fungal colonizers were identified: (1) fungi that occurred initially during early stages of decomposition and disappeared as decomposition progressed; (2) fungi that showed less initial activity but gradually increased their activity and declined in the final stages; (3) fungi that appeared in the late stages of decomposition.
Introduction
Litter decomposition is an important process of nutrient cycling in forest ecosystems (Charley and Richards Citation1983), playing a major role in the transfer of energy and nutrients (Toky and Singh Citation1983). It is now well established that the decomposition of plant litter on the soil surface is brought about by a variety of microorganisms, including bacteria, fungi and actinomycetes. Among the microbes, fungi are regarded as efficient decomposers of organic matter, especially plant litter (Dickinson and Pugh Citation1974).
There are numerous ecological studies of litter microfungi, especially the pattern of fungal colonization on naturally occurring plant debris above the soil. Chesters (Citation1950) gave a descriptive account of the succession of ascomycetes associated with the early colonization and decay of deciduous logs and branches. Mangenot (Citation1952) carried out a more extensive study of the fungi concerned in the progressive decay of dead deciduous tree trunks. There are extensive studies of fungal succession on various substrates; for example those by Visser and Parkinson (Citation1975) on Populus tremuloides, Mehrotra and Aneja (Citation1979) on Chenopodium album, Subramanian and Vittal (Citation1979, Citation1980) on Atlantia monophylla and Gymnosporia emarginata, Kuter (Citation1986) on Acer saccharum, Heredia (Citation1993) on Quercus germana, Q. sartorii and Liquidambar styraciflua, Rosenbrock et al. (Citation1995) on Alder glutinosa, Pasqualetti et al. (Citation1999) on Myrtus communis, Valenzuela et al. (Citation2001) on Nothofagus pumilio, Promputtha et al. (Citation2002) on Manglietia garrettii, Zhou and Hyde (Citation2002) on bamboos, Thorman et al. (Citation2003) on Sphagnum fuscum, Carex aquatilis, Salix planifolia, Sadaka et al. (Citation2003) on Quercus rotundifolia, Osono et al. (Citation2004) on Swida controversa, Tang et al. (Citation2005) on Castanopsis fissa, Paulus et al. (Citation2006) on Ficus pleurocarpa, Duong et al. (Citation2008) on Castanopsis diversifolia, Kodsueb et al. (Citation2008) on Magnolia lilifera, and Osono et al. (Citation2009) on Shorea obtusa.
Anacardium occidentale L. (cashew, family Anacardiaceae), native to South America, was introduced in to India four centuries ago. Cashew is an important cash crop and India ranks second to Brazil in production, with about 300,000 ha under cultivation. The plant is a slow-spreading, evergreen, perennial, bushy, low-branched tree, about 6–10 m tall, and the lower branches often bend to touch the ground. Cashew plantations have vast potential as organic biomass available for recycling, while the leaf litter is extensively used for composting. The quantity of litter available under different age groups of cashew plantations ranged from 1.38 to 5.2 tonnes/ha/year for 10–15 and 25–40-year-old plantations, respectively (Kumar and Hegde, Citation1999). Despite its high litter production and its utility in composting, no systematic attempts have been made to investigate the fungal diversity associated with leaves and litter of this plant.
Materials and methods
Study site
The study was carried out within the compound of a cashew plantation at the Theosophical Society, located at Adyar, South-East of Chennai along the Bay of Bengal coast. The Society is spread over an area of 266 acres with scattered buildings surrounded by luxuriant vegetation of three distinct types, namely mangroves, psammophytes and tropical dry evergreen elements. The soil is sandy loam with high porosity. The vegetation is mostly dominated by Anacardium occidentale occupying large areas. In addition, plant taxa such as Berrya cordifolia, Cassia fistula, Dillenia indica, Swietenia mahogoni, Terminalia catappa, Tectona grandis, Syzygium cumini, Mimusops elengi, Adansonia digitata, Sterculia foetida, Adenanthera pavonina, Couropita gigantea are found scattered in the campus. The climate is hot and humid for 8 months of the year. Rainfall is high during June and November due to the onset of the southwest and northeast monsoons, respectively. Annual precipitation during the study period was 903 mm with the average maximum and minimum temperatures being 33.8 and 24.6°C, respectively.
