Abstract
Saprotrophic cord-forming basidiomycetes are functionally important components of temperate woodland ecosystems, being major agents of organic matter decomposition and affecting the distribution of nutrients within soils. They also represent the primary nutrient source for soil-dwelling animals. We review the interactions between cord-forming basidiomycetes and soil invertebrates, and their implications for the soil-decomposer community and woodland ecosystem functioning. Top-down grazing pressures can influence mycelial growth and physiology with implications for fungal nutrient distribution and mineralisation. Bottom-up effects also determine the survival, population growth and behaviour of invertebrates. These top-down and bottom-up processes are intrinsically linked and interact to determine decomposer communities and their functioning.
Introduction
Saprotrophic basidiomycetes are among the primary regulators of belowground nutrient dynamics in temperate woodland ecosystems. They are one of a few groups of organisms capable of producing the lignocellulytic enzymes to access the extensive carbon and nutrient stores within dead wood and other recalcitrant plant material (Baldrian and Valášková Citation2008). Following resource acquisition, nutrients are conserved and re-distributed throughout extensive hyphal systems, which, in the case of non-unit-restricted species, grow through the upper soil and litter horizons (Boddy Citation1999). Some species are capable of producing cords (aggregations of parallel hyphae), which can extend tens of metres across the soil-litter interface (Cairney Citation2005). The potential of these cord-forming basidiomycetes to translocate carbon, nitrogen and phosphorous throughout their cords means that they are, to some extent, responsible for the distribution of nutrients within these woodland ecosystems.
Cord formation and extension beyond the woody resources inevitably leads to encounters with soil-dwelling fauna. Soil invertebrate biomass can often exceed 0.5 tonnes ha−1 (Killham Citation1994) and their diversity has led to soil ecosystems being termed ‘the poor man's tropical rainforest’ (Usher et al. Citation1979). These invertebrates contribute extensively to the functional diversity within woodland soils but, in terms of nutrient cycling and litter decomposition, the most important species include the nematodes, arthropods (including collembola, mites, diplopods and isopods), oligochaetes (earthworms and enchytraeid worms) and molluscs (slugs and snails) (Boddy and Jones Citation2008). Most of these ‘decomposer invertebrates’ feed directly or indirectly on fungi (Pollierer et al. Citation2009) and possess chitinases for degrading fungal cell walls (Berg et al. Citation2004).
The importance of the role of cord-forming basidiomycetes and mycophagous soil invertebrates in the functioning of woodland ecosystem has, over the past decade, been recognised and emphasised (Hattenschwiler et al. 2005; ). Generally, these studies () have included a variety of mycophagous invertebrate taxa (including micro-, meso- and macrofauna) and their effects on common basidiomycete species (including Hypholoma fasciculare, Phanerochaete velutina, Phallus impudicus and Resinicium bicolor) in temperate woodland soil. Top-down ‘grazer’ control can have direct consequences for fungal growth, physiology and community composition, while bottom-up factors (i.e. fungal resource availability) can affect invertebrate population growth and survivorship. This review considers the relative importance of these top-down and bottom-up effects, and highlights the functional significance of these fungus-invertebrate interactions for woodland ecosystem functioning.
Table 1. Mean percentage effects of different invertebrate grazers on hyphal coverage, extension rate and wood decay rate of cord–forming basidiomycete fungi compared to un-grazed controls growing in 2-D soil microcosms
Top-down effects
Mycelial growth and foraging
The most direct effects of invertebrate grazing are associated with the ingestion of hyphae and the limitation of mycelial growth across the soil. By severing cords and removing nutritious hyphal tips, invertebrates including collembola, oribatid mites, diplopods and isopods have the potential to reduce, or even prevent, cord extension throughout the soil (; e.g. Kampichler et al. Citation2004; Tordoff et al. Citation2006; A'Bear et al. Citation2010; Crowther et al. Citation2011a). This can limit the potential of cord-formers to forage for, and encounter, new litter resources. These effects vary between invertebrate communities, with different species and population densities exerting different grazing pressures on foraging systems. Crowther et al. (Citation2011a) showed that while micro- and mesofauna reduced hyphal coverage by ingesting fine hyphae, macrofauna exerted the strongest grazing pressures by severing thick cords and limiting extension rates. Other studies (e.g. Tordoff et al. Citation2006, 2008) have found stronger effects of mesofauna on cord extension but the species-specific nature of grazing effects suggest that invertebrate diversity is an important factor determining the growth and foraging potentials of basidiomycete cords in woodland soil.
