Abstract
Four early-phase (EP) and one late-phase (LP) ammonia fungi were co-cultured with three non-ammonia fungi on urea, ammonium, nitrite and nitrate agar media at different pH values (5.5, 7.0 and 9.0). The early stage EP species Amblyosporium botrytis and Ascobolus denudatus showed deadlock competition with the non-ammonia fungi Aspergillus niger and Penicillium citrinum on urea agar medium, and neutral intermingling or weak competing deadlock on ammonium, nitrite and nitrate agar media in all pH conditions. The late stage EP species, Coprinopsis phlyctidospora and Lyophyllum tylicolor, showed antagonistic deadlock and invaded the region containing mycelia of the non-ammonia fungi, P. citrinum and Trichoderma viride, on urea, nitrite and nitrate agar media in all pH conditions. The LP species, Hebeloma vinosophyllum, showed competing deadlock with Asp. niger and P. citrinum on urea medium and weak competition on nitrite and nitrate agar media at pH 9.0. These findings suggest that the successive occurrence of ammonia fungi is partly due to changes in the interactions between ammonia fungi and non-ammonia fungi based on changes in inorganic nitrogen.
Introduction
To understand the structure and function of fungal communities, it is important to understand the interactions between different types of fungi (Rayner and Boddy Citation1988; Christensen Citation1989). Ammonia fungi are defined as a chemo-ecological group of fungi that sequentially develop reproductive structures exclusively or relatively luxuriantly on soil after a sudden addition of ammonia, alkalis, or other nitrogenous materials that react directly as a base or indirectly as a base after decomposition (Sagara Citation1975).
As described in previous studies, when the forest floor was treated with a large amount of urea, the ammonium nitrogen concentration in the soil increased, thus increasing the pH, and then both gradually returned to original levels (Yamanaka Citation1995a–c; Suzuki Citation2000; Suzuki et al. Citation2002). In these situations, the occurrence of ammonia fungi generally proceeded as follows: anamorphic fungi and cup fungi in the Ascomycota occurred when the soil ammonium-nitrogen concentration decreased (associated with a decrease in pH from 8 to 7). These species that occur at an early stage of the early phase are hereafter referred to as early stage EP species. When the oxidation of ammonium nitrogen decreased the soil pH from 7 to 6, fungi in the Basidiomycota with smaller fruiting bodies became dominant. These species that occur at the late stage of early phase are hereafter referred to as late stage EP species. Further decreases in pH (to a level similar to that of the control at pH 3.5–6.0) were associated with the occurrence of fungi with larger fruiting bodies (Sagara 1992; Yamanaka Citation1995a–c; Suzuki Citation2000; Suzuki et al. Citation2002). These late-phase occurring species are hereafter referred to as LP species (Sagara 1992; Yamanaka Citation1995a–c; Suzuki Citation2000; Suzuki et al. Citation2002). The LP species disappeared within 2–3 years, when concentrations of nitrite- and nitrate-nitrogen declined to similar levels as those in control soil (Sagara 1992; Yamanaka Citation1995a–c). All EP species and several LP species are saprobic and most LP species are ectomycorrhizal (Sagara 1995; Sagara et al. Citation2008). Yamanaka (Citation1995a–c) suggested that ammonium nitrogen was the major nitrogen source for vegetative growth of LP species in urea-treated soils, and it was gradually replaced by nitrate-nitrogen. Licyayo et al. (Citation2007) suggested that the interactions among ammonia fungi were one of the main factors affecting their successive occurrence. However, little is known about the effects of interactions between ammonia and non-ammonia fungi on the successive occurrence of ammonia fungi. Therefore, to examine in detail the interactions between ammonia fungi and non-ammonia fungi, we co-cultured various fungi on agar media containing urea, ammonium-nitrogen, nitrite-nitrogen and nitrate-nitrogen, adjusted to several different pH values.
Materials and methods
Organisms
shows the fungal species used in these experiments. The fungi included four saprobic ammonia fungi, one ectomycorrhizal ammonia fungus that is commonly found in Japan after field applications of urea, two common soil fungi (saprobic non-ammonia fungi) and one mycoparasite (a saprobic non-ammonia fungus).
