Abstract
Colletotrichum horii causes serious anthracnose on persimmon (Diospyros kaki cv. Wuheshi). The taxon was previously identified as C. gloeosporioides and only recently revealed to be C. horii based on molecular data and comparisons to type specimens. This fungus provides an important new model for examining plant–fungus interactions in the perennial persimmon crop. In this paper, we review available information on C. horii, with special focus on symptoms, morphological characteristics, phylogenetic analysis, host-specificity and pathogenicity testing, infection processes, and the effects of environment factors on anthracnose development, including a discussion on future prospects.
Introduction
Diospyros kaki, known as shizi in Chinese, is the most widely used species of persimmon (oriental persimmon), having been cultivated in China for over 2500 years. More than 2000 different cultivars exist, with 960 of them being cultivated (Zhang Citation2008). Diospyros kaki cv. Wuheshi has been grown for more than 600 years in the Chunan area of Zhejiang Province (Zhang et al. Citation2003). However, there have been few records of persimmon anthracnose and local growers are unfamiliar with the disease. In 1992, the local government established 666 ha of persimmon orchard to stimulate the industry with the result that persimmon anthracnose has become increasingly common. In 1996, about half of the planted persimmon trees had died from anthracnose (Zhang et al. Citation2003). The disease has caused serious economic losses and become a major problem for the persimmon industry (Zhang Citation2008).
The pathogen causing persimmon anthracnose was previously identified as C. gloeosporioides (Zhang et al. Citation2005). Cytological research of the infection processes and intracellular infection structures have shown that Colletotrichum on persimmon is a hemibiotrophic species and, thus, is different from C. gloeosporioides sensu stricto (Cannon et al. Citation2008). During initial colonization of host cells, infection vesicles and primary hyphae are surrounded by an interfacial matrix that separates the fungal cell wall from the invaginated host plasma membrane, closely resembling that of C. lindemuthianum on Phaseolus vulgaris (Zhang et al. Citation2003, Citation2005; Zhang Citation2008).
Weir and Johnston (Citation2010) described the species causing persimmon anthracnose as C. horii Weir and Johnst., and our isolates from Chunan area were found to be conspecific to the ex-epitypes of C. horii. Weir and Johnston (Citation2010) considered C. horii as part of the C. gloeosporioides species complex, but recognized it as a distinct species from phylogenetic analysis based on ITS, EF1α, and GPDH sequences. Although Weir and Johnston (Citation2010) described the morphological characteristics of C. horii, other features, such as pathogenicity, host range and characteristics of appressoria and conidia in viva, were not detailed. Isolates from China (Chunan, Zhejiang) generally do not produce setae and a teleomorph was not observed on the host in natural or in artificial culture. Field and laboratory observations showed that C. horii (as C. gloeosporioides) was specific to different species, cultivars and organs of Diospyros (Zhang and Xu Citation2005), although its host range has not been fully determined.
Recent research has elucidated the etiology of anthracnose disease of persimmon (Zhang et al. Citation2005b), including the primary inoculum source (Zhang and Xu Citation2003), host-specificity (Zhang and Xu Citation2005), infection processes and the effects of environment factors on disease and fungal development (Zhang et al. Citation2005; Zhang Citation2008). Less attention has been paid to understanding the biological characteristics of this species. In this paper, we describe the morphological characteristics of this species in detail, determine its pathogenicity and host range, and investigate its phylogenetic relationships with closely related taxa based on multiple gene sequence analysis.
Synonyms of Colletotrichum horii
The fungus causing anthracnose on persimmon was first described by Shotaro Hori (Citation1910a,b) as Gloeosporium kaki based on its morphological characteristics, which were similar to Glomerella rufomaculans and Gloeosporium fruitigenum (Hori Citation1910b). In China, this disease was first recorded in Taiwan and noted as Gloeosporium kaki Hori (Sawada Citation1933). Maffei (Citation1921) had also described a leaf spot disease of persimmon from Italy and the pathogen was described as Colletotrichum kaki Maffei. The two taxa were distinguished by the presence of setae and pathogenicity. G. kaki was associated with lesions on young twigs and shoots and as spots on unripe fruit, while Colletotrichum kaki produced numerous setae and infected only leaves (Maffei Citation1921). However, there is no evidence that Gloeosporium kaki and Colletotrichum kaki are synonyms (Weir and Johnston Citation2010). Von Arx (Citation1957) placed G. kaki in synonymy with the conidial state of Glomerella cingulata, i.e. Colletotrichum gloeosporioides and Sutton (Citation1992) did not recognize G. kaki as a distinct species. For this reason, the pathogen-causing disease of persimmon in the Chunan area of Zhejiang Province was known as Colletotrichum gloeosporioides using morphological and pathogenic characters.
Phylogenetic analysis has increasingly becoming a useful tool for species delimitation in Colletotrichum (Shenoy et al. Citation2007a,b; Than et al. Citation2008; Cai et al. Citation2009). However, if type material is lost or in such poor condition that it cannot be used to extract DNA, molecular data cannot be obtained. Epitypification is a good way to resolve this problem and has been applied to several Colletotrichum species (Hyde and Zhang Citation2008; Cannon et al. 2009; Crouch et al. Citation2009). An isolate causing persimmon anthracnose was designated as the epitype of Gloeosporium kaki Hori and the taxon was transferred to C. horii (Weir and Johnston Citation2010).