The highly coordinated nature of fungal networks enables some species to exhibit morphological responses following disturbance, which can limit the negative effects of grazing. A switch from slow, exploitative foraging, to fast, explorative growth has been recorded in various fungal species during collembola grazing (Tordoff et al. Citation2006). This has been interpreted as a fugitive response, promoting growth away from disturbed areas. Hedlund et al. (Citation1991) recorded similar increased mycelial extension and hyphal fanning in un-grazed regions of mycelia of the zygomycete Mortierella isabellina, suggesting the presence of an ‘unknown factor’ that enabled signalling across mycelial systems. The same study also reported aerial growth of hyphae during collembola activity, which was also interpreted as a fugitive response to limit contact with grazers.
Increased mycelial growth in all directions following grazing has also been interpreted as a compensatory growth response, similar to that recorded in plants during herbivory (Boddy and Jones Citation2008). This has been recorded in numerous basidiomycete species following collembola and nematode grazing (Bretherton et al. Citation2006; Crowther et al. Citation2011b). Rather than limiting contact with grazers, the increased mycelial growth and branching enables fungi to obtain more nutrients from the soil and compensate for the negative effects of grazing (Crowther et al. Citation2011c). Fungal compensatory responses are invertebrate density-dependent. Low intensity grazing regularly stimulates mycelial growth, but beyond a certain density threshold, grazing effects overcome mycelial responses (Hedlund et al. Citation1991; Bretherton et al. Citation2006). Following removal of grazing pressure, however, it is high-intensity grazing events which induce the strongest growth responses; hyphal coverage of P. velutina mycelia following invertebrate removal by 80 collembola was greater than that following lower intensity (20 and 40 collembola) grazing (Bretherton et al. Citation2006). The variation in grazing pressures exerted by different invertebrate species (Crowther et al. Citation2011a,b) highlights that, in nature, grazer species composition also plays an important role in determining the morphological responses and, therefore, mycelial distribution of fungi within soil.
Fungal susceptibility to grazing varies depending on mycelial age and stage of development. During emergence from resources, and prior to cord formation, fine hyphae are at their most vulnerable and grazing can prevent cord development altogether (Tordoff et al. Citation2008; Crowther et al. Citation2011b). This is a particularly important phase in fungal development because it is not until the formation of cords that non-resource-unit-restricted basidiomycetes can persist and forage in woodland soil. Compensatory growth responses enable some fungi to exploit periods of low grazing intensity, increasing extension rates into uncolonised soil following invertebrate removal or death (Crowther et al. Citation2011b). Following emergence, the acquisition of fresh new litter resources leads to the formation of highly sclerotised interconnecting cords (Wood et al. Citation2006). Increased rind thickness and accumulation of toxic secondary metabolites are likely to make these cords less palatable than younger hyphae (Anderson and Healey Citation1972; Wiggins et al. Citation1979). Effects of collembola on these large networks are, therefore, limited in comparison to those on smaller, single-resource systems (Wood et al. Citation2006).
