Abstract
Our study evaluated the efficacy of a combination of fluconazole and the psychoactive compound sertraline against strains of Cryptococcus neoformans and Cryptococcus gattii. Using the chequerboard microdilution method based on CLSI M27-A2 guidelines, we determined the susceptibilities of each drug alone and in combination against 40 strains of serotypes A, D and AD in C. neoformans and 13 strains of serotype B C. gattii. The MIC ranged 10.8–43 and 2–64mg/l for sertraline and fluconazole, respectively. No difference in MICSertraline was observed among the serotypes. However, within C. neoformans, a significant difference in MICFluconazole was observed among the serotypes, as follows: AD >A > D (AD being the most resistant). Strains of C. gattii had MICFluconazole not significantly different from those of serotypes A and AD but significantly higher than serotype D. Synergy (FICI ≤ 0.5) was found for 31 strains, while the remaining 22 strains showed no difference. No evidence for antagonistic interaction was observed. Our study suggests the potential utility of fluconazole–sertraline combination therapy for cryptococcal infections.
Abbreviations
| DMSO | = |
dimethyl sulfoxide |
| CLSI | = |
clinical laboratory standards institute |
| MIC | = |
minimal inhibitory concentration |
| FIC | = |
fractional inhibitory concentration |
| MAPK | = |
mitogen-activated protein kinase |
| CFU | = |
colony formation units |
Introduction
Cryptococcus neoformans is a pathogenic basidiomycete yeast capable of causing meningoencephalitis in immunosuppressed individuals. It is a ubiquitous fungus that is commonly isolated from rotting wood and avian guano in the environment (Litvintseva et al. Citation2005; Nielsen et al. Citation2007). Cryptococcus gattii on the other hand is most frequently found in tropical and subtropical regions and is capable of infecting immunocompetent individuals. The largest outbreak of C. gattii occurred in British Colombia, Canada in 1999 (Kidd et al. Citation2005). Since then, C. gattii has been isolated from several parts of Pacific North America indicating a changing demographic for the organism (MacDougall et al. Citation2007; Dixit et al. Citation2009). Similarly, a recent study regarding the infection patterns in China discovered that C. neoformans might also be capable of infecting healthy, immunocompetent individuals (Chen et al. Citation2008). Infections by both species can result in significant morbidity and mortality, with mortality rates reaching as high as 60% 6 months after a meningeal infection for some populations. The high mortality serves as a grim reminder that our arsenal and tactics against this disease need to be expanded (Kambugu et al. Citation2008).
Fluconazole remains the drug of choice for treating cryptococcosis and many other fungal infections. It has a high bioavailability, is well tolerated by most patients and can be administered orally. However, the maximum attainable plasma concentration with a 400-mg oral capsule is 13.5 mg/l or 4.92–17.6 μg/g in the brain (Fischman et al. Citation1993; Thaler et al. Citation1995). As such, infections with resistant strains may result in poor treatment outcomes unless other more potent drugs but with significant toxicities (i.e. amphotericin B) are used. In these scenarios, combination therapies may be necessary and preferable. The idea behind combination therapy is that a greater therapeutic efficacy can be achieved by producing a potent fungicidal outcome with reduced toxicity (due to reduced drug dosage) experienced by the patient (Barcheisi et al. Citation2000). Moreover, the use of combinations of drugs may even present a synergistic result whereby the antifungal effect is more pronounced than any drug alone at the same concentration. Additionally, the use of combination treatments can help reduce the emergence of resistance. One of the most commonly used combination therapy against human pathogenic fungi is that of the amphotericin B/flucytosine combinations (Schwarz et al. Citation2006, Citation2007).
In this study, a combination of sertraline hydrochloride and fluconazole was tested for efficacy and interaction against strains of C. neoformans and C. gattii. This drug combination was selected based on several recent reports showing that sertraline displayed antifungal activities against Saccharomyces, Candida and Aspergillus species (Lass-Flörl et al. Citation2001a,Citationb, 2003; Ericson et al. Citation2008). However, no studies have been conducted, to the best of our knowledge, on the efficacy of sertraline against any Cryptococcus species. Sertraline is a commonly prescribed psychotropic drug that functions as a selective serotonin re-uptake inhibitor (SSRI). It acts specifically on the 5-HT neuronal transporter protein (SERT) found in humans (Barchiesi et al. Citation2005). It is a competitive inhibitor of serotonin that functions on the serotonin-binding domain of the 5-HT protein (Koe et al. Citation1990).