Symptoms
Anthracnose is a destructive disease of persimmon nurseries in the field. The fungus attacks young twigs, leaves (petioles and veins) and fruits, leading to anthracnose lesions, which comprises twig blight, leaf defoliation, fruit drop and fruit rot (Zhang and Xu Citation2005). If twig blight becomes significant, the growth of a tree may decline and the entire tree can be killed within two or three years ().
Figure 1. Symptoms of persimmon anthracnose on Diospyros kaki cv. Wuheshi. A-B, disease lesions on newly-formed twigs. A, large numbers of lesions. B, infection of the whole twig. C, dieback. D, twig canker. E, persimmon tree killed by anthracnose fungus. F, lesions (arrowed) on leaf petioles and veins. G, a fruit lesion on a young fruit. H, premature fruit drop. I, lesions on premature fruits. Note that the cracks are produced longitudinally on fruits.
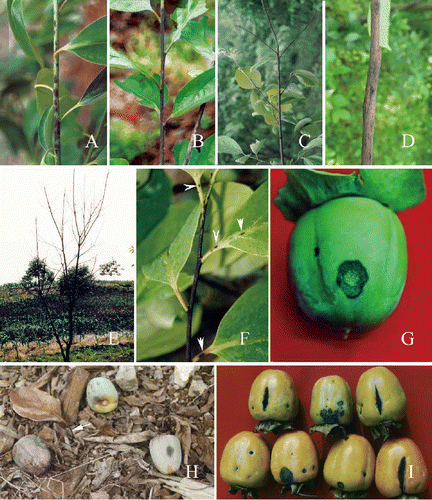
Anthracnose symptoms on twigs, leaves and fruits first appear in the spring as darkish, oval or elliptic spots, or as pin-pricks on newly-formed twigs. The minute spots develop into dark purple or dark brown lesions (), with a sharp line of demarcation between diseased and symptomless tissues. Pale orange conidial masses are frequently produced in the lesion centre. Under favorable conditions, adjacent lesions may coalesce, increasing in size until the entire twig is infected (). When a twig is girdled or completely infected, then dieback results (). The lesions may become dormant under unfavorable conditions but, in this situation, the fungus still continues to extend into the xylem, resulting in collapse with longitudinal cracking and finally forming cankers on a twig (). Leaf defoliation occurs if lesions develop at the base of petioles.
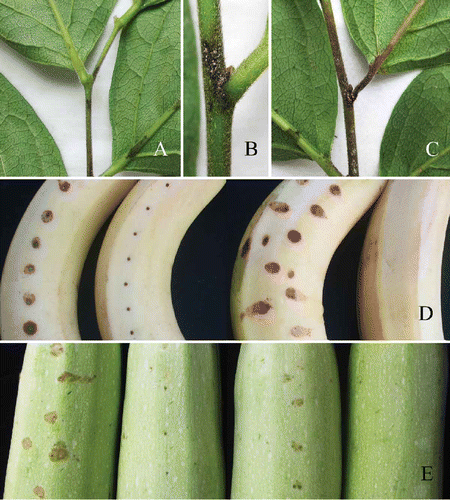
Figure 4. Pathogenicity testing. A-C, Diospyros kaki cv. Wuhesh. Visible symptoms produced after three days after inoculation. B, Yellow-pink conidial masses producing on the central part of a lesion after five days. C, Symptoms after seven days. D, Symptoms on wounded (left) and unwounded (right) banana after five days. E, Symptoms on wounded (left) unwounded (right) marrow after five days.
The pathogen infects petioles and leaf veins to produce the small, round or ovoid, sunken, purple to dark brown spots (), but they form later than those on young twigs. These small spots develop into the larger lesions, but they rarely coalesce on the petioles and leaf veins. If a petiole is infected, the leaf may continue to develop and remain green for an extended period, but may easily defoliate in the wind.
The persimmon fruits can be infected throughout the entire fruit-growing season. In young fruits, the lesions are often circular or oval, 3–8 mm in diameter, purple to dark purple, and occasionally slightly depressed. As the disease progresses, sometimes fruit lesions reach ∼20 mm in diameter (). The centre of the lesions becomes grey-white over time, while the broad margins remain dark purple. Pale orange conidial masses are produced in the lesion centre. Under dry conditions, the diseased lesions are sunken, and a longitudinal crack often occurs through the centre (Zhang Citation2008). If fruits are badly infected, they may drop in an unripe state (). In pre-mature fruits, the diseased lesions are often dark brown or purple dark, oval, sunken, with small cracks. Larger cracks often form and almost all deep cracks are produced in a longitudinal direction (). Anthracnose of persimmon fruits also occurs in market shelves and storage warehouses, resulting in fruit rot (Zhang Citation2008).