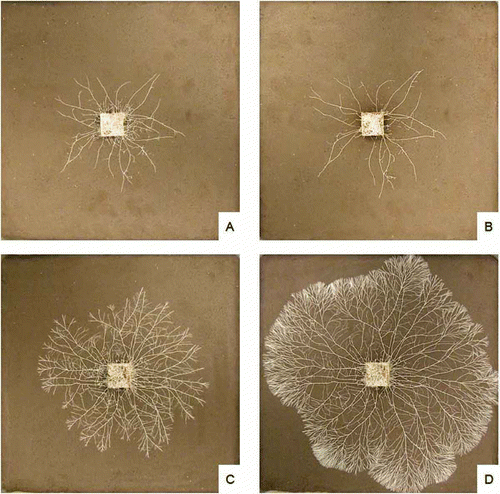
The size and quality of litter resources can also determine fungal susceptibility to grazers. Fungi extending from large and nutrient-rich wood blocks commonly produce dense, luxurious mycelial systems which are less susceptible to grazing than those extending from smaller resources (Harold et al. Citation2005). It is likely that enhanced mycelial networking increases the resilience of these larger systems; the formation of cross-links, connecting radial cords can limit the destructive effects of grazing collembola (; Rotheray et al. Citation2009; Boddy et al. Citation2010). It currently remains unclear whether this networking will limit the effects of micro- and macrofauna, but the increased potential of highly interconnected systems to relocate nutrients and respond to disturbance (Bebber et al. Citation2007) highlights the importance of resource nutrient status in determining the effects of grazers on fungal growth in soil.
Figure 1. Digital images of Phallus impudicus growing from 2 × 2 × 1 cm wood blocks across 2D soil microcosms. Images show a poorly interconnected system after 1 (A) and 10 (B) days of collembola (Folsomia candida) grazing and a highly interconnected network after 1 (C) and 10 (D) days of grazing. Interconnected systems were less affected by grazing.
Mycelial physiology
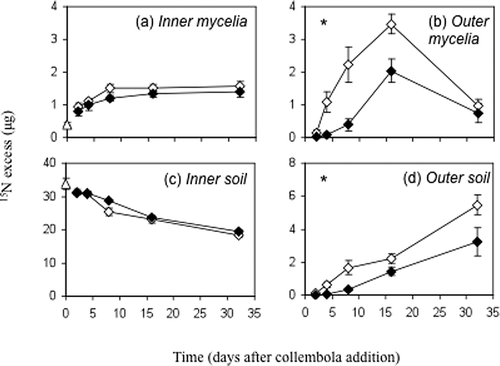
By altering mycelial growth and morphology, grazers directly affect nutrient distribution in soil, but invertebrates can also modify the allocation of nutrients within cord systems. Grazing by collembola, Protaphorura armata, for example, disrupted the translocation of 15N throughout cords of the basidiomycete P. velutina (Tordoff et al. Citation2011), reducing the amount of N detected in the growing hyphal tips and the ‘outer-zone’ soil beneath them (). Earthworm activity has also been shown to disrupt the distribution of carbon within mycelial systems (Butenschoen et al. Citation2007). The potential of cords to extend tens of metres across the soil–litter interface suggests that these localised grazing events can influence nutrient dynamics in un-grazed regions of soil.
Figure 2. 15N excess (mean ± SE) of inner (a) and outer (b) zone mycelia, and inner (c) and outer (d) zone soil across the 32-day study period. Treatments are ungrazed (◊) and grazed (⧫) systems. 15N excess values at t 0 (▵) are immediately before grazing commenced. Significant time × grazing treatment interactions (RM ANOVA) are indicated; *p ≤ 0.05. Note different y-axis scales. Figure modified from Tordoff et al. (Citation2011).
Physiological responses of fungi to grazing can also influence the breakdown and mineralisation of nutrients in woodland soil. Fungal enzyme production is responsible for the deconstruction of complex organic matter in the soil and litter horizons. Crowther et al. (Citation2011c) found that grazing generally increased the activity of enzymes associated with the cycling of carbon, phosphorous, nitrogen and sulphur in soil colonised by four different cord-forming basidiomycetes. Enzymes responsible for the breakdown of cellulose (cellobiohydrolase and β-glucosidase), chitin (N-acetylglucosaminidase) and phosphorus-containing compounds (acid phosphatase and phosphodiesterase) were most frequently affected, but responses varied dramatically between fungi. While the activity of the unpalatable fungus, H. fasciculare, was stimulated by grazing, enzyme production by the palatable species, R. bicolor, was always reduced due to the removal of functioning hyphae (Crowther et al. Citation2011c). Differential responses to nematode, Panagrellus redivivus, activity were also recorded in the basidiomycetes, Stereum hirsutum and P. velutina; the former increased protease and acid phosphatase production, while production of esterase and acid phosphatase were reduced in the latter (Dyer et al. Citation1992). These enzymatic responses have implications for soil nutrient availability but also influence fungal-mediated wood decomposition. Decay rates of basidiomycete-colonised wood blocks have been shown to increase (; e.g. Crowther et al. Citation2011a), and decrease (e.g. Tordoff et al. Citation2008) during extra-resource mycelial grazing, depending on fungal susceptibility to grazing and stage of development.