To examine the effects of sertraline and fluconazole/Sertraline combination on C. neoformans and C. gattii, we screened 40 strains of C. neoformans representing three distinct serotypes A, D, and AD. In addition, 13 strains of C. gattii, a close relative of C. neoformans were included. The C. gattii strains all belonged to serotype B. We were interested in whether the different species or serotypes would show different patterns of drug responses to fluconazole, sertraline and/or to a combination of the two drugs. Any observed differences would also provide evidence that each serotype might employ different regulatory mechanisms to deal with cell stress and might be related to their different virulence properties in mammalian hosts (Barchiesi et al. Citation2005). Since fluconazole targets 14-α-demethylase and sertraline does not (Lass-Flörl et al. 2003; Rodero et al. Citation2003), we expect no evidence of cross-resistance between the two drugs. Moreover, due to the distinction in targets, we expect to see a synergistic interaction between the drugs particularly against fluconazole-resistant isolates. As was shown for the amphotericin B/flucytosine combinations, the two drugs interacted more synergistically against more flucytosine-resistant strains (Schwarz et al. Citation2006, Citation2007).
A finding of synergy would help in the development of a more aggressive treatment option against cerebral mycotic infections. Additionally, the potential patterns of antifungal drug responses among strains and serotypes might lead to the development of therapies targeted towards specific serotypes, which may help improve the success rate of treating systemic cryptococcal infections. Aside from the health benefits, targeted therapies could also reduce costs. A recent report has indicated that the treatment of mycotic infections amounts to $2.6 billion USD annually in US healthcare spending alone (Cowen and Steinbach Citation2008).
Materials and methods
Preparation of drug stock solutions
Sertraline was obtained from Sigma-Aldrich Canada and Fluconazole (Diflucan®) was purchased from Pfizer (New York, USA). Sertraline was dissolved in DMSO to a final concentration of 2870 mg/l. Fluconazole was bought as a prepared i.v. solution at 2000 mg/l in saline. The stock solutions were stored at room temperate (25 °C) as indicated by the manufacturers.
Isolates
A total of 53 isolates were tested in this study, including 40 C. neoformans strains and 13 C. gattii strains (). These strains were originally obtained from the Center for Disease Control and Prevention in the US and the University of British Columbia (Kidd et al. Citation2005; Brandt et al. Citation1996; Yan et al. Citation2002). We also included two CLSI recommended quality control (QC) reference strains Candida parapsilosis ATCC22019 and Candida krusei ATCC6258. The type strain of C. neoformans var. grubii, H99, was also included as a third QC strain throughout our testing. Each strain was removed from the −80 °C freezer and subcultured onto yeast extract peptone dextrose (YEPD: 2% dextrose, 1% yeast extract, 1% peptone) plates. The subcultures were further streaked onto fresh YEPD and allowed to grow at 30 °C for 3–5 days. Three to five colonies were removed from each plate, inoculated into 4 ml of YEPD broth (Difco) and incubated overnight (∼12 h) in a rotating incubator at 120 rpm at 37 °C. The OD530 of the overnight cultures were obtained and standardized to 0.5 McFarland units in YEPD broth prior to inoculation for drug susceptibility testing.