Table 1. Fungal isolates used in these experiments
Medium preparation
The basal medium was composed of 22.22 g glucose, 0.33 g KH2PO4, 0.33 g MgSO4·7H2O, 0.11 g CaCl2·H2O, 0.33 mg ZnSO4·7H2O, 0.15 mg FeSO4·7H2O, 0.10 mg CuSO4·5H2O, 0.10 mg MnSO4·5H2O, 0.02 mg Na2MoO4·2H2O, 0.50 mg thiamine hydrochloride, 0.10 mg nicotinic acid, and 1000 ml distilled water (Kitamoto et al. Citation1972). Urea, ammonium chloride, sodium nitrite, or sodium nitrate was added to the basal medium at concentrations ranging from 0.041 to 4.18 g N/l, according to the optimal concentrations for each ammonia fungus (). All four types of media were adjusted to three different pH levels (5.5, 7.0 and 9.0) with 1 M NaOH and 1 M HCl, to determine the effects of pH on interactions between fungi. The medium adjusted to pH 5.5 served as the control because the soil pH in most Japanese forests is between 3.6 and 6.0 (Sagara Citation1975; Yamanaka Citation1995a–c; Fukiharu et al. Citation1997; Sato and Suzuki Citation1997; Suzuki Citation2000; Suzuki et al Citation2002; He and Suzuki Citation2004). Hereafter, the basal media containing urea, ammonium chloride, sodium nitrite, and sodium nitrate are described as urea agar medium, ammonium agar medium, nitrite agar medium, and nitrate agar medium, respectively. For all media, double-strength medium was sterilized by filtration through a membrane filter (cellulose nitrate; 0.22 μm pore size; Advantec, Tokyo, Japan). Agar medium [30 g agar (Nacalai Tesque, Kyoto, Japan)/1000 ml distilled water] was sterilized for 15 min at 120°C and kept at 60°C. Then, an equal volume of the agar medium and each double-strength test medium was mixed, keeping the mixture at 60°C before dispensing 20-ml aliquots of the medium into sterilized Petri dishes (90 mm in diameter).
Table 2. Nitrogen concentrations used in co-culturing experiments
Co-culturing of ammonia fungi and non-ammonia fungi
Agar discs (4 mm diameter) were cut from the sub-peripheral region of actively growing mycelial colonies of each fungal isolate. The discs were then cultured at room temperature separately on urea, ammonium, nitrite or nitrate agar media. Two discs from different fungal species were inoculated 55 mm apart on the agar media with different pH (5.5, 7.0 and 9.0). Slow-growing fungi were inoculated onto the medium before the fast-growing fungi (), because slow-growing fungi can be completely suppressed by fast-growing fungi when they are inoculated at the same time (Shaw et al. Citation1995). The co-culture experiments included saprobic EP species and the three saprobic non-ammonia fungus species, and also the ectomycorrhizal LP species H. vinosophyllum, which was included because it is a facultative mycorrhizal fungus, i.e. it is somewhat saprobic (Suzuki Citation2009). Pairs of the same isolates were also prepared as a control. All procedures were conducted under aseptic conditions. Co-cultures were incubated at 20.0±0.5°C under light irradiation at ∼500 lux with a white light florescent lamp (Hitachi, Tokyo, Japan). All co-cultures had five replicates.
Table 3. Inoculation schedule of each fungus on different nitrogen agar media
Observations and measurements
We studied the interactions between five ammonia fungi and three non-ammonia fungi () co-cultured on different kinds of nutrient agar media. We categorized the interactions observed in co-cultures based on the features observed at the contact zone (CZ) of the mycelial colonies and those observed at the opposite sides to contact zone (OCZ) of the mycelial colonies. The types of interactions were recorded after 25–30 days of incubation. We also measured mycelial expansion (radial growth) on each medium after 2 to 30 days of incubation. The ratio between the mycelial colony radius in the CZ direction to that in the OCZ direction indicated the degree of growth inhibition.
Assessment of colony interaction types
Mycelial colony interactions were described according to Rayner and Boddy (Citation1988), with modifications. When the mycelia of both inoculated fungi completely intermingled with each other, this was defined as neutral intermingling (N) (). When co-cultured fungi first showed contact zone inhibition but then intermingled, this was defined as Ci(N). When either of the inoculated fungi invaded past the CZ into the mycelial region of the other fungi and the invasion stopped within a few centimetres, this was defined as competing deadlock (Dc) (). When a CZ formed between the mycelia of the two fungi and low mycelial density was observed behind the CZ, this was defined as antagonistic deadlock (Da) (). When a significant growth inhibition was observed before the mycelia of the two fungi came into contact, this was defined as inhibition deadlock (Di) ( and ). When the mycelia of either fungus covered the colony of the other fungus on the nutrient agar plate, this was defined as covering (C).
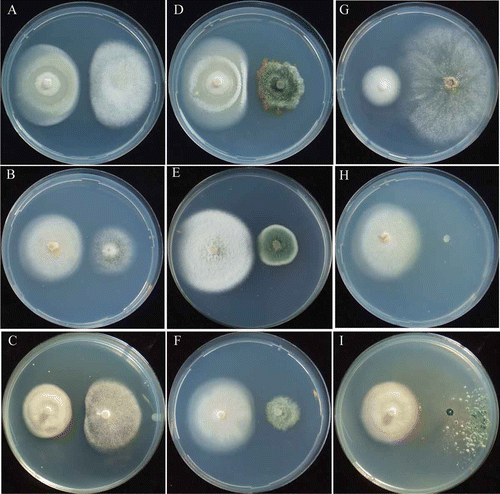
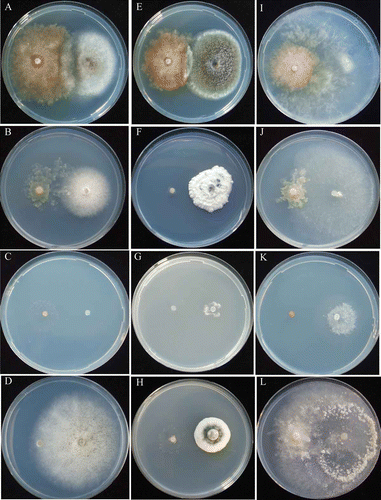
Figure 2. Mycelial interactions between Ascobolus denudatus and non-ammonia fungi. Asc. denudatus /Asp. niger at pH 5.5 (A) urea, 12th day; (B) NH4-N, 12th day; (C) NO2-N, 9th day; (D) NO3-N, 9th day. Asc. denudatus/P. citrinum at pH 5.5 (E) urea, 9th day; (F) NH4-N, 12th day; (G) NO2-N, 18th day; (H) NO3-N, 12th day. Asc. denudatus/T. viride at pH 5.5 (I) urea, 9th day; (J) NH4-N, 19th day; (K) NO2-N, 18th day; (L) NO3-N, 12th day.