Fungal community composition
The variation in fungal susceptibility and responsiveness to grazing suggests that invertebrates can exert selective pressures on fungal community composition. Newell et al. (Citation1984a,b) highlighted the potential of collembola to influence the composition of basidiomycete communities in litter; selective grazing on the palatable fungus, Marasmius androsaceus, increased the relative abundance of a less palatable species, Mycena galopus, in Sitka spruce (Picea sitchensis) litter. The potential for collembola to influence competitive interactions between basidiomycetes was supported by Rotheray et al. (Citation2011) who showed that grazing can stimulate the rate at which P. velutina replaced H. fasciculare in soil. Although both these studies highlighted the mechanisms by which collembola can influence the development of competitive interactions, neither study showed that mesofaunal grazing can lead to the complete replacement of the formerly dominant competitor.
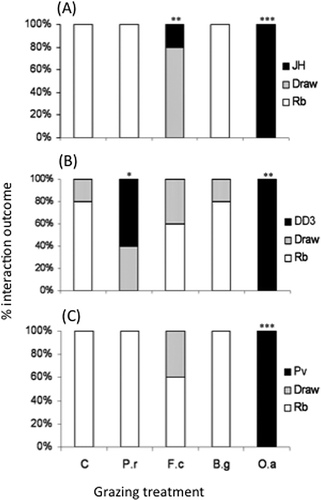
Larger, more destructive invertebrates have since been shown to exert stronger grazing pressures on microbial communities (Crowther and A'Bear Citation2011). By selectively removing entire systems of the dominant fungus, R. bicolor, woodlice prevented the competitive exclusion of all competing fungi, completely reversing the outcomes of combative interactions with two H. fasciculare strains and P. velutina in soil and wood (Crowther et al. Citation2011d). Microfauna also have the potential to alter the balance of these competitive interactions, but by an opposing mechanism. Low-intensity nematode grazing stimulated growth of the less competitive fungus, enabling it to overgrow and replace dominant species (). Species-specific fungal enzyme production and respiration rates highlight that these grazer-induced changes in fungal species composition will have direct consequences for litter decomposition and the flux of CO2 from the soil (Newell Citation1984b; Crowther et al. Citation2011d). The varying effects of different invertebrate species and functional groups suggest that grazer community composition may play an important role in regulating these processes in woodland ecosystems.
Figure 3. Outcomes of competitive fungal interactions with Resinicium bicolor (Rb) against Hypholoma fasciculare JH (Hf JH), Hypholoma fasciculare DD3 (Hf DD3) and Phanerochaete velutina (Pv) in soil during control (C), Folsomia candida (Fc), Oniscus asellus (Oa), Blaniulus guttulatus (Bg) and Panagrellus redivivus (Pr) grazing treatments. “Draw” indicates that neither fungus replaced its opponent. Stars indicate significant differences (logistic regression) compared to un-grazed controls (***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05). Figure modified from Crowther et al. (Citation2011d).