Table 1. List of strains
Drug susceptibility testing
A modified version of the CLSI M27-A2 broth microdilution method was employed to test the drug combination. Briefly, serial two-fold dilutions of both fluconazole and sertraline were performed with concentrations ranging 0.25–128 mg/l and 1.3–86 mg/l, respectively. Sertraline was diluted in dimethyl sulfoxide (DMSO), while fluconazole was diluted in double distilled water. Both drugs were distributed in a 96-flatwell clear-bottomed microtitration plate (Costar 96-well tissue culture plate; Corning Inc., New York, NY, USA) with the final DMSO concentration not exceeding 3% (v/v) per well. A preliminary test involving a chequerboard of varying concentrations of DMSO in combination with fluconazole demonstrated that DMSO inhibited growth by 50% at the concentration of 5% (v/v) and caused complete growth inhibition at concentrations ≥ 7.5% (v/v) against C. neoformans strain H99. However, there were no synergy between DMSO and fluconazole (FICI = 1.03). Each well was inoculated with approximately 1.0 × 103–2.0 × 103 CFU/ml. The plates were incubated at 37 °C for 72 h. The MIC for both fluconazole and sertraline was determined as the initial most prominent decrease in turbidity as indicated by CLSI. The fractional inhibitory concentration (FIC) was determined as the well that showed the most prominent decrease in turbidity relative to the other combination wells. If more than one well showed similar decreases in turbidity, the well that yielded the lowest FIC Index (FICI) value was used since this would represent the optimal fluconazole:sertraline ratio for in vitro growth inhibition. The FICI value was calculated for each strain using the formula: FICI = (FICFluconazole/MICFluconazole) + (FICSertraline/MICSertraline), where the FIC represents the MIC of the drug in combination. A FICI ≤ 0.5 was considered synergistic, >0.5-4.0 indifference and > 4.0 antagonistic (Odds Citation2003).
Statistical analysis
The Minitab Database Software (MDS) 14.0 was used to perform Mann–Whitney tests to determine serotype differences in their MICs to each drug alone and in combination (i.e. FIC). The Mann–Whitney test was also used to determine serotype differences in drug interactions by comparing the FICI values for strains of each serotype. A p < 0.05 was considered statistically significant.
The MDS 14.0 software was also used to calculate Pearson correlation coefficient values to determine the relationship between the fluconazole or sertraline susceptibility and the FICI within this population of 53 strains. A p < 0.05 was considered significant for all tests.
Results and discussion
Efficacy of the sertraline/fluconazole combination
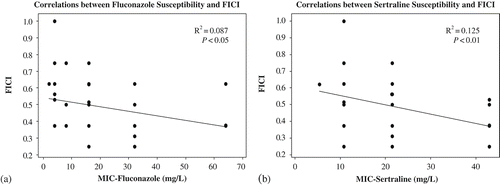
While there are exceptions in correlating in vitro data with in vivo outcomes for most antibiotics, including antifungals, the general trend for combination therapy shows that synergy or indifference demonstrated in vitro is usually indicative of positive outcomes in vivo (Johnson et al. Citation2004; Mukherjee et al. Citation2005). Our study found no evidence of antagonistic interaction (FICI ≥ 4.0) between the sertraline and fluconazole combination against strains of the C. neoformans species complex. A large number of isolates (31/53) responded with synergy (FICI ≤ 0.5) to the sertraline/fluconazole combination. Of these 31 strains, 10 belonged to serotype A (of 15 serotype A strains total tested), nine to serotype AD (of 13 strains total), nine to serotype B (of 13 strains total) and three to serotype D (of 12 strains total). The remaining 22 strains demonstrated no significant interaction (i.e. 0.5 < FICI < 4.0). Thus, we expect this combination to work effectively in vivo to produce positive treatment outcomes. Moreover, our linear regression analysis found a weak, but significant, negative correlation between the susceptibility to fluconazole and the FICI within this population of isolates (a). A similar trend was also observed with regards to a strain's susceptibility to sertraline (b). The Pearson's correlations for fluconazole and sertraline MICs to the FICI were –0.296 (p < 0.05) and –0.355 (p < 0.01), respectively. Our analyses indicate that the more resistant a strain is to either fluconazole or sertraline, the more likely it will respond synergistically to the combination therapy (a and b). Consequently, this combination could be ideal to treat non-responsive infections.
Figure 1. (a) Linear regression graph showing a strain's susceptibility to fluconazole and its FICI indicate a weak negative correlation. (b) Same as (a) but with a strain's susceptibility to sertraline. Filled circles indicate the MIC versus FICI point for individual strains.