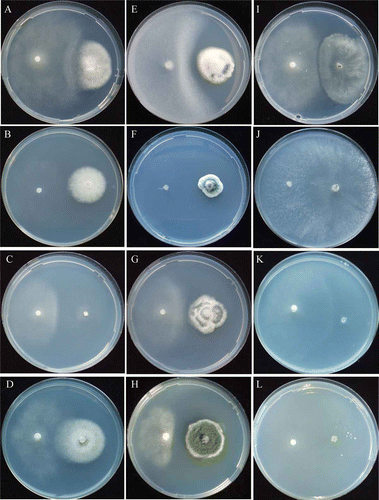
Figure 4. Mycelial interactions between Lyophyllum tylicolor and non-ammonia fungi. L. tylicolor /Asp. niger at pH 5.5 (A) urea, 12th day; (B) NO2-N, 18th day; (C) NO3-N, 12th day. L. tylicolor/P. citrinum at pH 5.5 (D) urea, 9th day; (E) NO2-N, 18th day, (F) NO3-N, 12th day. L. tylicolor/T. viride at pH 5.5 (G) urea, 12th day; (H) NO2-N, 18th day; (I) NO3-N 12th day.
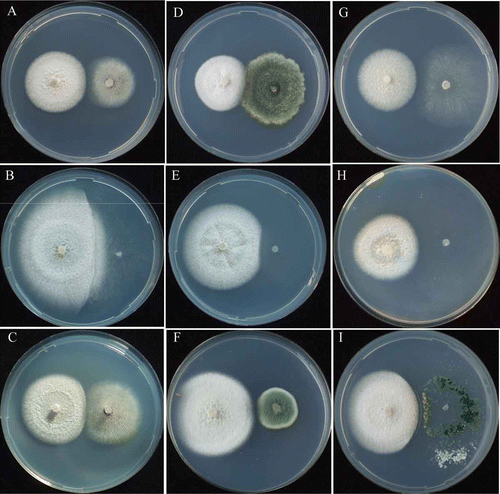
Figure 5. Mycelial interactions between Hebeloma vinosophyllum and non-ammonia fungi. vinosophyllum /Asp. niger at pH 5.5 (A) urea, 12th day; (B) NO2-N, 18th day; (C) NO3-N, 12th day. H. vinosophyllum/P. citrinum at pH 5.5 (D) urea, 9th day; (E) NO2-N, 18th day; (F) NO3-N, 12th day. H. vinosophyllum/T. viride at pH 5.5 (G) urea, 12th day; (H) NO2-N, 18th day; (I) NO3-N 12th day.
Results
Intra-species co-culturing on media containing different nitrogen sources
Co-culturing of the early stage EP species, Amblyosporium botrytis and Ascobolus denudatus, with the late stage EP species, Coprinopsis phlyctidospora, resulted in neutral intermingling on urea, nitrite and nitrate agar media (Tables ). When the late stage EP species, Lyophyllum tylicolor, and the LP species, Hebeloma vinosophyllum, were co-cultured, they first showed contact zone inhibition and finally intermingled on urea, nitrite and nitrate agar media (, , and ).
Table 4. Mycelial interactions between ammonia fungi and non-ammonia fungi on agar media containing different nitrogen sources at pH 5.5
Table 5. Growth response of fungi interacting on urea agar medium at three different pH levels
Table 6. Growth response of fungi interacting on ammonium agar medium at three different pH levels
Table 7. Growth response of fungi interacting on nitrite agar medium at three different pH levels
Table 8. Growth response of fungi interacting on nitrate agar medium at three different pH levels
When the non-ammonia fungi, Aspergillus niger, Penicillium citrinum and Trichoderma viride, were co-cultured, they first showed contact zone inhibition and finally intermingled on urea, ammonium, nitrite and nitrate agar media in all pH conditions (Tables ). The ratio between of mycelial colony radius in the CZ direction to that in the OCZ direction showed growth inhibition even the same species (Tables ).