Sampling procedure
The fungal diversity and pattern of colonization were studied by the litter bag technique (Kshattriya et al. Citation1994) over a period of 1 year (January 2002 to November 2003). Forty eight nylon bags, each measuring 25 × 20 cm and with mesh size of 2 mm were used. Twenty air-dried senescent leaves of Anacardium occidentale collected directly from the tree were packed in each bag and the bags were placed randomly on the forest floor underneath the trees () during December 2001. A second batch of another 12 bags, each containing 5 g of the senescent leaves (20 leaves) were also placed on the floor underneath the trees. These were to monitor weight loss during the course of decomposition, which was measured by oven-drying at 80°C for 48 h (Lousier and Parkinson Citation1976).
Four randomly selected litter bags were removed from the forest floor each at 2-month intervals and taken to the laboratory for enumeration of fungal diversity. The first set of samples was removed during the last week of January 2002. The leaf litter samples were incubated in moist chambers for 48 h and then examined under a stereomicroscope (). Sporulating fungi were recorded and identified to species level. Furthermore, the ‘periodicity of occurrence’ (POC) and ‘frequency of occurrence’ (FOC) for each fungal species was calculated. The POC denotes the number of samplings in which a taxon was recorded as against total samplings (12). FOC was calculated on the basis of the number of leaves on which a particular taxon was recorded as against the total number of leaves examined (80). The data is expressed as percentage periodicity of occurrence (%POC) and percentage frequency of occurrence (%FOC). Based on %POC, the fungi were classified as ‘most common’ 76–100%, ‘common’ 51–75%, ‘occasional’ 26–50% and ‘sporadic’ 1–25%.
Results
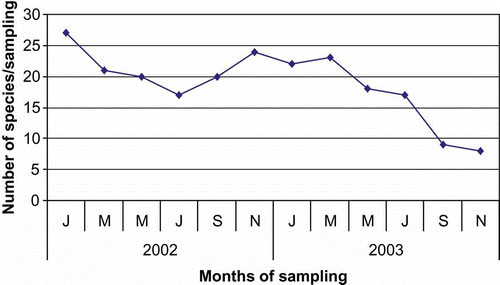
Altogether, 960 leaves were examined during the 2-year period of study. These yielded 77 species belonging to 57 genera: one taxon belonging to Myxomycota, seven to Zygomycota, three to Ascomycota and 66 anamorphic fungi. The latter included six coelomycetes and 60 hyphomycetes (). The number of fungal taxa recorded per sampling varied from 8 to 27 (). Higher numbers were recorded in the initial stages, and the number of fungi decreased as decomposition progressed.
Table 1. List of fungi recorded and percentage frequency of occurrence in different samplings
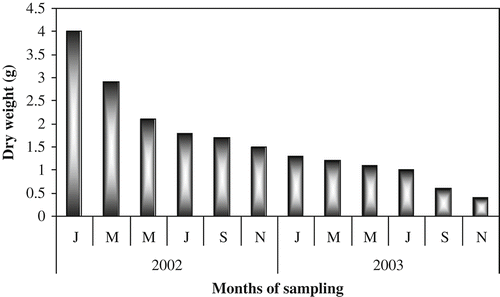
The dry weight values showed rapid weight loss during the initial 8-month period of incubation, with gradual loss thereafter (). The weight loss was almost 93% in the final set of bags collected during November 2003; the leaves were fragmentary and in an advanced stage of decomposition ().