Sertraline hydrochloride is a psychoactive drug that can be used to treat obsessive compulsive disorder, panic disorder and depression. The dosage administered ranges from 50 to 200 mg/day in oral form. The 50 mg/day dose yields a mean 24 h plasma concentration of 0.006 mg/l, while the 200 mg/day dose yields a mean plasma concentration of approximately 0.107 mg/l (Pfizer Inc. Citation2002; Nagy et al. Citation2004). However, the concentration achievable in the brain is greater than 40 times that in the plasma (DeVane Citation1992). In our collection of strains, the highest concentration of sertraline required in the in vitro combination treatment was 10.75 mg/l. Concordantly, of the 28 strains (MICFluconazole ≥ 16 mg/l) that were predicted to fail with fluconazole monotherapy, eight strains could potentially be successfully treated by the sertraline/fluconazole combination (FICFluconazole<16 mg/l and FICSertraline < 4.28 mg/l). This is based on the assumption that the in vitro and in vivo sertraline susceptibilities are directly correlated. Despite a salvage rate of approximately 28% (8/28), short-term use of sertraline has more manageable adverse effects than the use of amphotericin B. In a study by Nagy et al. (Citation2004), the most significant adverse effect due to sertraline was one case of eisonophilia among 17 healthy volunteers. The most frequently observed adverse effects were nausea and diarrhea (6/17). This might be preferable to acute renal toxicity commonly experienced with amphotericin B treatment. Our analyses suggest that further studies regarding the in vivo susceptibilities of C. neoformans and C. gattii to sertraline/fluconazole combination therapy are warranted. Moreover, if the functional groups of sertraline could be modified to improve its pharmacokinetic parameters and increase its fungicidal potency, the salvage rate might increase significantly.
Analysis of serotype differences
There has been some debate as to whether differences exist among the serotypes of the C. neoformans species complex in their antifungal susceptibilities. Several studies reported that serotype B strains were less susceptible to fluconazole than serotype A strains (Khan et al. Citation2007; Gomez-Lopez et al. Citation2008; Torres-Rodriguez et al. Citation2008), while others found no difference (Chang et al. 2006; Tay et al. Citation2006; Thompson et al. Citation2008). In our study, a significant difference was found between the susceptibility of serotypes A, AD, D and B to fluconazole alone. Specifically, serotype AD strains were more resistant to fluconazole than serotype A strains (p < 0.05) which in turn were more resistant than serotype D strains (p < 0.01). Additionally, serotype B strains were also significantly more resistant to fluconazole than serotype D strains (p < 0.01) but no difference was found between serotype B and the other two C. neoformans serotypes A and AD. Interestingly, this serotype-specific difference was statistically not significant when comparing FICFluconazole between the three C. neoformans serotypes A, D and AD. However, while C. gattii serotype B had a similar value to serotype AD, its FICFluconazole was significantly higher than both serotype A and serotype D (p < 0.05). shows their geometric means and ranges.
Table 2. Geometric means (range) of the minimum inhibitory concentrations (MIC), fractional inhibitory concentrations (FIC), and fractional inhibitory concentration index (FICI) among the four serotypes of the C. neoformans species complex
In contrast to those for fluconazole, we found no differences in susceptibility to sertraline among the serotypes in the C. neoformans species complex. This pattern was also seen for the concentration of sertraline required in the fluconazole/sertraline combination as well, with the exception of a significant difference between serotypes AD and D. Specifically, the FICSertraline for serotype AD strains were significantly higher than those for serotype D strains (p < 0.05). Furthermore, there was also no evidence of cross-resistance between sertraline and fluconazole – no significant correlation was found between MICfluconazole and MICsertraline or between FICfluconazole and FICsertraline (data not shown).
We would like to mention that the number of strains for each examined serotype is relatively small and that different samples might show different patterns. However, typically it is more difficult to obtain statistically significant differences with small sample sizes, as seen above. Possibly, when more strains are investigated, a greater difference among the serotypes might be observed.