Inter-species co-culturing on media containing different nitrogen sources
The late stage EP species, C. phlyctidospora and L. tylicolor, and the LP species, H. vinosophyllum, grew poorly on ammonium agar medium. Therefore, we did not co-culture these species with non-ammonia fungi in the following experiments ().
Interactions between ammonia fungi and non-ammonia fungi on urea agar medium
The early stage EP species, Am. botrytis showed antagonistic deadlock with the non-ammonia fungi, Asp. niger ( and ; ) and P. citrinum ( and ; ), but neutral intermingling with another non-ammonia fungus, T. viride ( and ; ). T. viride grew poorly on urea agar medium containing the concentration of urea that was optimal for Am. botrytis (; ; Barua et al. 2012.). Another early stage EP species, Asc. denudatus, showed antagonistic deadlock with non-ammonia fungi in all pH conditions ( and ; , and ). After 30 days of incubation, the mycelium of Am. botrytis was slightly overgrown by those of Asp. niger and P. citrinum (; ), whereas the mycelium of Asc. denudatus was covered gradually by those of all tested non-ammonia fungi in all pH conditions (). When Am. botrytis was co-cultured with non-ammonia fungi, it formed abundant conidiophores at pH 5.5 and pH 7.0 but few at pH 9.0 (). Asp. niger and P. citrinum formed abundant conidiophores after their mycelia contacted those of Am. botrytis at pH 5.5 and pH 7.0, but formed a few conidiophores at pH 9.0. Asc. denudatus did not form fruiting bodies in intra-species and inter-species co-cultures, whereas Asp. niger and P. citrinum showed poor conidiophore formation when co-cultured with Asc. denudatus in all pH conditions ( and ). T. viride did not form conidiophores when co-cultured with Am. botrytis and Asc. denudatus in all pH conditions ( and ).
Figure 3. Mycelial interactions between Coprinopsis phlyctidospora and non-ammonia fungi. C. phlyctidospora /Asp. niger at pH 5.5 (A) urea, 9th day; (B) NO2-N, 18th day; (C) NO3-N, 12th day. C. phlyctidospora/P. citrinum at pH 5.5 (D) urea, 12th day; (E) NO2-N, 18th day; (F) NO3-N, 12th day. C. phlyctidospora/T. viride at pH 5.5 (G) urea, 12th day; (H) NO2-N, 18th day; (I) NO3-N 12th day.
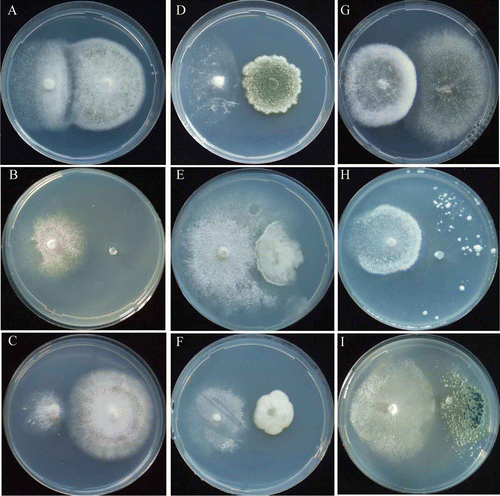
Figure 1. Mycelial interactions between Amblyosporium botrytis and non-ammonia fungi. Am. botrytis/As. niger at pH 5.5 (A) urea, 12th day; (B) NH4-N, 12th day; (C) NO2-N, 9th day; (D) NO3-N, 9th day. Am. botrytis/P. citrinum at pH 5.5 (E) urea, 12th day; (F) (NH4-N, 12th day; (G) NO2-N, 9th day, (H) NO3-N 9th day. Am. botrytis/T. viride at pH 5.5 (I) urea, 12th day; (J) NH4-N, 9th day; (K) (NO2-N, 9th day; (L) NO2-N, 9th day.
The late stage EP species C. phlyctidospora and L. tylicolor showed antagonistic deadlocks with non-ammonia fungi ( and ; , and ). The mycelium of C. phlyctidospora was not covered by those of P. citrinum and T. viride even 30 days of incubation, but covered by Asp. niger, at any pH conditions ( and ; ). The mycelium of L. tylicolor was covered gradually by those of the non-ammonia fungi. C. phlyctidospora and L. tylicolor did not form fruiting bodies during intra-species and inter-species co-culturing, whereas Asp. niger showed poor conidiophore formation when co-cultured with C. phlyctidospora ( and ). P. citrinum formed conidiophores when co-cultured with L. tylicolor in all pH conditions ( and ). T. viride did not form conidiophores when co-cultured with C. phlyctidospora and L. tylicolor in all pH conditions ( and ).
The LP species H. vinosophyllum showed competing deadlock with Asp. niger and P. citrinum, and neutral intermingling with T. viride in all pH conditions ( and ; ). The mycelium of H. vinosophyllum was rapidly covered by that of T. viride within 3 days after the two species came into contact. However, the mycelium of H. vinosophyllum was not covered by those of Asp. niger and P. citrinum even after 30 days of incubation in all pH conditions ( and ). H. vinosophyllum did not form fruiting bodies during intra-species and inter-species co-culturing, whereas Asp. niger and P. citrinum formed abundant conidiophores when co-cultured with H. vinosophyllum at pH 5.5 and 7.0, and but a few at pH 9.0. Mycelium of T. viride covered that of H. vinosophyllum and then formed conidiophores in all pH conditions ().