Of the 77 species recorded, four taxa, viz. Beltrania rhombica, Beltraniella portoricensis, Gyrothrix circinata and Subramaniomyces fusisaprophyticus were ‘most common’ and recorded in all the 12 samplings. Among the six species classed as ‘common’, Acremonium strictum, Circinotrichum falcatisporum and Parasympodiella laxa showed high periodicity of occurrence. Similarly, Pestalotiopsis theae and Cladosporium cladosporioides occurred with high periodicity of occurrence among the 11 taxa recorded occasionally. Fifty-six species comprising 73% of the total species recorded were ‘sporadic’ in occurrence and they were confined to 1–3 samplings.
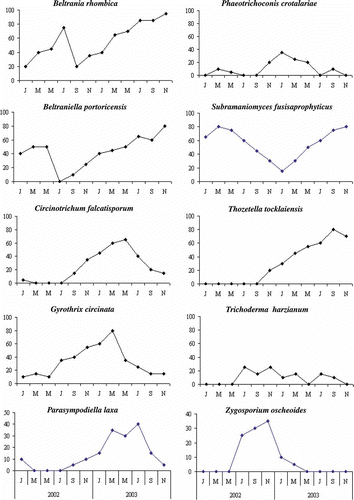
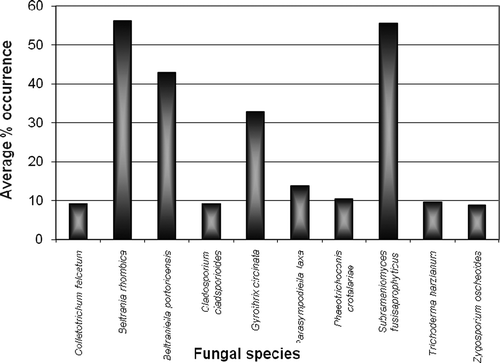
The data recorded on the frequency of occurrence of individual taxa over the 2-year period displayed no specific pattern. However, a critical analysis of the data revealed that fungi such as Beltrania rhombica and Beltraniella portoricensis, although present in all the samplings, were more abundant in the latter stages of decomposition (). On the other hand, the frequency of occurrence of Circinotrichum falcatisporum, Gyrothrix circinata, Parasympodiella laxa, Phaeotrichoconis crotalariae and Zygosporium oscheoides, although low during the initial stages, gradually increased as decomposition progressed and then declined in the final stages of decomposition. Thozetella tocklaiensis made its appearance during the mid-stages of decay and its frequency of occurrence increased as decomposition progressed. Subramaniomyces fusisaprophyticus, although one of the most dominant colonizers of Anacardium litter, consistently declined during the mid-stage of decomposition, before steadily increasing towards the end. The average percentage frequency of occurrence (average of 12 sampling) of selected fungi is compared in .
Taking the average percent occurrence of each species (computed as an average of the number of samplings in which a particular fungus was present) as an index of colonizing efficiency, the most active colonizers of Anacardium litter were Beltrania rhombica (56.3%), Subramaniomyces fusisaprophyticus (55.4%), Beltraniella portoricensis (42.9%), Gyrothrix circinata (32.9%), Circinotrichum falcatisporum (25%), Parasympodiella laxa (13.8%), Acremonium strictum (10.4%) and Phaeotrichoconis crotalariae (10.4%).
Discussion
The study of the pattern of fungal colonization on leaf litter using litter bags was previously demonstrated by Tempesta et al. (Citation2003), who carried out succession of microfungi in Phillyrea angustifolia litter in a Mediterranean maquis in Sardinia. They described two main groups of fungi involved in the process of litter degradation, whose presence was correlated with the successive decomposition stages of the substrate and with seasonal variations. Osono et al. (Citation2009) studied fungal succession and decomposition on Shorea obtusa leaves using polyethylene litter bags to examine the effect of colonization by ligninolytic and cellulolytic fungi in the overall process of decomposition. In the present study, nylon bags of mesh diameter size 2 mm were used to incubate the leaf litter on the forest floor.