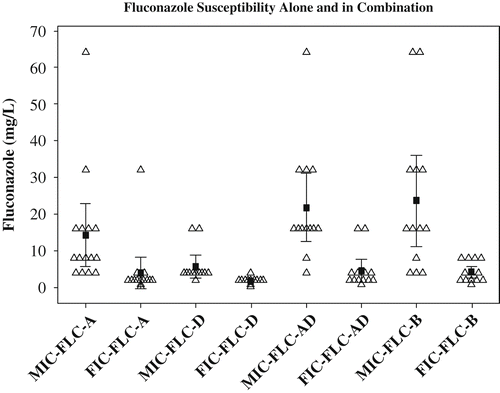
Our results showed fluconazole tolerance of C. neoformans is affected by the presence of sertraline more so than C. gattii (). Sertraline has been shown to inhibit the activity of extracellular phospholipases in Candida albicans (Lass-Flörl et al. Citation2003). Assuming this holds true with Cryptococcus, phospholipases might play a bigger role in drug stress response in C. neoformans than in C. gattii. One of the more important phospholipases is phosphatidylinositol-specific phospholipase C. This enzyme is involved in the activation of both the MAPK/Protein kinase C and calcium/calcineurin pathway through the release of phospholipase B1 (Plb1) (Chayakulkeeree et al. Citation2008). Both pathways have shown to contribute to fluconazole tolerance in Cryptococcus species (Kraus et al. Citation2003; Del Poeta et al. 2004; Stie and Fox Citation2008). Specifically, inhibition of calcineurin made fluconazole fungicidal (Del Poeta et al. Citation2000; Onyewu et al. Citation2003). Moreover, these pathways seemed to play slightly different roles among the serotypes of C. neoformans (Cruz et al. Citation2000; Bahn et al. Citation2005). Since there were no significant differences in the susceptibility to sertraline alone, the role of phospholipases is likely conserved among the serotypes. The serotype differences in susceptibility to fluconazole are, thus, likely due to divergent signaling roles of downstream pathways activated by phospholipases among the serotypes. Consequently, the divergence in signaling leads to differential thresholds of tolerance for fluconazole. This is consistent with the lack of serotype differences in fluconazole susceptibility in the presence of sertraline. When the drug stress response pathways in C. neoformans were inhibited, all the serotypes would revert to baseline susceptibility (). This hypothesis is consistent with the observation that the fluconazole/sertraline combination is more synergistic with increased resistance to individual drugs. For example, the more resistant a strain is to fluconazole, the larger the drop to baseline and consequently the lower the FICI ().
Figure 2. Fluconazole tolerance alone (MIC-FLC) and in the presence of sertraline (FIC-FLC) for each serotype. The serotypes revert to their respective baseline fluconazole susceptibility in the presence of sertraline. The open triangles represent the respective value for individual strains in each category; the filled squares represent the respective mean value for each category with a 95% confidence interval bar.
Conclusions
Our study demonstrated that fluconazole/sertraline combinations could be a promising duo in the treatment of Cryptococcal meningitis. Moreover, the more resistant a strain is to either sertraline or fluconazole, the more likely it is to respond synergistically to the combination. We also revealed that the four serotypes of the C. neoformans species complex showed overall different susceptibilities to fluconazole with AD>A>D and B>D (AD being the most resistant). However, this pattern of differences disappeared in the presence of sertraline for C. neoformans. Interestingly, C. gattii was more resistant to fluconazole than serotypes A and D in the presence of sertraline. Since sertraline inhibits extracellular phospholipase activity, we hypothesize that the variability in fluconazole susceptibility might be due to differential roles of phospholipase-activated pathways among the serotypes.
Acknowledgements
We are grateful to the students and staff of the Xu Laboratory for their help and support in this project. We are also grateful to the Michael G. DeGroote Institute for Infectious Disease Research at McMaster University for their financial support through student research scholarships. This study was supported by grants from the Michael G. DeGroote Institute for Infectious Disease Research (IIDR) at McMaster University, National Sciences and Engineering Research Council (NSERC) of Canada, the Canadian Foundation for Innovation (CFI) and the Ontario Innovation Trust (OIT).
References
- Bahn , Y , Kojima , K , Cox , GM and Heitman , J. 2005 . Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans . Mol Biol Cell , 16 : 2285 – 2300 .