Interactions between ammonia fungi and non-ammonia fungi on ammonium medium
The early stage EP species Am. botrytis showed neutral intermingling with non-ammonia fungi (). Another early stage EP species, Asc. denudatus, also showed neutral intermingling with Asp. niger and T. viride, but inhibition deadlock with P. citrinum in all pH conditions (; ). After 30 days of incubation, the mycelia of Am. botrytis and Asc. denudatus were covered by those of non-ammonia fungi (). Am. botrytis showed poor conidiophore formation when co-cultured with Asp. niger and T. viride at pH 5.5 and pH 7.0, but not at pH 9.0 (). Am. botrytis did not form conidiophores when co-cultured with P. citrinum in all pH conditions (). Asc. denudatus did not form fruiting bodies during intra-species and inter-species co-culturing. All of the tested non-ammonia fungi formed abundant co-nidiophores when co-cultured with Am. botrytis and Asc. denudatus in all pH conditions.
Interactions between ammonia fungi and non-ammonia fungi on nitrite medium
The early stage EP species, Am. botrytis grew poorly and showed neutral intermingling with non-ammonia fungi at pH 7.0 and pH 9.0. Am. botrytis started to grow 18 days after inoculation on medium at pH 5.5 ( and ). Another early stage EP species, Asc. denudatus, showed weak antagonistic deadlock with non-ammonia fungi in all pH conditions ( and ; ). After 30 days of incubation, the mycelia of Am. botrytis and Asc. denudatus were covered by those of non-ammonia fungi (). Am. botrytis showed poor conidiophore formation when co-cultured with Asp. niger at pH 5.5 () but no conidiophore formation at pH 7.0 and 9.0. Am. botrytis did not form conidiophores with P. citrinum and T. viride at any pH conditions. Asc. denudatus did not form fruiting bodies during intra-species and inter-species co-culturing.
The late stage EP species, C. phlyctidospora and L. tylicolor, showed weak antagonistic deadlock with non-ammonia fungi ( and ; , , and ). The mycelium of C. phlyctidospora was slightly covered by that of Asp. niger, but not by those of P. citrinum and T. viride (; ). The mycelium of L. tylicolor was gradually covered by those of Asp. niger and T. viride, but not by that of P. citrinum in all pH conditions (; ). C. phlyctidospora and L. tylicolor did not form fruiting bodies during intra-species and inter-species co-culturing. The non-ammonia fungus T. viride showed poor conidiophore formation when co-cultured with C. phlyctidospora and L. tylicolor, and no conidiophore formation of non-ammonia fungi Asp. niger and P. citrinum when co-cultured with C. phlyctidospora and L. tylicolor in all pH conditions ().
The LP species H. vinosophyllum showed competition deadlock when co-cultured with Asp. niger and P. citrinum at pH 5.5 and 7.0, and neutral intermingling at pH 9.0 (). H. vinosophyllum showed neutral intermingling with T. viride in all pH conditions (). T. viride formed conidiophores surrounding the mycelium of H. vinosophyllum after its mycelium was covered by that of T. viride (). The mycelium of H. vinosohpyllum was covered by those of Asp. niger and P. citrinum in all pH conditions (; ). When co-cultured with H. vinosophyllum, the non-ammonia fungus, Asp. niger formed conidiophores poorly at pH 5.5 () and pH 7.0, but formed abundant conidiophores at pH 9.0. The non-ammonia fungus, P. citrinum, showed poor conidiophore formation when co-cultured with H. vinosophyllum at pH 5.5 (), and formed no conidiophore when cultured at pH 7.0 and 9.0. The non-ammonia fungus, T. viride, did not form conidiophores when co-cultured with H. vinosophyllum at any pH ().
Interactions between ammonia fungi and non-ammonia fungi on nitrate medium
The early stage EP species Am. botrytis grew weakly and showed neutral intermingling with the non-ammonia fungi, Asp. niger, P. citrinum and T. viride ( and ; ). Another early stage EP species, Asc. denudatus, showed antagonistic deadlock with Asp. niger and T. viride in all pH conditions (). Asc. denudatus showed strong competing deadlock with P. citrinum and was not completely invaded (). The mycelia of Am. botrytis and Asc. denudatus were gradually covered by those of non-ammonia fungi in all pH conditions ( and ). Am. botrytis showed poor conidiophore formation when co-cultured with P. citrinum, and did not form conidiophores with Asp. niger and T. viride in all pH conditions ().