Morphological characteristics
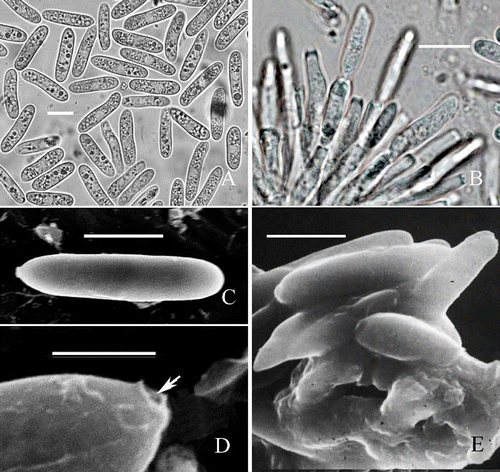
Conidiophores (11–) 11.5–25–35 (–50)×3–3.5–4.5. μm (n = 30), congregated, produced in acervuli, fasciculate, straight or occasionally geniculate (), 1–3–septate, dark grey at the base, reduced to a single hyaline conidiogenous cell on the natural host. Conidiogenous cells (7.5–) 9.5–13–15 (–16) × 3–3.5–4.5. μm, produced at the apex of conidiophores, cylindrical, rarely ampulliform, smooth, with a dark collarette () or occasionally annellidic at the conidiogenous sites. Conidia (16.5–) 17–19.5–20.5 (–22.5) × (4.5–) 5–5.5 –6 (–6.5) μm (n = 30), formed in yellowish-orange masses, holoblastic, cylindrical, straight or slightly curved, non-septate, smooth, apex obtuse, obtuse at both ends, with a hilum-like low protuberance at the base (). Under scanning electron microscopy, apex of conidia obtuse, base truncate (), with a truncate hilum that is hollow ().
Figure 2. Morphological characteristics of C. horii from Diospyros kaki cv. Wuhesh. A-B, conidia on natural host. A, conidia. B, conidiophores and conidia. C-E, transmission electron micrographs showing conidia. C, straight conidium with obtuse apex and truncate end. Bar = 10 mm. D, a conidium with broken hilum at its base (arrow). E, conidia embedded within and surrounded by the mucilage in acervulus. Bar = 10 μm.
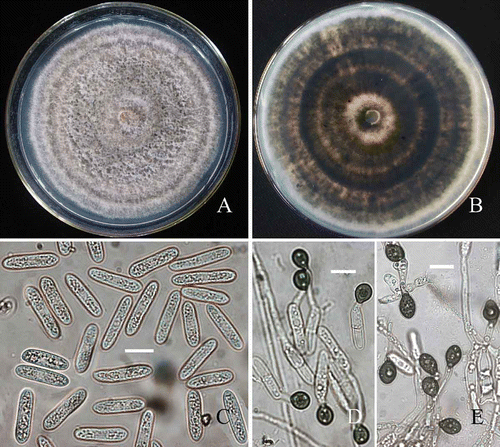
Colonies on PDA velvety, floccose, grey to dark grey, with the large numbers of yellowish-orange conidial masses, edge regular (), reverse dark grey to dark brown, with concentric zonation (). Margin of colony regular and the mean daily growth rate at 25°C was 12.8 ± 0.8 mm per day. Conidia produced across the whole colony, forming slimy, pale orange conidial masses amongst the aerial mycelium. Conidiophores 3.5–5 μm diam., short-cylindrical. Conidiogenous cells 10–15 × 3–5 μm, cylindrical. Conidia (17–) 18.5–20–21.5 (–22.5) × (4.5–) 5–5.4–5.7 (–5.9) μm (n = 30), cylindrical (). Appressoria were 8–9 × 7.8–8.7 μm, smooth, globose and dark brown () on polystyrene Petri dishes, as described by O¢Connell et al. (2004) and Sun and Zhang (Citation2009). On coverslips, appressoria were dark, globose to subglobose, smooth, and similar to those on plastic Petri dishes (), as described by Cei et al. (2009).
Figure 3. Morphological characteristics of C. horii (from Diospyros kaki cv. Wuhesh). A-C, on PDA. A, view of colony. B, reverse view of colony. C, conidia. D, appressoria formed on polystyrene Petri dish. E, appressoria formed on coverslips. Bar = 10 μm.
Material examined : CHINA: Zhejiang Province, Chunan County, Weiping Town (118° 20′ E, 118º 32′ N), on Diospyros kaki cv. Wuheshi (Ebenaceae), 30 Apr. 2009, J.Z. Zhang and L. Xie (HMAS 197044 ). The living cultures (TSG001, TSG002, TSG003, TSG004 and TSG005) are deposited in the collection of Biotechnology Institute, Zhejiang University, Zhejiang Province, China. Sources of isolates used in this study are given in .
Table 1. Sources of isolates used in this study
Notes : Numerous conidia aggregate on the acervuli in cone-shaped masses (Zhang et al., Citation2005B) and the conidia are embedded within and surrounded by mucilage (). In the process of conidial formation, transmission electron microscopy clearly showed that the outer wall of an apical conidiogenous locus breaks and forms the collarette, but its inner wall takes part in the formation of the outer wall of a new conidium (, ).