- Barcheisi , F , Schimizzi , A , Caselli , F , Novelli , A , Fallani , S , Giannini , D , Arzeni , D , Di Cesare , S , di , Falconi , Francesco , L , Fortuna , M , Giacometti , A , Carle , F , Mazzei , T and Scalise , G. 2000 . Interactions between triazoles and amphotericin B against Cryptococcus neoformans . Antimicrob Agents Chemother. , 44 ( 9 ) : 2435 – 2441 .
- Barchiesi , F , Cogliati , M , Esposto , MC , Spreghini , E , Schimizzi , AM , Wickes , BL , Scalise , G and Viviani , MA. 2005 . Comparative analysis of pathogenicity of Cryptococcus neoformans serotypes A, D and AD in murine cryptococcosis . J Infect. , 51 : 10 – 16 .
- Brandt , ME , Hutwagner , LC , Klug , LA , Baughman , WS , Rimland , D , Graviss , EA , Hamill , RJ , Thomas , C , Pappas , PG , Reingold , AL and Pinner , RW. 1996 . Molecular subtype distribution of Cryptococcus neoformans in four areas of the United States . J Clin Microbiol. , 34 : 912 – 917 .
- Chang , W , Huang , C , Lei , C , Lee , P , Chien , C , Chang , H , Chang , C and Lu , C. 2004 . Serotypes of clinical cerebrospinal fluid Cryptococcus neoforans isolates from southern Taiwan and their in vitro susceptibilities to amphotericin B, fluconazole and voriconazole . Jpn J Infect Dis. , 57 : 113 – 115 .
- Chayakulkeeree , M , Sorrell , T , Siafakas , RA , Wilson , CF , Pantarat , N , Gerik , KJ , Boadle , R and Djordjevic , JT. 2008 . Role and mechanism of phosphatidylinositol-specific phospholipase C in survival and virulence of Cryptococcus neoformans . Mol Microbiol. , 69 : 809 – 826 .
- Chen , J , Varma , A , Diaz , MR , Litvintseva , AP , Wollenberg , KK and Kwon-Chung , KJ. 2008 . Cryptococcus neoformans strains and infection in apparently immunocompetent patients, China . Emerg Infect Dis. , 14 ( 5 ) : 755 – 762 .
- Cowen , L and Steinbach , W. 2008 . Stress, drugs, and evolution: The role of cellular signaling in fungal drug resistance . Eukaryotic Cell , 7 ( 5 ) : 747 – 764 .
- Cruz , C , Sia , R , Olson , M , Cox , G and Heitman , J. 2000 . Comparison of the roles of calcineurin in physiology and virulence in serotype D and serotype A strains of Cryptococcus neoformans . Infect Immun. , 68 ( 2 ) : 982 – 985 .
- Del Poeta , M , Cruz , C , Cardenas , M , Perfect , JR and Heitman , J. 2000 . Synergistic antifungal activities of bafilomycin A1, fluconazole, and the pneumocandin MK-0991/Caspofungin acetate (L-743,873) with calcineurin inhibitors FK506 and L-685,818 against Cryptococcus neoformans . Antimicrob. Agents Chemother. , 44 ( 3 ) : 739 – 746 .
- DeVane , C. 1992 . Pharmacokinetics of the selective serotonin reuptake inhibitors . J Clin Psychiatry , 53 : 13 – 20 .
- Dixit , A , Carroll , SF and Qureshi , ST. 2009 . Cryptococcus gattii: An emerging cause of fungal disease in North America . Interdisciplinary Perspectives on Infectious Diseases 2009 , 840452, doi:10.1155/2009/840452
- Ericson , E , Gebbia , M , Heisler , L , Wildenhain , J , Tyers , M , Giaever , G and Nislow , C. 2008 . Off-target effects of psychoactive drugs revealed by genome-wide assays in yeast . PLoS Genetics [Internet]. , [revised 4(8)]
- Fischman , A , Alpert , N , Livni , E , Ray , S , Sinclair , I , Callahan , R , Correia , J , Webb , D , Strauss , W and Rubin , R. 1993 . Pharmacokinetics of 18F-labeled fluconazole in healthy human subjects by positron emission tomography . Antimicrob Agents Chemother. , 37 ( 6 ) : 1270 – 1277 .