The late stage EP species C. phlyctidospora and L. tylicolor showed inhibition deadlock when co-cultured with non-ammonia fungi ( and ; ,F,I and 4C,F,I). The mycelium of C. phlyctidospora was gradually covered by that of Asp. niger, but not by those of P. citrinum and T. viride (; ). The mycelium of L. tylicolor was gradually covered by those of non-ammonia fungi in all pH conditions (; ). C. phlyctidospora and L. tylicolor did not form fruiting bodies in intra-species and inter-species co-cultures. The non-ammonia fungus, T. viride, showed poor conidiophore formation when co-cultured with C. phlyctidospora and L. tylicolor. Non-ammonia fungi Asp. niger and P. citrinum did not form conidiophores when co-cultured with C. phlyctidospora and L. tylicolor in all pH conditions ().
The LP species H. vinosophyllum showed competing deadlock with Asp. niger and P. citrinum and neutral intermingling with T. viride (; ). The mycelia of Asp. niger and P. citrinum covered those of H. vinosophyllum in all pH conditions (; H). Asp. niger formed abundant conidiophores when co-cultured with H. vinosophyllum at pH 9.0, but a few at pH 7.0 and 5.5 (). P. citrinum formed conidiophores poorly when co-cultured with H. vinosophyllum in all pH conditions (). T. viride formed conidiophores surrounding the mycelium of H. vinosophyllum after its mycelium covered that of H. vinosophyllum in all pH conditions ().
Discussion
Ammonia fungi occur sequentially on soil after a sudden addition of alkalis, urea, aqueous ammonia, or nitrogenous materials that release ammonia during decomposition and cause alkalinisation of the soil (Sagara Citation1975). In Castanopsis and Quercus-dominated mixed forest, addition of urea to soil (800 g/m2) increased the amount of ammonium-nitrogen to 30–40 mg N/g dry soil. This was associated with an increase in pH to 8–10. Subsequent oxidation of ammonium-nitrogen into nitrate-nitrogen via nitrite-nitrogen decreased the amount of ammonium nitrogen to levels similar to that of the control (0.05–0.28 mg N/g dry soil; pH ∼5.5) (Suzuki et al. Citation2002). The early stage EP species, Am. botrytis and Asc. denudatus and the late stage, C. phlyctidospora and L. tylicolor, became dominant when the pH increased from 6.6 to 9.4 due to an increase in the amount of ammonium-nitrogen (1.5–25 mg N/g dry soil) derived from hydrolysis of urea. During the occurrence of EP species, the amounts of nitrite-nitrogen and nitrate-nitrogen in urea-treated soil were 0.280–0.930 and 1.1–10 mg-N/g dry soil, respectively, although these amounts of nitrogen were less than that of ammonium-nitrogen. The LP species, Hebeloma spoliatum, occurred on urea-treated soil during a temporary increase in amounts of nitrite-nitrogen (0.080–0.230 mg-N/dry soil) and nitrate-nitrogen (0.93–1.2 mg-N/g dry), which was associated with a return of the pH to a level similar to that of the control (Suzuki et al. Citation2002). Similar patterns in the changes in the amounts of inorganic nitrogen and pH in urea-treated soil, and the successive occurrence of ammonia fungi, were reported after nitrogen disturbances in Pinus densiflora forest (Yamanaka Citation1995a,Citationb), and in mixed forests dominated by Quercus serrata (Fukiharu et al. Citation1997) or Q. serrata and Abies firma (Suzuki Citation2000). The first flush of H. vinosophyllum occurs alongside the appearance of other Hebeloma spp. including H. spoliatum, and Alnicola lactariens and Laccaria bicolor on urea-treated forest floors (Sagara Citation1975; Yamanaka Citation1995a; Fukiharu and Horigome Citation1996; Suzuki Citation2000; Imamura and Yumoto Citation2004). At this time, the soil pH returns to a level similar to that of the control, although the soil contains more ammonium-nitrogen and more nitrate-nitrogen than the control (Yamanaka Citation1995b; Suzuki Citation2000). This suggests that the soil conditions suitable for the colonization and occurrence of H. vinosophyllum would be similar to those described above for H. spoliatum. Imamura et al. (Citation2006) reported a rapid increase in the activity of ureases in urea-treated soil, especially in the O layer, and also reported that there were similar patterns of increases in the amount of ammonium-nitrogen among different vegetation types and among different urea application times.
In vitro, the mycelia of the late stage EP species and the LP species showed extremely sparse radial growth on agar media containing higher concentrations (0.041–0.41 g N/l) of ammonium chloride in all pH conditions (). Ellouze et al. (Citation2009) also reported that ammonium chloride inhibited radial growth. Most saprobic EP species colonized vigorously on urea-containing media under weak alkaline to neutral conditions, and showed strong extracellular urease activities under neutral conditions (Barua et al. Citation2011). These observations suggested that EP species could decompose urea into ammonium-nitrogen, thereby altering the concentration of ammonium-nitrogen to a level suitable for their vegetative growth. In this present study, therefore, we evaluate the growth responses of fungi on urea agar medium instead of ammonium agar medium to avoid the influence of inhibitive effect of chloride ion.