Bottom-up effects
Population growth and survival
As highly concentrated nutrient sources, cord-forming basidiomycetes can directly influence grazer population growth. Collembola, oribatid mite, enchytraeid and isopod population numbers were, for example, greater while feeding on the palatable fungus, R. bicolor, than those in unsterilized soil (Crowther et al. Citation2011a,b). Cord formation is, however, an energetically expensive process and damage to these nutrient channels can be extremely costly to fungal functioning and survival (see above). Many basidiomycetes produce toxins and anti-feedants to deter or kill predators. Sesquiterpenes, secondary metabolites synthesised in fungal cell walls, are produced by P. velutina, H. fasciculare and P. impudicus (Hynes et al. Citation2007), and are used in the defence against fungivores (Ladygina et al. Citation2006; Kempken and Rohlfs Citation2010). Population numbers of most invertebrate species were lower while feeding on these fungi, than in uncolonised soil; only specialist invertebrate species survived whilst grazing these toxic fungi (Crowther et al. Citation2011a). Calcium oxalate crystals are also deposited on the surface of cords of wood decay fungi, and are likely to deter invertebrates (Rotheray et al. Citation2009). These chemical and physical defences can have direct consequences for invertebrate population dynamics (Crowther et al. Citation2011a, b).
Behaviour
Variation in fungal nutrient quality, structure and chemical defences determine invertebrate feeding preferences. Collembola, isopods and diplopods all show preferences for specific cord-forming systems (Crowther et al. Citation2011d) and these appear to relate to invertebrate survival and population growth; populations increase when feeding on preferred fungi (Tordoff et al. Citation2008; Crowther et al. Citation2011a). It is likely that the detection of volatile organic compounds enables these invertebrates to identify and move towards their selected resources (Rotheray et al. Citation2009). These preferences are what enable invertebrates to exert selective pressures on fungal communities. Most invertebrates are thought to show preferences for similar fungi (particularly nutritious or palatable species; Maraun et al. Citation2003). Recent work, however, suggests that some species specialise in feeding on resources that are toxic to most other grazers (Crowther et al. Citation2011d). Nematode (Panagrellus redivivus) populations, for example, increased in number whilst grazing P. impudicus, a toxic fungus that is generally avoided by other invertebrate species (Crowther et al. Citation2011a). Their potential to form clumps (where individuals in the centre are protected from toxic environments by those on the edge) may, potentially, enable nematodes to exploit the fungal resource, which is not available to other grazers. This resource partitioning may be an important factor contributing to the huge abundance and diversity of decomposer invertebrates in temperate woodland soils. It may also highlight that factors which alter soil invertebrate community composition will also drive changes in soil fungal communities as a result of altered selective grazing pressures.
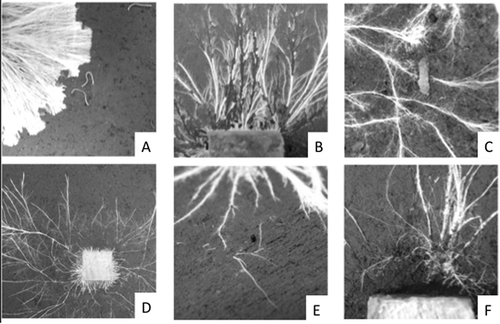
The feeding preferences of soil invertebrates are not constant, but change depending on the physiological state of the available fungal resources. When grown separately, P. velutina is preferred to H. fasciculare by collembola, Folsomia candida (Crowther et al. Citation2011d). During interactions between the two fungal species, however, the damaged hyphal tips of H. fasciculare become more palatable to the grazers (Rotheray et al. Citation2011). Via this process (selectively grazing the less competitive fungal species), collembola can stimulate progression rates of competitive interactions. Most invertebrates also show preferences for specific regions within individual mycelial systems. Collembola (F. candida) and millipedes (Blanniulus guttulatus), for example, feed selectively on the tips of extending basidiomycete cords, while oribatid mites (Euzetes globulus) and woodlice (Oniscus asellus) prefer to graze within mycelial systems, severing, interconnecting cords (). Such fine scale niche partitioning could suggest that different invertebrates have complimentary roles in regulating fungal growth and development.