- Gomez-Lopez , A , Zaragoza , O , Dos , M , Martins , A , Melhem , MC , Rodriguez-Tudela , JL and Cuenca-Estrella , M. 2008 . In vitro susceptibility of Cryptococcus gattii clinical isolates . Clin Microbiol Infect. , 14 ( 7 ) : 727 – 730 .
- Hirano , K , Kimura , R , Sugimoto , Y , Yamada , J , Uchida , S , Kato , Y , Hashimoto , H and Yamada , S. 2005 . Relationship between brain serotonin transporter binding, plasma concentration and behavioural effect of selective serotonin reuptake inhibitors . Br J Pharmacol. , 144 : 695 – 702 .
- Johnson , M , MacDougall , C , Ostrosky-Zeichner , L , Perfect , JR and Rex , JH. 2004 . Combination antifungal therapy . Antimicrob Agents Chemother. , 48 : 693 – 715 .
- Kambugu , A , Meya , DB , Rhein , J , O'Brien , M , Janoff , EN , Ronald , AR , Kamya , MR , Mayanja-Kizza , H , Sande , MA , Bohjanen , PR and Boulware , DR. 2008 . Outcomes of cryptococcal meningitis in Uganda before and after the availability of HAART . Clin Infec Dis , 46 ( 11 ) : 1694 – 1701 .
- Khan , ZU , Randhawa , HS , Kowshik , T , Chowdhary , A and Chandy , R. 2007 . Antifungal susceptibility of Cryptococcus neoformans and Cryptococcus gattii isolates from decayed wood of trunk hollows of Ficus religiosa and Syzygium cumini trees in north-western India . J Antimicrob Chemother. , 60 : 312 – 316 .
- Kidd , S , Guo , H , Bartlett , K , Xu , J and Kronstad , J. 2005 . Comparative gene genealogies indicate that two clonal lineages of Cryptococcus gattii in British Columbia resemble strains from other geographical areas . Eukaryotic Cell , 4 : 1629 – 1638 .
- Koe , B , Lebel , L and Welch , W. 1990 . [3H]-sertraline binding to rat brain membranes . Psychopharmacology , 100 ( 4 ) : 470 – 476 .
- Kraus , P , Fox , D , Cox , G and Heitman , J. 2003 . The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function . Mol Microbiol. , 48 ( 5 ) : 1377 – 1387 .
- Lass-Flörl , C , Dierich , MP , Fuchs , D , Semenitz , E , Jenewein , I and Ledochowski , M. 2001a . Antifungal properties of selective serotonin reuptake inhibitors against Aspergillus species in vitro . J Antimicrob Chemother. , 48 ( 6 ) : 775 – 779 .
- Lass-Flörl , C , Dierich , MP , Fuchs , D , Semenitz , E and Ledochowski , M. 2001b . Antifungal activity against Candida species of the selective serotonin-reuptake inhibitor, sertraline . Clin Infect Dis. , 33 ( 12 ) : 135 – 136 .
- Lass-Flörl , C , Ledochowski , M , Fuchs , D , Speth , C , Kacani , L , Dierich , MP , Fuchs , A and Würzner , R. 2003 . Interaction of sertraline with Candida species selectively attenuates fungal virulence in vitro . FEMS Immunol Med Microbiol. , 35 ( 1 ) : 11 – 15 .
- Litvintseva , AP , Kestenbaum , L , Vilgalys , R and Mitchell , TG. 2005 . Comparative analysis of environmental and clinical populations of Cryptococcus neoformans . J Clin Microbiol. , 43 ( 2 ) : 556 – 564 .
- MacDougall , L , Kidd , SE , Galanis , E , Mak , S , Leslie , MJ , Cieslak , PR , Kronstad , JW , Morshed , MG and Bartlett , KH. 2007 . Spread of Cryptococcus gattii in British Columbia, Canada, and detection in the Pacific Northwest, USA . Emerg Infect Dis. , 13 ( 1 ) : 42 – 50 .
- Mukherjee , PK , Sheehan , DJ , Hitchcock , CA and Ghannoum , MA. 2005 . Combination treatment of invasive fungal infections . Clin Microbiol Rev. , 18 : 163 – 194 .