Most conidia on PDA are straight and less curved than that on the natural host, but they were similar in size with mean dimension of 19.5 × 5.5 μm on the natural host and 20 × 5.4 μm on PDA. Similarly, conidia size of isolates from Japan on PDA were (13–) 15–21 (–23) × 4–5.5 μm (mean = 17.5 × 4.8 μm) and those from New Zealand were 16–29.5 (–35) × (4–) 4.5–6 (–7) μm (mean = 22 × 5 μm) (Weir and Johnston Citation2010). There is a large variation in conidial size in isolates from different locations. Colletotrichum horii is morphologically similar to C. gloeosporioides, but can be differentiated as the latter epitype by conidial size of 17–20.5 × 5–6 μm for the epitype of C. horii compared to 12–17 × 4.5–6 μm for the epitype of C. gloeosporioides (Cannon et al. Citation2008).
Host specificity and results of pathogenicity testing
Colletotrichum horii isolates from the Chunan area of Zhejiang Province have been tested for host specificity in the field and laboratory (Zhang and Xu Citation2005). They found that a cultivar of Diospyros glaucifolia from the Chunan area was completely resistant; D. kaki cv. Wuheshi, was very susceptible; D. kaki cv. Dongshi was susceptible and the fruit is infected; while D. kaki var. sylvestris (wild persimmon), whose twigs were infected, was only slightly susceptible.
Pathogenicity testing was conducted in the laboratory on both healthy young twigs of D. kaki cv. Wuheshi and the fruits of several other plant species, including Musaceae (banana, Musa acuminata), Solanaceae (tomato, Lycopersicon esculentum; green pepper, Capsicum annuum), Cucurbitaceae (pumpkin, Cucurbita pepo; marrow, Cucurbita pepo), Rutaceae (orange, Citrus sinensis), Anacardiaceae (mango, Mangifera indica), and Leguminosae (common bean, Phaseolus vulgari; cowpea, Vigna unguiculata). An aqueous suspension 1 × 105 conidia/ml was prepared from 8–10-day cultures. The inoculated fruits were washed three times with sterile water, and 50% were pricked with insect needles prior to inoculation. The wounded/unwound plants materials were then inoculated with a 100 μl conidial suspension as described by Zhang and Xu (Citation2005).
Five isolates showed strong virulence, with anthracnose symptoms occurring on unwounded/wounded persimmon twigs and leaf veins within 3 days following inoculation (). Serious symptoms resulted within 5 days. Lesion size varied greatly on inoculated twigs and leaf veins, depending on the age of the persimmon twigs. Average lesion size on the unwounded/wounded persimmon twigs was 3.75 (±0.94) cm and 3.68 (±1.73) cm, respectively; and 2.07 ± 0.67 (±0.67) cm and 2.26 (±0.72) cm on the unwounded/wounded leaf veins. Large numbers of yellow-pink conidial masses were produced on the central parts of lesions (). Seven days after inoculation, the lesions extended to almost entire twigs or main leaf veins (). In contrast, smaller lesions were found on unwounded/wounded banana (0.68 ± 0.08/0.8 ± 0.07 cm) () and marrow (0.76 ± 0.09/0.92 ± 0.08 cm) fruits (), as well as on unwounded green pepper fruits (confined to inoculation sites), 5 days after inoculation. No conidial masses were produced on the lesions until 7 days and no infected symptoms were observed on the other plants inoculated.
Pathogenicity testing indicated that D. horii infected unwounded banana and marrow fruits. Interestingly, marrow plants are grown in the region but no infection has been reported under field conditions. Banana plants do not grow in this location. Artificial host inoculation is usually not reliable enough for determining host-specificity (usually being determined by natural infection), but may indicate the potential for infection (Freeman et al. 1998), cross-infection potential and cytological characteristics of the infection process.
Infection process
On persimmon
The infection process of C. horii on susceptible Diospyros kaki cv. Wuheshi has been well documented (Zhang et al. Citation2004; Zhang Citation2008). C. horii exhibits an infection strategy of intracellular colonization and fungal hyphae growth within the cell lumen without penetrating host protoplasts. This is similar to that of C. lindemuthianum on beans and C. sublineolum on sorghum (O'Connell et al. Citation1985; Wharton et al. Citation2001). This research also revealed that cytological and ultrastructural characters are similar in the process of infection of twigs and petioles, closely resembling that of C. lindemuthianum on bean (Zhang et al. Citation2003, Citation2005). In contrast, conidia of C. horii germinate on twigs of the resistant Zhejing persimmon (D. glaucifolia) and produce germ tubes that form dark appressoria which, in turn, produce infection pegs and penetrate the cuticle within 24 h of inoculation. The fungus then became quiescent and did not develop further and no visible symptoms of disease were observed (Zhang and Xu Citation2005). This phenomenon may be similar to that of quiescent infection in unripe avocado fruits attacked by Colletotrichum gloeosporioides (Prusky Citation1996).