Figure 4. Digital images showing grazing styles of millipede, Blanniulus guttulatus, on Hypholoma fasciculare (A), woodlouse, Porcellio scaber, on Resinicium bicolor (B), Panagrellus redivivus on Phanerochaete velutina (C), Folsomia candida (D), Euzetes globulus (E) and Enchytraeus crypticus on R. bicolor (F). Figure modified from Crowther et al. (Citation2011a).
Conclusions
Fungus–invertebrate interactions determine the growth, population dynamics and community composition of both fungal and invertebrate components of the soil-decomposer subsystem with direct implications for woodland nutrient dynamics. These effects are also interactive and it is likely that there will be a number of feedbacks which affect soil communities in the long term. The grazing of nutritious fungi can increase invertebrate population growth, but biochemical responses of fungi to high intensity grazing (e.g. calcium oxalate crystal production) are likely to limit grazing with knock on effects for fungal communities and nutrient cycling. It is likely that the top-down and bottom-up processes are intrinsically linked, but controlled by different components of the decomposer community. Macrofauna exert stronger grazing pressures on mycelial systems than micro- and mesofauna (Crowther and A'Bear Citation2012). These larger invertebrates exert the top-down selective pressures that determine fungal community compositions (Crowther et al. Citation2011d). It is, however, the changes in fungal community composition and diversity, that can result from macrofauna grazing, that dictate the abundance and population dynamics of the smaller, more responsive, micro- and mesofauna communities in soil (Jones et al. Citation1998; TW Crowther et al. unpublished data). Exploring the effects of these interactive top-down and bottom-up effects in combination may provide valuable insights into the processes controlling belowground communities and woodland ecosystem functioning.
Global climate change is predicted to drive shifts in the soil fungal and invertebrate communities (Jones et al. Citation1998; Wolters et al. Citation2000; He et al. Citation2010). Grazing interactions are likely to influence, and respond to, these changes in the soil communities. Elevated temperature, for example, has been shown to stimulate collembola (F. candida) population growth, leading to increased top-down determination of basidiomycete community composition (TW Crowther et al. unpublished data). Collembola, via this process, also reversed the effects of warming alone, restoring fungal communities to those recorded at ambient temperature. In this case, grazing mitigated the effects of elevated temperature on fungal communities, but it is possible that, in different fungal communities, the effects of climate could be amplified by changes in the invertebrate community. Interactive effects of biotic and abiotic factors are likely to be key in determining decomposer community composition, and both should be considered in combination in the future when exploring the effects of climate change on belowground ecosystem functioning.
Acknowledgements
The work was funded by the Natural Environment Research Council (NE/G523420/1).
References
- A'Bear , AD , Boddy , L , Raspotnig , G and Jones , TH. 2010 . Non-trophic effects of oribatid mites on cord-forming basidiomycetes in soil microcosms . Ecol Entomol. , 35 : 477 – 484 .
- Anderson , JM and Healey , IN. 1972 . Seasonal and interspecific variation in major components of the gut contents of some woodland Collembola . J Anim Ecol. , 41 : 359 – 368 .
- Baldrian , P and Valášková , V. 2008 . Degradation of cellulose by basidiomycetous fungi . FEMS Microbiol Rev. , 32 : 501 – 521 .
- Bebber , DP , Hynes , J , Darrah , PR , Boddy , L and Fricker , MD. 2007 . Biological solutions to transport network design . Proc R Soc B. , 274 : 2307 – 2307 .
- Berg , MP , Stoffer , M and van der Heuvel , HH. 2004 . Feeding guilds in Collembola based on digestive enzymes . Pedobiologia , 48 : 589 – 601 .
- Boddy , L. 1999 . Saprotrophic chord-forming fungi: meeting the challenge of heterogeneous environments . Mycologia , 91 : 13 – 32 .
- Boddy , L and Jones , TH. 2008 . “ Interactions between Basidiomycota and Invertebrates ” . In Ecology of Saprotrophic Basidiomycetes , Edited by: Boddy , L , Frankland , JC and van West , P . 155 – 179 . San Diego : Academic Press .
- Boddy , L , Wood , J , Redman , E , Hynes , J. and Fricker , MD. 2010 . Fungal network responses to grazing . Fung Gen Biol. , 47 : 522 – 530 .