The mycelia of the early stage EP species, Am. botrytis and Asc. denudatus, showed vigorous radial growth on urea agar medium and invaded into the territory of the non-ammonia fungi in all pH conditions (; and ). In contrast, Am. botrytis showed poor radial growth and was covered by mycelia of non-ammonia fungi on ammonium, nitrite, and nitrate agar media in all pH conditions (Tables ; ). On all of the tested media and in all pH conditions, Asc. denudatus showed vigorous radial growth, but did not invade into the territory of the non-ammonia fungi. The mycelium of Asc. denudatus was covered by mycelia of non-ammonia fungi on urea, ammonium, nitrite and nitrate agar media in all pH conditions (Tables ; ), except for P. citrinum, which did not invade into the territory of Asc. denudatus on urea and ammonium agar media. ( and ; ). When co-cultured with non-ammonia fungi, Am. botrytis formed abundant conidiophores on urea agar medium in all pH conditions ().
These findings indicate that the early stage EP species would have an advantage over non-ammonia fungi to colonize in the presence of a large amount of ammonium-nitrogen, which is associated with neutral and weak alkaline conditions. This also suggests that period in which the early stage EP species, Am. botrytis and Asc. denudatus, occur would be shortened in the field as a result of a decrease in their abilities to colonize and form conidiophores when competing with non-ammonia fungi. Occurrence of the non-ammonia fungi is associated with increased concentrations of nitrate-nitrogen, which result from oxidation of ammonium-nitrogen after a nitrogen disturbance. In other words, nitrate-nitrogen would be a key factor for the disappearance of the early stage EP species. The mycelia of the late stage EP species, C. phlyctidospora and L. tylicolor, invaded into the territory of the non-ammonia fungi on urea agar medium ( and ; and ) and weakly invaded or intermingled on nitrite and nitrate agar media in all pH conditions ( and ; ). In contrast, none of the other saprobic and ectomycorrhizal ammonia fungi evaluated showed such invasion abilities. The late stage EP species showed strong invasion abilities, and were able to compete against non-ammonia fungi, including mycoparasites. This was particularly evident in the presence of a large amount of ammonium-nitrogen. These strong competitive abilities would be an advantage for establishment of late stage EP species, and would allow them to retain their territory for a longer period than early stage EP species. This is because the late stage EP species can compete with non-ammonia fungi even under high concentrations of nitrite-nitrogen and nitrate-nitrogen. The mycelium of the LP species, H. vinosophyllum, did not invade into the territories of the non-ammonia fungi, Asp. niger, P. citrinum and T. viride, on urea, nitrite and nitrate agar media in any pH conditions (Tables ; ). The mycelium of H. vinosophyllum was not covered by those of Asp. niger and P. citrinum on urea agar medium, but was covered by their mycelia on nitrite and nitrate agar media in all pH conditions. The mycelium of H. vinosophyllum was slightly covered by that of T. viride on urea agar medium, but was completely covered with its mycelium on nitrite and nitrate agar media in all pH conditions (). These findings suggest that LP species would not be able to retain their territories and/or survive without mycorrhizal symbiosis. In other words, they rely on their mycorrhizal symbioses due to the drastic loss of their ability to compete with non-ammonia fungi when the ammonium-nitrogen is converted into nitrite-nitrogen and nitrate-nitrogen in the field.
Suzuki (Citation1989) also proposed that the sequential occurrence of ammonia fungi results from changes in the ammonium-nitrogen concentration and pH. Yamanaka (Citation1999, Citation2003) suggested that the successive change in the species composition of ammonia fungi from EP to LP species may be affected by changes in pH and the form of inorganic nitrogen. Suzuki (Citation2006) and Licyayo et al. (Citation2007) suggested that the successive occurrence of ammonia fungi was caused not only by the physiochemical soil characteristics of soil, such as pH and nitrogen concentration, but also by the interspecific interactions among ammonia fungi associated with changes in pH and nitrogen concentration.
In conclusion, the changes in the interactive abilities of ammonia fungi and non-ammonia fungi, namely the increasing in invasion abilities of non-ammonia fungi as the levels of ammonium-nitrogen, nitrite-nitrogen and nitrate-nitrogen decrease to similar levels to those of undisturbed soil, is an important factor in determining the occurrence and persistence of each ammonia fungus.
Acknowledgements
We sincerely thank Naohiko Sagara (Professor Emeritus, Kyoto University) and the Medical Mycology Research Center, Chiba University, for providing most of the fungal isolates used in this study.
References
- Barua , BS , Suzuki , A , Pham , HND and Inatomi , S. 2012 . Effects of urea on vegetative growth of ammonia fungi . J Agric Technol , 8 : 173 – 189 .
- Christensen , M. 1989 . A views of fungal ecology . Mycologia , 8 : 1 – 19 .
- Ellouze , M , Aloui and Sayadi , S. 2009 . Effect of high ammonia concentrations on fungal treatment of Tunisian landfill leachates . Desalination , 248 : 147 – 156 .
- Fukiharu , T and Horigome , R. 1996 . Ammonia fungi in the Abukuma Mountains and its biogeographical distribution around Japan . Mem Natl Sci Mus Tokyo , 29 : 105 – 111 . in Japanese with English summary
- Fukiharu , T , Sato , Y and Suzuki , A. 1997 . The occurrence of ammonia fungi, and changes in soil conditions and decay rate of bamboo in response to application of a large amount of urea in a bamboo grove in Chiba Prefecture, central Japan . Bull Fac Edu. Chiba Univ (III Nat Sci). , 45 : 61 – 67 .