Infection process on banana and marrow
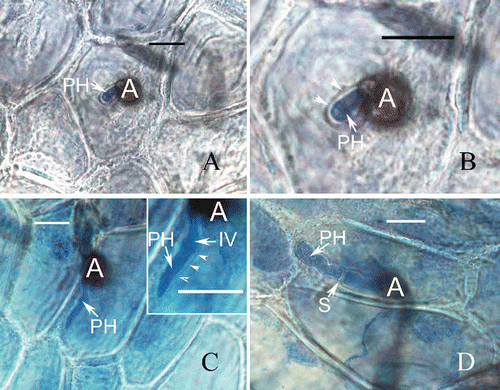
The infection process of C. horii on banana (Musa acuminata) and marrow (Cucurbita pepo) was observed with fruits inoculated with a conidial suspension, as described above. Pieces were cut from the inoculated banana and marrow skins and decolourized in a 0.15% (w/v) solution of trichloroacetic acid in a 3:1 (v/v) mixture of ethanol and chloroform for 14 h. They were then stained in a 0.025% (w/v) solution of aniline blue in lactophenol for 3–4 h, as described by Sun and Zhang (Citation2009). Light microscopic examination was made with a Zeiss Axiophot 2 microscope with Axiocam CCD camera and Axiovision digital imaging software (AxioVision Software Release 3.1, ver. 3–2002; Carl Zeiss Vision Imaging Systems). The preinfection stages of C. horii isolates on banana are very similar to those on Diospyros kaki (Zhang et al. Citation2003, Citation2005), in which conidia adhere to and germinate on the plant surface, producing germ tubes that form appressoria 12 h after inoculation, which in turn produced infection pegs 24 h after inoculation and penetrate the cuticle directly. About 48 h after inoculation, the inoculated tissue sites became brown, but no infection vesicle were seen; these appeared 60 h after inoculation. Ninety six hours after inoculation, the primary hypha formed in the initial infection cell () and was surrounded by an invaginated plasma membrane. The primary hyphae did make contact with the plasma membrane and were separated by an interfacial matrix (), closely resembling that on Diospyros kaki (Zhang et al. Citation2005; Zhang Citation2008). The host plasma membrane surrounding the primary hypha became thick and was probably deposited by the opaque material, as previously described on Diospyros kaki () (Zhang et al. Citation2003). However, in contrast to Diospyros kaki (Zhang Citation2008), the disease development was limited and the primary hyphae developed very slowly. Even 120 h after inoculation, the lesions were only slightly larger than the inoculated sites, and dark brown. The primary hyphae was confined to the initial infection cell but did not produce branches and septa (), and an interfacial matrix separated the cell wall of the primary hyphae from the host plasma membrane (). Then, 144 h after inoculation, the primary hypha produced septa, but was still restricted to the invading cell and no secondary hyphae were produced from it. At the same time, the interfacial matrix disappeared () and the host cell was killed.
Figure 5. Development of fungal infection structures on host cells during interaction of banana with C. horii isolates. A, a primary hypha in an initial infection cell 96 h after inoculation. B, enlarged portion of (A). Note that a primary hypha is separated by an interfacial matrix from the invaginated plasma membrane (arrowhead) surrounding it. C, a primary hypha in an initial infection cell 120 h after inoculation. Note that an enlarged portion of (C) at the right top, in which a primary hypha did not make contact with the host plasma membrane (arrowlead). D, a primary hypha in an initial infection cell 144 h after inoculation. A, appressorium; PH, primary hyphae; IV, infection vesicle; S, septum; Bar = 10 μm.
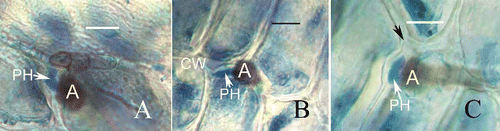
The infection pegs produced from appressoria penetrated the cuticle to invade the host cell and also entered the intercellular tissues () or the host cell walls (,). Similarly, development of the primary hyphae was evidently restrained and no secondary hyphae were produced. In contrast to hyphal length, the primary hyphae (7.12 ± 0.22 μm) in the intercellular tissues and host cell walls were shorter than in the invading cells (13.8.12 ± 0.20 μm) and no septum was produced until 132 h after inoculation (). The walls of primary hyphae made contact with the cell walls of the host and occupied the space between the cell walls (). After invading the host cell wall, swollen primary hypha may produce cell wall-degrading enzymes and exert mechanical pressure with its development, causing the host cell wall to rupture (). Such observations clearly indicate that the hyphal growth of C. horii isolates were inhibited at ∼3 days after inoculation, compared with the extensive hyphal development on Diospyros kaki (Zhang et al. Citation2003, Citation2005), and no secondary hyphae differentiation was produced after primary hyphae differentiation at the transition between the biotrophic and necrotrophic phases. We have demonstrated that an interfacial matrix also was formed in the interaction between C. horri isolates and banana plant.
Figure 6. Development of fungal infection structures in host intercellular space and cell walls during interaction of banana with C. horii isolates. A, a primary hypha in intercellular space 120 h after inoculation. Note that a primary hyphae is in close contact with the host cell walls. B, a primary hypha in cell wall 120 h after inoculation. C, a primary hypha in cell wall 132 h after inoculation. Note that swollen primary hypha has caused the host cell wall to curve and rupture (arrowhead).
Similarly, the initial stages of C. horii isolates on marrow are very similar to that on banana, in which the appressoria produce infection pegs 24 h after inoculation, penetrating the cuticle directly. However, the infection pegs seemed to cease development until 144 h after inoculation, as described on twigs of the resistant Zhejing persimmon (D. glaucifolia) (Zhang and Xu Citation2005).