- Bretherton , S , Tordoff , GM , Jones , TH and Boddy , L. 2006 . Compensatory growth of Phanerochaete velutina mycelial systems grazed by Folsomia candida (Collembola) . Microb Ecol. , 58 : 33 – 40 .
- Butenschoen , O , Poll , C , Langel , R , Kandeler , E , Marhan , S and Scheu , S. 2007 . Endogeic earthworms alter carbon translocation by fungi at the soil-litter interface . Soil Biol Biochem. , 39 : 2854 – 2864 .
- Cairney , JWG. 2005 . Basidiomycetes mycelia in forest soils: dimensions, dynamics and roles in nutrient distribution . Mycol Res. , 109 : 7 – 20 .
- Crowther , TW and A’ Bear , AD. 2012 . Impacts of grazing soil fauna on decomposer fungi are species-specific and density-dependent . Fung Ecol. , doi: 10.1016/j.funeco.2011.07.006
- Crowther , TW , Boddy , L and Jones , TH. 2011a . Species-specific effects of soil fauna on fungal foraging and decomposition . Oecologia , 167 : 535 – 545 .
- Crowther , TW , Jones , TH and Boddy , L. 2011b . Species-specific effects of grazing invertebrates on mycelial emergence and growth from woody resources into soil . Fung Ecol. , 5 : 333 – 341 .
- Crowther , TW , Jones , TH , Boddy , L and Baldrian , P. 2011c . Invertebrate grazing determines enzyme production by basidiomycete fungi . Soil Biol Biochem. , 43 : 2060 – 2068 .
- Crowther , TW , Boddy , L and Jones , TH. 2011d . Outcomes of fungal interaction are determined by soil invertebrate grazers . Ecol Lett. , 14 : 1125 – 1133 .
- Dyer , HC , Boddy , L and Preston-Meek , CM. 1992 . Effect of the nematode Panagrellus redivivus on growth and enzyme production by Phanerochaete velutina and Stereum hirsutum . Mycol Res. , 96 : 1019 – 1028 .
- Harold , S , Toroff , GM , Jones , TH and Boddy , L. 2005 . Mycelial responses of Hypholoma fasciculare to collembola grazing: effect of inoculum age, nutrient status and resource quality . Mycol Res. , 109 : 927 – 935 .
- He , Z , Xu , M , Deng , Y , Kang , S , Kellogg , L , Wu , L , Van Nostrand , JD , Hobbie , SE , Reich , PB and Zhou , J. 2010 . Metagenomic analysis reveals a marked divergence in the structure of belowground microbial communities at elevated CO2 . Ecol Lett. , 13 : 564 – 575 .
- Hedlund , K , Boddy , L and Preston , CM. 1991 . Mycelial responses of the soil fungus Mortierella isabellina to grazing by Onychiurus armatus (Collembola) . Soil Biol Biochem. , 36 : 591 – 599 .
- Hynes , J , Muller , CT , Jones , TH and Boddy , L. 2007 . Changes in volatile production during the course of fungal mycelial interactions between Hypholoma fasciculare and Resinicium bicolor . J Chem Ecol. , 33 : 43 – 57 .
- Jones , TH , Thompson , LJ , Lawton , JH , Bezemer , TM , Bardgett , RD , Blackburn , TM , Bruce , KD , Cannon , PF , Hall , GS , Hartley , SE , Howson , G , Jones , CG , Kampichler , C , Kandeler , E and Ritchie , DA. 1998 . Impacts of rising atmospheric carbon dioxide on model terrestrial ecosystems . Science , 280 : 441 – 443 .
- Kampichler , C , Rolschewski , L , Donnelly , DP and Boddy , L. 2004 . Collembolan grazing affects the growth strategy of chord-forming fungus Hypholoma fasciculare . Soil Biol Biochem. , 36 : 591 – 599 .
- Kempken , F. and Rohlfs , M. 2010 . Fungal secondary metabolite biosysthesis – a chemical defence strategy against antagonistic animals? . Fung Ecol. , 3 : 1 – 8 .