Different methodologies were carried out to study fungal diversity on the leaf litter in different communities. The methodology of indirect isolation often results in more taxa (including non-sporulating morphospecies) than direct observation (Bills and Polishook Citation1994; Bills Citation1996; Polishook et al. Citation1996; Paulus and Hyde Citation2003). Indirect isolation methods have been criticized (Paulus and Hyde Citation2003) because they recover both active fungi and dormant spores that do not participate in leaf decomposition. Incubating the litter samples in moist chambers and observing the fungi developing thereon still remains the best way of analyzing mycoflora (Webster Citation1956 Citation1957; Hering Citation1965; Hogg and Hudson Citation1966; Promputtha et al. Citation2002; Kodseub et al. Citation2008), as one can observe many of the typical litter fungi in sporulating condition and which rarely show up in cultural methods. The direct method, in which the substratum is examined in the field or laboratory for fruiting bodies, is regarded as the most common approach for taxonomic purposes (Paulus et al. Citation2003). Therefore, the direct observation method is preferred in the present study in identifying fungal communities on leaf litter.
Fungal diversity
The highest fungal diversity occurred during the first month of sampling with the most species being present (). Then, fungal diversity gradually declined over the next 3 months and then increased from the fifth and sixth month, and fell thereafter till the leaves were completely decomposed. From the seventh month of incubation, the leaves were found to be skeletonized; thus the fungal communities had decreased in number. The time for fungal communities to reach a peak of species diversity or fungal activity has varied in different studies. For example, complete decomposition of sugarcane bagasse needed 20 weeks and maximum colony counts were recorded during weeks 6–13 (Sandhu and Sidhu Citation1980). In a 2-year study of the decomposition of leaf and root litter of pineapple, the number of species, the number of viable propagules associated with the litter and the rate of weight loss were maximal during the middle phase of litter decomposition (Tiwari et al. Citation1994). In the decomposition of the senescent leaves of Manglietia garretti, highest species diversity was observed on day 40 of the decomposition (Promputtha et al. Citation2002).
Pattern of fungal colonization
It is evident from the data that some of the fungi belonging to Zygomycota, and the anamorphic fungi such as Cladosporium, Curvularia, Drechslera, Pithomyces and Nigrospora were recorded only during the initial one or two samplings, suggesting that they may be carry over species from the phylloplane. Their frequency of occurrence on surfaces of green foliage has been reported previously (Subramanian & Vittal Citation1979, Citation1980) and they are considered primary saprophytes, which are ubiquitous inhabitants of aerial plant surfaces (Hudson Citation1968). The activity of these fungi was limited to freshly fallen litter and any records of these species in later stages of decomposition would be sporadic. Based on the hierarchical clustering, it was found that Beltrania rhombica, Beltraniella portoricensis, Subramaniomyces fusisaprophyticus and Gyrothrix circinata were typical litter colonizers in the initial stages of decomposition. These are considered as true ‘litter fungi’. As decomposition progressed, these taxa were joined by a second group of colonizers, such as Acremonium strictum, Parasympodiella laxa and Trichoderma harzianum. Finally, in the later stages of decomposition, a third group of fungi, consisting of Phaeotrichoconis crotalariae and Thozetella tocklaiensis, appeared and persisted. The significant features of colonization of Anacardium litter by microfungi, as elucidated in the present study, revealed that the primary colonizers are the ‘foliicolous fungi’, which arrive on the host surface as airborne spores and act as initial colonizers after abscission. These are later joined by ‘true litter fungi’, which are perhaps those that colonize leaves after they are shed and show activity for varying periods thereafter.