Species delineation in Colletotrichum is confused, being based on few morphological characters and host relationships. Cytological studies clearly show that the infection process and intracellular infection structures of C. horii are different from that of C. gloeosporioides (Sutton Citation1992) and similar to hemibiotrophic species of Colletotrichum (Perfect et al. Citation1999), but more closely related to that of C. lindemuthianum on bean (O'Connell et al. Citation1985; Zhang et al. Citation2003, Citation2005; Zhang Citation2008) and banana. However, morphologically, C. horii is different from C. lindemuthianum when conidial size 9.5–11.5 × 3.5–4.5 μm is compared (Sutton Citation1992).
Effects of environment factors on growth and development of C. horii
Colletotrichum horii overwinters mainly in lesions of living twigs (Zhang and Xu Citation2003). Although it was believed that pathogen could overwinter on dead leaves and fruits, no experimental data has been provided (Jia et al. Citation1997). In the field, the disease first appears in the vicinity of previously diseased twigs and no symptoms are visible on newly-formed twigs near the ground. Consequently it is speculated the pathogen may disappear when leaves and fruits rot on the ground. Detection of pathogen from various plant parts showed that the survival rate of C. horii was 16.67% in lesions of living twigs, and 1.56% in the segments of dead diseased twigs (Zhang and Xu Citation2003). Mycelia in diseased tissues are, therefore, considered to be an important source of inocula-producing primary conidia in field. In spring, mycelia in diseased tissues produce conidia and they are dispersed by rain splash and wind to newly formed twigs. The pathogen can also be dispersed in symptomless seedlings over long distances (Zhang and Xu Citation2003). Symptomless persimmon seedlings from diseased areas produced anthracnose lesions within 1 year on newly formed twigs. According to the position of the lesions, it was believed that the infection source was related to the bud scales that carried the pathogen. In the spring, when favorable weather conditions occur, conidia that develop from the overwintering mycelium act as the primary source of inocula. Successful infection involves various environment factors, such as temperature, humidity, pH and nutrition on the surface of the host, which determines disease occurrence.
The effect of temperature on growth of mycelium is significant. The optimal temperature for growth in C. horii is ˜25°C; higher temperature inhibits mycelium growth (Zhang and Hu Citation2004). While conidia germinate and form appressoria over a wide range of pH 2.0–9.0, the optimal pH for conidial germination and appressorial formation was between pH 5.0 and 6.0. When combining temperature and pH, pathogenicity testing showed interesting results. Visible symptoms of anthracnose occurred on new twigs at 23°C within pH 4.0–8.0 with conidial masses on lesions after 80–90 h; at 17°C and pH 6.0 without spore masses after 7 days, but were absent at temperatures lower than 17°C and pH 5.0–6.0 (Zhang and Hu Citation2004).
Glucose also influences conidial germination and appressoria formation (Zhang and Hu Citation2004). The percentage of conidial germination increases with increasing concentrations of glucose and time (). The percentage of conidial germination increased between 21 and 59 % with the concentration of glucose (0.1–5%) after 12 h and, subsequently, the percentage in all treatments increased with time. After 48 h, the percentage of conidia germination went from 57 to 87% with increasing glucose concentrations.
Table 2. Rate of conidial germination in different concentration of a glucose solution
Glucose inhibited appressorial formation (), which decreased from 32 to 0% after 12 h with glucose concentrations (0.1–5%); similarly, with increasing time, the percentage also increased correspondingly. After 48 h, the percentage climbed from 31 to 87% with increasing concentrations of glucose.
Table 3. Percentage of appressorial formation in different concentrations of a glucose solution
PCR, sequencing and phylogenetic analysis
Partial actin (ACT), β-tubulin (TUB2), calmodulin (CAL), glutamine synthetase (GS), glyceraldehyde-3-phosphate dehydrogenase (GPDH) genes and the complete rDNA-ITS (ITS) region from five Colletotrichum strains were amplified by PCR, as described by Prihatsuti et al. (2009). The amplified DNA fragments were purified by an Axygen PCR purification kit. Purified PCR products for partial actin, beta-tubulin, calmodulin and glutamine synthetase were ligated into a pGEM-T vector (TaKaRa Co., Japan), and the ligated products were transformed into DH5α. The positive clone was propagated, the recombinant plasmids were extracted according to the manufacturer's instructions (Axygen Bioscience), and identified by PCR and restriction endomuclease enzyme digestion. The sequence determination of PCR products and recombinant plasmid was carried out by Hangzhou Genomics Institute for sequencing in both directions. DNA sequencings were performed at the SinoGenoMax Company Limited. The accession numbers of all sequences are listed in . Phylogenetic analyses were performed using PAUP* 4.0b10 (Swofford Citation2002) and MrBayes 3.0b4 (Huelsenbeck and Ronquist Citation2001), with details outlined by Cai et al. (2006).
The combined dataset included 20 sequences with 2291 characters after alignment. Parsimony analysis resulted in six equally parsimonious trees. The KH test showed that these trees were not significantly different. One of these trees is shown in . Phylogenetic analysis showed that the five tested isolates of C. horii clustered together with the reference isolates of C. kahawae (IMI 363578 and IMI 319418), with 98% bootstrap support. Two species could be well differentiated by the conidia size, as C. horii has much larger conidia than C. kahawae (12.5–19.0 × 4.0 μm) (Waller et al. Citation1993).