- Killham , K. 1994 . Soil Ecology , Cambridge University Press .
- Ladygina , N , Dedyukhina , EG and Vainshtein , MB. 2006 . A review on microbial synthesis of hydrocarbons . Process Biochem. , 41 : 1001 – 1014 .
- Maraun , M , Martens , H , Migge , S , Theenhaus , A and Scheu , S. 2003 . Adding to ‘the enigma of soil animal diversity’: Fungal feeders and saprotrophic soil invertebrates prefer similar food substrates . Eur J Soil Biol. , 39 : 85 – 95 .
- Newell , K. 1984a . Interaction between two decomposer Basidiomycetes and a collembolan under Sitka spruce: grazing and its potential effects on fungal distribution and litter decomposition . Soil Biol Biochem. , 16 : 235 – 239 .
- Newell , K. 1984b . Interaction between two decomposer Basidiomycetes and a collembolan under Sitka spruce: distribution, abundance and selective grazing . Soil Biol Biochem. , 16 : 223 – 233 .
- Pollierer , MM , Langel , R , Scheu , S and Maraun , M. 2009 . Compartmentalization of the soil animal food web as indicated by dual analysis of stable isotope ratios (15N/14N and 13C/12C) . Soil Biol Biochem. , 41 : 1221 – 1226 .
- Rotheray , TD , Boddy , L and Jones , TH. 2009 . Collembola foraging responses to interacting fungi . Ecol Entomol. , 34 : 125 – 132 .
- Rotheray , TD , Chancellor , M , Jones , TH and Boddy , L. 2011 . Grazing by collembola affects the outcome of interspecific mycelial interactions of cord-forming basidiomycetes . Fung Ecol. , 4 : 1 – 14 .
- Tordoff , GM , Boddy , L and Jones , TH. 2006 . Grazing by Folsomia candida (collembola) differently affects mycelial morphology of the chord-forming basidiomycetes Hypholoma fasciculare, Phanerochaete velutina, and Resinicium bicolor . Mycol Res. , 110 : 335 – 345 .
- Tordoff , GM , Boddy , L and Jones , TH. 2008 . Species-specific impacts of collembola grazing on fungal foraging ecology . Soil Biol Biochem. , 40 : 434 – 442 .
- Tordoff , GM , Chamberlain , PM , Crowther , TW , Black , HIJ , Jones , TH , Stott , A and Boddy , L. 2011 . Nitrogen partitioning in the mycelia of Phanerochaete velutina: an investigation into the influence of collembola grazing using stable isotopes . Soil Biol Biochem , doi: 10.1016/j.soilbio.2011.07.005
- Usher , MB , Davis , P , Harris , J and Longstaff , B. 1979 . “ A profusion of species? Approaches towards understanding the dynamics of the populations of microarthropods in decomposer communities ” . In Population dynamics , Edited by: Anderson , RM , Turner , BD and Taylor , LR . 359 – 384 . Oxford : Blackwell .
- Wiggins , EA , Curl , EA and Harper , JD. 1979 . Effects of soil fertility and cotton rhizosphere on populations of collembola . Pedobiologia , 19 : 77 – 82 .
- Wood , J , Tordoff , GM , Jones , TH and Boddy , L. 2006 . Reorganization of mycelial networks of Phanerochaete velutina in response to new woody resources and collembola (Folsomia candia) grazing . Mycol Res. , 110 : 985 – 993 .
- Wolters , V , Silver , WL , Bignell , DE , Coleman , DC , Lavelle , P , Van Der Putten , WH , De Ruiter , P , Rusek , J , Wall , DH , Wardle , DA , Brussard , L , Dangerfield , JM , Brown , VK , Giller , KE , Hooper , DU , Tiedje , J , Sala , O and Van Veen , JA. 2000 . Effects of global changes on above- and belowground biodiversity in terrestrial ecosystems: implications for ecosystem functioning . Bioscience , 50 : 1089 – 1098 .