- Nagy , CF , Kumar , D , Perdomo , CA , Wason , S , Cullen , EI and Pratt , RD. 2004 . Concurrent administration of donepezil HCl and sertraline HCl in healthy volunteers: Assessment of pharmacokinetic changes and safety following single and multiple oral doses . Br J Clin Pharmacol. , 58 : 25 – 33 .
- Nielsen , K , De Obaldia , AL and Heitman , J. 2007 . Cryptococcus neoformans mates on pigeon guano: Implications for the realized ecological niche and globalization . Eukaryotic Cell , 6 ( 6 ) : 949 – 959 .
- Odds , FC. 2003 . Synergy, antagonism, and what the chequerboard puts between them . J Antimicrob Chemother. , 52 : 1
- Onyewu , C , Blankenship , J , Del Poeta , M and Heitman , J. 2003 . Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei . Antimicrob Agents Chemother. , 47 ( 3 ) : 956 – 964 .
- Inc , Pfizer . 2002 . FDA approved labeling for zoloft® for the treatment of social anxiety disorder attachment to FDA approval letter for NDA 19-839/S-045: ZOLOFT (sertraline hydrochloride) tablets and oral concentrate; 69-4721-00-4.2 . US FDA Approval Letters 2002 , : 1 – 35 .
- Rodero , L , Mellado , E , Rodriguez , CA , Salve , A , Guelfand , L , Cahn , P , Cuenca-Estrella , M , Davel , G and Rodriguez-Tudela , JL. 2003 . G484S amino acid substitution in lanosterol 14-a-demethylase (ERG11) is related to fluconazole resistance in a recurrent Cryptococcus neoformans clinical isolate . Antimicrob Agents Chemother. , 47 ( 11 ) : 3653 – 3656 .
- Schwarz , P , Dromer , F , Lortholary , O and Dannaoui , E. 2006 . Efficacy of amphotericin B in combination with flucytosine against flucytosine-susceptible or flucytosine-resistant isolates of Cryptococcus neoformans during disseminated murine cryptococcosis . Antimicrob Agents Chemother. , 50 : 113 – 120 .
- Schwarz , P , Janbon , G , Dromer , F , Lortholary , O and Dannaoui , E. 2007 . Combination of amphotericin B with flucytosine is active in vitro against flucytosine-resistant isolates of Cryptococcus neoformans . Antimicrob Agents Chemother. , 51 ( 1 ) : 383 – 385 .
- Stie , J and Fox , D. 2008 . Calcineurin regulation in fungi and beyond . Eukaryotic Cell , 7 ( 2 ) : 177 – 186 .
- Tay , ST , Haryanty , TT , Ng , KP , Rohani , MY and Hamimah , H. 2006 . In vitro susceptibilities of Malaysian clinical isolates of Cryptococcus neoformans var. grubii and Cryptococcus gattii to five antifungal drugs . Mycoses , 49 : 324 – 330 .
- Thaler , F , Bernard , B , Tod , M , Jedynak , CP , Petitjean , O , Derome , P and Loirat , P. 1995 . Fluconazole penetration in cerebral parenchyma in humans at steady state . Antimicrob Agents Chemother. , 39 ( 5 ) : 1154 – 1156 .
- Thompson , G , Wiederhold , N , Fothergill , A , Vallor , A , Wickes , B and Patterson , T. 2008 . Antifungal susceptibility among different serotypes of Cryptococcus gattii and Cryptococcus neoformans . Antimicrob Agents Chemother. , doi:10.1128/AAC.01216-08
- Torres-Rodriguez , JM , Alvarado-Ramirez , E , Murciano , F and Sellart , M. 2008 . MICs and minimum fungicidal concentrations of posaconazole, voriconazole and fluconazole for Cryptococcus neoformans and Cryptococcus gattii . J Antimicrob Chemother. , 62 : 205 – 210 .
- Yan , Z , Li , X and Xu , J. 2002 . Geographic distribution of mating type alleles of Cryptococcus neoformans in four areas of the United States . J Clin Microbiol. , 40 : 965 – 972 .