Figure 7. Phylogram generated from parsimony analysis based on combined actin, Bt2, calmodulin, GS and ITS sequences. Data were analysed with random addition sequence, unweighted parsimony and treating gaps as missing data. Bootstrap values ≥50% are shown above or below branches. Thickened branches indicate Bayesian posterior probabilities ≥95%. The tree is rooted with Colletotrichum falcatum.
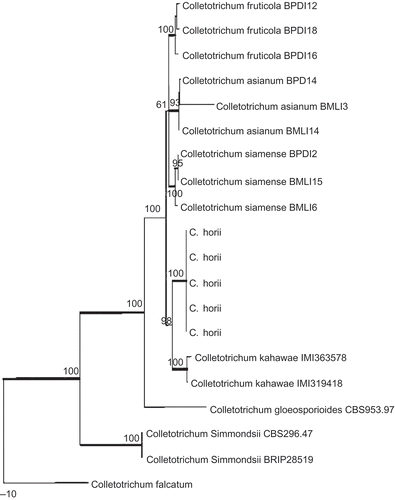
The phylogenetic relationship between C. horii and its closely related species in Colletotrichum is analyzed based on combined partial actin, beta-tubulin, calmodulin, glutamine synthetase and ITS/5.8S gene sequence data. The results of phylogenetic analysis clearly indicated that C. horri is a distinct species, closely related to C. kahawae.
Future prospects
In this paper, we review the available information on Colletotrichum horii, which will facilitate its correct and accurate identification. Improved agricultural production requires accurate identification of pathogens to enable more effective disease control and management (Than et al. Citation2008). Previously, we had found that symptomless seedlings of persimmon carried C. horii, which lead to its dispersal over long distances (Zhang and Xu Citation2003). As this pathogen was previously identified as Colletotrichum gloeosporioides, which has a wide range of hosts, it has not been added to list of quarantine pests in China. In addition, the percentage of bud scales that carried the pathogen is very low, ranging from 3.3 to 4% (Zhang and Xu Citation2003). Although latent infection by C. horii in persimmon seedlings is undergoing extensive research (http://www.cab.zju.edu.cn/instswjs/people/zhang-jz/c3.htm), methods that enable its rapid and accurate detection from a large numbers of persimmon seedlings need to be improved.
The infection process in C. horii is well understood. C. horii provides an excellent pathosystem for studying the molecular basis for infection and fungal–plant interactions. To understand the molecular mechanisms of interactions between the pathogen and host, a genomic library of C. horii has been constructed and cloning of pathogenesis-related genes have also been performed by Agrobacterium tumefaciens - mediated transformation technique (Sun et al. Citation2008). A few mutants have been shown to be related to pathogenicity, and corresponding gene fragments had have been cloned (Sun et al. Citation2008). Identification and functional analysis of pathogenesis-related genes are a major undertaking for future studies but will provide new insights into the molecular mechanism of infection structure differentiation and fungal–plant interactions.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 30571208).
References
- Arx JA von . 1957 . Die Arten der Gattung Colletotrichum Cda . Phytopathologische Zeitschrift , 29 : 413 – 468 .
- Cai , L , Hyde , KD , Taylor , PWJ , Weir , BS , Waller , JM , Abang , MM , Zhang , JZ , Yang , YL , Phoulivong , S , Liu , ZY , Prihastuti , H , Shivas , RG , McKenzie , EHC and Johnston , PR. 2009 . A polyphasic approach for studying Colletotrichum . Fungal Diversity , 39 : 183 – 204 .
- Cannon , PF , Buddie , AG and Bridge , PD. 2008 . The typification of Colletotrichum gloeosporioides . Mycotaxon , 104 : 189 – 204 .
- Crouch , JA , Beirn , LA , Cortese , LM , Bonos , SB and Clarke , BB. 2009 . Anthracnose disease of switchgrass caused by the novel fungal species Colletotrichum navitas . Mycol Res. , 113 : 1411 – 1421 .
- Hori , S. 1910a . Kaki no Shinbyogai Tansobyo . Engei no Tomo , 6 ( 1 ) : 58 – 61 .
- Hori , S. 1910b . Kaki no Shinbyogai Tansobyo . Engei no Tomo , 6 ( 2 ) : 21 – 24 .
- Huelsenbeck , JP and Ronquist , FR. 2001 . MRBAYES: Bayesian inference of phylogenetic trees . Biometrics , 17 : 754 – 755 .
- Jia , KF , Cheng , Y and Wang , LH. 1997 . Epidemic factors and control techniques of Japanese sweet kaki persimmon anthracnose . J Zhejiang Forestry College , 14 ( 1 ) : 45 – 49 .
- Johnston , PR and Jones , D. 1997 . Relationship among Colletotrichum isolates from fruit rots assessed using rDNA sequences . Mycologia , 89 : 420 – 430 .
- Hyde , KD and Zhang , Y. 2008 . Epitypification: should we epitypify? . J Zhejiang Univ Sci. , B 9 : 842 – 846 .