- He , XM and Suzuki , A. 2004 . Effects of urea treatment on litter decomposition in Pasania edulis forest soil . J Wood Sci. , 50 : 266 – 270 .
- Imamura , A and Yumoto , T. 2004 . The time of urea treatment and its effects on the succession of ammonia fungi in two warm temperate forests in Japan . Mycoscience , 45 : 123 – 130 .
- Imamura , A and Yumoto , T. 2006 . Urease activity in soil as factor affecting the succession of ammonia fungi . J For Res. , 11 : 131 – 135 .
- Kitamoto , Y , Suzuki , A and Furukawa , S. 1972 . An action spectrum for light-induced primordium formation in basidiomycete, Favolus arcularius (Fr) Ames . Plant Physiol. , 49 : 338 – 340 .
- Licyayo , DC and Suzuki , A. 2006 . Growth responses of ammonia fungi to different concentration of ammonium-nitrogen . Mush Sci Biotechnol , 14 : 145 – 156 .
- Licyayo , DCM , Suzuki , A and Matsumoto , M. 2007 . Interactions among ammonia fungi on MY agar medium with varying pH . Mycoscience , 48 : 20 – 28 .
- Rayner , ADM and Boddy , L. 1988 . Fungal communities in the decay of wood . Adv Microb Ecol. , 10 : 115 – 166 .
- Sagara , N. 1975 . Ammonia fungi: a chemoecological grouping of terrestrial fungi . Contrib Biol Lab Kyoto Univ. , 24 : 205 – 276 .
- Sagara , N , Yamanaka , K and Tibbett , M. 2008 . “ Soil fungi associated with graves and latrines: toward a forensic mycology ” . In Soil analysis in forensic taphonomy: chemical and biological effect of buried human remains , Edited by: Tibbett , M and Carter , DO . 67 – 107 . Boca Raton , FL : CRC Press .
- Sato , Y and Suzuki , A. 1997 . The occurrence of ammonia fungi, and changes in soil conditions and wood decay rate in response to application of a large amount of urea in a Quercus serrata dominated mixed forest in Meguro , 53 – 59 . Tokyo : Bull Fac Educ Chiba Univ. 45(III Nat Sci) .
- Shaw , TM , Dighton , J and Sanders , FE. 1995 . Interactions between ectomycorrhizal and saprotrophic fungi on agar in association with seedling of lodgepole pine (Pinus contorta) . Mycol Res. , 29 : 159 – 165 .
- Suzuki , A. 1989 . “ Analyses of factors affecting the occurrence and succession of the ammonia fungi ” . In Recent advances in microbial ecology (ISME 5) , Edited by: Hattori , T , Ishida , Y , Maruyama , Y , Morita , RY and Uchida , A . 275 – 279 . Tokyo : Japanese Science Society Press .
- Suzuki , A. 2000 . A survey of species assemblage of ammonia fungi. Nat Hist Plant Planta , 68 : 27 – 35 . in Japanese
- Suzuki , A. 2006 . Experimental and physiological ecology of ammonia fungi: studies using natural substrates and artificial media . Mycoscience , 47 : 3 – 17 .
- Suzuki , A , Motoyoshi , N and Sagara , N. 1982 . Effects of ammonia, ammonium salts, urea, and potassium salts on basidiospore germination in Coprinus cinereus and Coprinus phlyctidosporus . Trans Mycol Soc Jpn. , 23 : 217 – 224 .
- Suzuki , A , Uchida , M and Kita , M. 2002 . Experimental analyses of successive occurrence of ammonia fungi in the field . Fungal Divers. , 10 : 141 – 165 .
- Suzuki , A. 2009 . Propagation strategy of ammonia fungi . Mycoscience , 50 : 39 – 51 .
- Yamanaka , T. 1995a . Changes in soil conditions following treatment with a large amount of urea to enhance fungal fruiting-body production in a Japanese red pine forest . Bull Jpn Soc Microb Ecol. , 10 : 67 – 72 .
- Yamanaka , T. 1995b . Nitrification in a Japanese pine forest soil treated with a large amount of urea . J Jpn For Soc , 77 : 232 – 238 .
- Yamanaka , T. 1995c . Changes in organic matter composition of forest soil treated with a large amount of urea to promote ammonia fungi and the abilities of these fungi to decompose organic matter . Mycoscience , 36 : 17 – 23 .
- Yamanaka , T. 1999 . Utilization of inorganic and organic nitrogen in pure cultures by saprotrophic and ectomycorrhizal fungi producing sporophores on urea-treated forest floor . Mycol Res. , 103 : 811 – 816 .
- Yamanaka , T. 2003 . The effect of pH on the growth of saprotrophic and ectomycorrhizal ammonia fungi in vitro . Mycology , 95 : 584 – 589 .