The pattern of colonization is in agreement with the scheme of fungal succession proposed by Garrett (Citation1963). According to him, the primary saprotrophs to invade are members of the Zygomycota, commonly referred to ‘sugar fungi’, which are non-cellulolytic and rely upon readily available sugars, such as hexoses and pentoses, and other carbon sources simpler than cellulose, such as pectins and starch. Hudson (Citation1986) himself has suggested that the mucorales, which appear in the final stages of decomposition, are mostly soil inhabiting and certainly not using any simple carbohydrates initially present in the leaves, as these would have been utilized already. The significant features of colonization of leaves and litter by microfungi, as elucidated in the present study, would then be as follows: the primary colonizers (primary saprophytes of Hudson' scheme) are the ‘foliicolous fungi’. These are soon joined by ‘true litter-inhabiting fungi’ which are perhaps those that colonize leaves after they are shed and show activity for varying periods thereafter. However, whether foliicolous species, which continue to be active on dead leaves and thus show a saprophytic phase, can be excluded from this category is a moot point. This latter group can be distinguished from the former by the fact that their activity begins on the living leaf, whereas the ‘true litter-inhabiting fungi’ work on dead leaves. Perhaps, this is a good enough distinction for the ecological behavior of these two categories of fungi. On this basis, species belonging to genera such as Beltrania, Beltraniella, Circinotrichum, Dicyma, Gyrothrix, Hansfordia, Helicoma, Helicosporium, Monodictys, Polyscytalum, Pseudobeltrania, Selenosporella, Subramaniomyces, Tetraploa, Torula, Thozetella, Wiesneriomyces and Zygosporium are considered ‘true litter-inhabiting fungi’. B. rhombica was reported to be a dominant fungus on leaf litter of tropical plants (Kiffer et al. Citation1981; Rambelli et al. Citation1983; Heredia Citation1993).
The duration of fungal decomposition varies with leaf litter. For instance, in cool temperate pine forests, it may take 10 years to fully decompose pine needles, less than 1 year for decomposition of leaves in ash and sycamore woods, and only a few weeks in a tropical forest (Hudson Citation1980). The time for decomposition of monocotyledons in tropical regions has been shown to be relatively short, e.g. 14 months for leaves of sugarcane (Hudson Citation1962), 19 months for stems of couch grass (Hudson and Webster Citation1958), 2 years for litter of pineapple (Tiwari et al. Citation1994), 1 year for leaves and rachis-tips of Phoenix hanceana and 18 months for mid-rachides and rachis-bases of Phoenix hanceana (Yanna et al. Citation2001). In contrast, the decomposition of senescent leaves of Anacardium occidentale in this study was rapid, leaves were completely decayed within 1 year.
References
- Bills , GF. 1996 . “ Isolation and analysis of endophytic fungal communities from woody plants ” . In Endophytic fungi in grasses and woody plants: systematics, ecology and evolution , Edited by: Redlin , SC . 31 – 65 . St. Paul, MN : APS Press .
- Bills , GF and Polishook , JD. 1994 . Abundance and diversity of microfungi in leaf litter of lowland forest in Costa Rica . Mycologia , 86 : 187 – 198 .
- Charley , JL and Richards , BN. 1983 . “ Nutrient allocation in plant communities. Mineral cycling in terrestrial ecosystems ” . In Physiological Plant Ecology IV. Ecosystem process. Mineral cycling, Productivity and Man's Influence , Edited by: Lange , OL , Nobel , PS , Osmond , CB and Ziegler , H . 5 – 45 . New York : Springer .
- Chesters , CGC. 1950 . On the succession of microfungi associated with the decay of logs and branches . Trans Br Mycol Soc. , 12 : 129 – 135 .
- Dickinson , CH and Pugh , GJF , eds. 1974 . Biology of Plant Litter Decomposition , Vol. I and II , London : Academic Press .
- Duong , LM , McKenzie , EHC , Lumyong , S and Hyde , KD. 2008 . Fungal succession on senescent leaves of Castanopsis diversifolia in Doi Suthep-Pui National Park, Thailand . Fungal Divers. , 30 : 23 – 36 .
- Garrett , SD. 1963 . Soil fungi and soil fertility , 165 Oxford : Pergamon Press .
- Heredia , G. 1993 . Mycoflora associated with green leaves and leaf litter of Quercus germana, Quercus sartorii and Liquidambar styraciflua in a Mexican cloud forest . Cryptogamie Mycol. , 14 : 171 – 183 .