- Maffei , L. 1921 . Una malattia delle foglie del “Kaki” dovuta al Colletotrichum kaki n. sp . Riv Patol Veg. , 11 : 116 – 118 .
- O'Connell , RJ , Bailey , JA and Richmond , DV. 1985 . Cytology and physiology of infection of Phaseolus vulgaris by Colletotrichum lindemuthianum . Physiol Plant Pathol. , 27 : 75 – 98 .
- Perfect , SE , Hughes , HB , O¢Connell , RJ and Green , JR. 1999 . Colletotrichum: A model genus for studies on pathology and fungal–plant interactions . Fung Genet Biol. , 27 : 186 – 198 .
- Prusky , D. 1996 . Quiescent infections by postharvest pathogens . Annu Rev Phytopathol. , 34 : 413 – 434 .
- Sawada , K. 1933 . Report of survey on fungi in Taiwan . Report of Agriculture Ministry of Taiwan Center Institute No. , 61 : 1 – 99 .
- Shenoy , BD , Jeewon , R , Lam , WH , Bhat , DJ , Than , PP , Taylor , PWJ and Hyde , KD. 2007 . Morpho-molecular characterisation and epitypification of Colletotrichum capsici (Glomerallaceae, Sordariomycetes), the causative agent of anthracnose in chilli . Fungal Diversity , 27 : 197 – 211 .
- Shenoy , BD , Jeewon , R and Hyde , KA. 2007 . Impact of DNA sequence-data on the taxonomy of anamorphic fungi . Fungal Diversity , 26 ( 1 ) : 1 – 54 .
- Sreenivasaprasad , S , Mills , PR , Meehan , B and Brown , AE. 1996 . Phylogeny and systematics of 18 Colletotrichum species based on ribosomal DNA sequences . Genome , 39 : 499 – 512 .
- Sun , H , Xu , T and Zhang , JZ. 2008 . Genome library construction of anthracnose pathogen on persimmon and screening of pathogenesis related mutants for analysis , 151 Zhejiang, , China : PhD thesis, Zhejiang University .
- Sun , H and Zhang , JZ. 2009 . Colletotrichum destructivum from cowpea infecting Arabidopsis thaliana and its identity to C. higginsianum . Eur J Plant Pathol. , 125 : 459 – 469 .
- Sutton , BC. 1992 . “ The genus Glomerella and its anamorph Colletotrichum ” . In Colletotrichum: Biology, Pathology and Control , Edited by: Bailey , J. A. and Jeger , M. J. 1 – 27 . Wallingford : CAB International .
- Swofford , DL. 2002 . PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods). Version 4b10 , Sunderland, MA : Sinauer Associates .
- Than , PP , Prihastuti , H , Phoulivong , S , Taylor , PWJ and Hyde , KD. 2008 . Review: Chilli anthracnose disease caused by Colletotrichum species . J Zhejiang Univ. , 9 : 764 – 778 .
- Waller , JM , Bridge , PD , Black , RL and Hakiza , G. 1993 . Characterization of the coffee berry disease pathogen, Colletotrichum kahawae Sp. Nov . Mycol Res. , 97 : 989 – 994 .
- Weir , BS and Johnston , PR. 2010 . Characterisation and epitypification of Gloeosporium kaki Hori as Colletotrichum horii nom. nov. Mycotaxon (in press)
- Wharton , PS , Julian , AM and O'Connell , RJ. 2001 . Ultrastructure of the infection of Sorghum bicolor by Colletotrichum sublineolum . Phytopathology , 91 : 149 – 158 .
- Zhang , JZ. 2008 . Anthracnose of persimmon caused by Colletotrichum gloeosporioides in China . Asian Australasian J Plant Sci Biotechnol. , 2 ( 2 ) : 50 – 54 .
- Zhang , JZ and Hu , DW. 2004 . Effects of environment factors on conidia germination, appresorium formation and pathogenicity of the persimmon anthracnose pathogens Collectotrichum gloeosporioides . Acta Phytopathol Sinica , 22 ( 4 ) : 645 – 652 .
- Zhang , JZ and Xu , T. 2003 . Various stages and amount of Colltotrichum gloeosporiodes on overwintering twigs of persimmon . J Plant Prot. , 30 ( 4 ) : 437 – 438 .
- Zhang , JZ and Xu , T. 2005 . Cytological characteristics of the infection in different species, varieties and organs of persimmon by Colletotrichum gloeosporioides . Mycosystema , 24 ( 1 ) : 116 – 122 .
- Zhang , JZ , Hu , DW and Xu , T. 2003 . Studies on cytology of the infection of persimmon by Colletotrichum gloeosporioides . Mycosystema , 22 ( 4 ) : 645 – 652 .
- Zhang , JZ , Hu , DW and Xu , T. 2005a . Ultrastructure of infection of persimmon petiole by Collectotrichum gloeosporioides . Acta Phytopathol Sinica , 35 ( 5 ) : 434 – 441 .
- Zhang , JZ , Xu , T and He , LP. 2005b . Anthracnose pathogen on Diospyros kaki cv . Wuheshi and its nuclear behavior in process of appressorium formation. Mycosystema , 24 ( 3 ) : 446 – 456 .