- Hering , TF. 1965 . Succession of fungi in the litter of a Lake District oakwood . Trans Br Mycol Soc. , 48 : 391 – 408 .
- Hogg , BM and Hudson , HJ. 1966 . Microfungi on leaves of Fagus sylvatica. I. The microfungal succession . Trans Br Mycol Soc. , 49 : 185 – 192 .
- Hudson , HJ. 1962 . Succession of micro-fungi on aging leaves of Saccharum officinarum . Trans Br Mycol Soc. , 45 : 395 – 423 .
- Hudson , HJ. 1968 . The ecology of fungi on plant remains above the soil . New Phytol. , 67 : 837 – 874 .
- Hudson , HJ. 1980 . Fungal saprophytism , London : Camelot Press .
- Hudson , D. 1986 . “ Fungal Biology ” . In Fungi as decomposers of leaves , 298 London : Chaucer Press . p.
- Hudson , HJ and Webster , J. 1958 . Succession of fungi on decaying stems of Agropyron repens . Trans Br Mycol Soc. , 41 : 165 – 177 .
- Kodsueb , R , McKenzie , EHC , Lumyong , S and Hyde , KD. 2008 . Fungal succession on woody litter of Magnolia liliifera (Magnoliaceae) . Fungal Divers. , 30 : 55 – 72 .
- Kiffer , E , Puig , H and Kilbertus , GG. 1981 . Biodégradation des feuilles d'Eperia falcate Aubl. Enforêt tropicale humide (Guyane Française) . Rev Ecol Biol Soc. , 18 : 135 – 151 .
- Kshattriya , S , Sharma , GD and Mishra , RR. 1994 . Fungal succession and microbes on leaf litters in two degraded tropical forests of North-East India . Pedobiologia , 38 : 125 – 137 .
- Kumar , DP and Hegde , M. 1999 . Recycling of plant nutrients through cashew leaf litter . The Cashew , 13 : 2 – 8 . (April–June)
- Kuter , GA. 1986 . Microfungal populations associated with the decomposition of sugar maple leaf litter . Mycologia , 78 : 114 – 126 .
- Lousier , JD and Parkinson , D. 1976 . Litter decomposition in a cool temperate deciduous forest . Can J Bot. , 54 : 419 – 436 .
- Mangenot , F. 1952 . Recherches methodiques sur les champignons de certains bois en decomposition . Rev Gen Bot. , 59 : 381
- Mehrotra , RS and Aneja , KR. 1979 . Microbial decomposition of Chenopodium album litter. I. Succession of decomposers . J Indian Bot Soc. , 58 : 189 – 196 .
- Osono , T , Bhatta , BK and Takeda , H. 2004 . Phyllosphere fungi on living and decomposing leaves of giant dogwood . Mycoscience , 45 : 35 – 41 .
- Osono , T , Ishii , Y , Takeda , H , Seramethakun , T , Khamyong , S , To‐Anun , C , Hirose , D , Tokumasu , S and Kakishima , M. 2009 . Fungal succession and lignin decomposition on Shorea obtusa leaves in a tropical seasonal forest in northern Thailand . Fungal Divers. , 36 : 101 – 119 .
- Pasqualetti , M , Mulas , B , Zucconi , L and Rambelli , A. 1999 . Succession of microfungal communities on Myrtus communis leaf litter in a Sardinian Mediterranean maquis ecosystem . Mycol Res. , 103 : 724 – 728 .
- Paulus , B and Hyde , KD. 2003 . Estimation of microfungal diversity in tropical rainforest leaf litter using particle filtration: the effects of leaf storage and surface treatment . Mycol Res. , 107 : 748 – 756 .
- Paulus , B , Gadek , P and Hyde , KD. 2006 . Successional patterns of microfungi in fallen leaves of Ficus pleurocarpa (Moraceae) in an Australian tropical rain forest . Biotropica , 38 : 42 – 51 .
- Polishook , JD , Bills , GF and Lodge , DJ. 1996 . Microfungi from decaying leaves of two rain forest trees in Puerto Rico . J Ind Microbiol. , 17 : 284 – 289 .
- Promputtha , I , Lumyong , P , McKenzie , EHC and Hyde , KD. 2002 . Fungal succession on senescent leaves of Manglietia garrettii in Doi Suthep-Pui National Park, northern Thailand . Fungal Divers. , 10 : 89 – 100 .
- Rambelli , A , Persiani , AM , Maggi , O , Lunghino , D , Onofri , S , Riess , G , Dowgiallo , B and Puppi , G. 1983 . “ Comparative studies on microfungi in tropical ecosystems ” . In Mycological Studies in South Western Ivory Coast Forest , Rome : MAB-UNESCO . Report No. 1
- Rosenbrock , P , Buscot , F and Munch , JC. 1995 . Fungal succession and changes in the fungal degradation potential during the initial stages of litter decomposition in a black alder forest (Alder glutinosa L. Gaertn.) . Eur J Soil Biol. , 31 : 1 – 11 .
- Sadaka , N , Nassima , B and Ponge , JF. 2003 . Fungal colonization of phyllosphere and litter of Quercus rotundifolia Lam. in a holm oak forest (High Atlas, Morocco) . Biol Fertil Soils , 39 : 30 – 36 .
- Sandhu , DK and Sidhu , MS. 1980 . Fungal succession on decomposing sugarcane bagasse . Trans Br Mycol Soc. , 75 : 281 – 286 .
- Subramanian , CV and Vittal , BPR. 1979 . Studies on litter fungi. II. Fungal colonization of Atlantia monophylla Corr. leaves and litter . Nova Hedwigia Beiheft Zur. , 63 : 361 – 369 .
- Subramanian , CV and Vittal , BPR. 1980 . Studies on litter fungi. IV. Fungal colonization of Gymnosporia emarginata leaves and litter . Trans Mycol Soc Jpn. , 21 : 339 – 334 .
- Tang , AMC , Jeewon , R and Hyde , KD. 2005 . Succession of microfungal communities on decaying leaves of Castanopsis fissa . Can J Microbiol. , 51 : 967 – 974 .
- Tempesta , S , Pasqualetti , M , Fonck , M and Mulas , B. 2003 . Succession of microfungi in Phillyrea angustifolia litter in a Mediterranean maquis in Sardinia . Plant Biosyst. , 137 : 149 – 154 .
- Toky , OP and Singh , V. 1983 . Litter dynamics in short-relation high density tree plantations in an arid region of India . Agric Ecol. Environ. , 45 : 129 – 145 .
- Thormann , MN , Currah , RS and Bayley , SE. 2003 . Succession of microfungal assemblages in decomposing peatland plants . Plant Soil , 250 : 323 – 333 .
- Tiwari , SC , Tiwari , BK and Mishra , RR. 1994 . Succession of microfungi associated with the decomposing litters of pineapple (Ananas comosus) . Pedobiologia , 38 : 185 – 192 .
- Valenzuela , E , Leiva , S and Godoy , R. 2001 . Seasonal variation and enzymatic potential of microfungi associated with the decomposition of Nothofagus pumilio leaf litter . Revista Chilena de Historia Natural , 74 : 737 – 749 .
- Visser , S and Parkinson , D. 1975 . Fungal succession on aspen poplar leaf litter . Can J Bot. , 53 : 1640 – 1657 .
- Webster , J. 1956 . Succession of fungi on decaying cocksfoot culms . I. J Ecol. , 44 : 517 – 544 .
- Webster , J. 1957 . Succession of fungi on decaying cocksfoot culms . II. J Ecol. , 45 : 1 – 30 .
- Yanna , H , Ho , WH , Hyde , KD and Goh , TK. 2001 . Occurrence of fungi on tissues of Livistona chinensis . Fungal Divers. , 6 : 167 – 180 .
- Zhou , DQ and Hyde , KD. 2002 . Fungal succession on bamboo in Hong Kong . Fungal Divers. , 10 : 213 – 227 .