ABSTRACT
Fomitopsis officinalis, also known as Laricifomes officinalis, is a medicinal polypore used for millennia (Agarikon) to contrast several diseases, particularly the pulmonary ones. A rich literature has dealt with its ethno-mycological aspects, but isolation and chemical characterisation of single compounds has only recently significantly developed, as well as in vitro tests for bioactivity. According to several reports there is evidence of a broad-spectrum antibacterial and antiviral activity by F. officinalis, including pathogens like Mycobacterium tuberculosis, Yersinia pseudotuberculosis and Staphylococcus aureus, as well as Ortopox virus. Chlorinated coumarins from mycelia and lanostane triterpenoids from basidiomes have been demonstrated to be directly responsible for antiviral-antibacterial and trypanocidal activity, respectively. A wider literature deals instead with crude extracts including an undetermined mixture of metabolites, whose efficacy in vitro is yet far from being standardised as extraction and treatment methodology are highly variable. Nevertheless, in vivo tests on bees provided promising results in order to develop sustainable solutions against the pathogens responsible for colony collapse disorders. Despite increasing attention has been paid to other medicinal aspects of this polypore, such as immune-tropic or antitumor, this review rationally reports and critically analyses the available knowledge by focusing on aspects of antimicrobial properties.
1. Introduction
Plants and fungi represent a major source of bioactive molecules showing antimicrobial properties. At least a part of such metabolites evolved as a response against a broad spectrum of microbial and nonmicrobial antagonists and this makes them particularly interesting also when dealing with pathogenic targets of human and animal concern (Wasser Citation2014; Mithöfer and Boland Citation2012; Gargano et al. Citation2017).
From a scientific point of view, species that have been reported as effective by traditional medicine when separately administered represent a more promising topic than species adopted in multi-ingredient formulates, where the single contributes are hardly distinguishable.
Fomitopsis officinalis (Batsch) (Bondartsev & Singer) is a polyporoid fungus recognised as medicinal for millennia all over its distribution range, particularly against pulmonary diseases. The first written report about the so-called Agarikon is by the protopharmacologist Dioscorides Pedanios (I century AD) but the use of this unmistakable species has thereafter been documented on Alps, Central and Eastern Europe, Urals, Siberia and North America, where it sometimes acquired mythic and ritual significance (Blanchette et al. Citation1992).
Far beyond the folk superstition, bioactive molecules have been recently isolated from this fungus and proved to show remarkable antimicrobial effect (Grienke et al. Citation2014; Stamets Citation2018).
This species is also known by the name of Laricifomes officinalis (Batsch) (Kotl. & Pouzar), that has been proposed to replace F. officinalis since molecular data suggest a separate systematic position from Fomitopsidaceae (Han et al. Citation2016b).
Showing a holartic distribution, F. officinalis is a wood-decay species developing its basidiomata on Pinaceae, where it causes heart cubic brown rot as a slow-growing necrotrophic parasite. Interestingly, several conifers are reported as host in North America, whereas in Eurasia F. officinalis almost exclusively occurs on Larix spp. Basidiomata are perennial and forward their growth for several years, sometimes reaching a considerable size. Apart from the morphological features of fully developed specimens, F. officinalis is immediately distinguished from other species by the chalky consistence, flavour and smell even in primordial stage (Bernicchia Citation2005; Ryvarden and Melo Citation2014).
Thus, due to its easy identification and consolidated use in folk medicine, F. officinalis has gained popularity both at international and local levels. Nevertheless, scientific literature dealing with chemical characterisation of metabolites and in vitro tests on biological properties has only recently developed.
Moreover, only a few purified compounds have been up to now singularly tested, that is the respective contribute of each compound to bioactivity has been poorly investigated.
Aim of the present review is to provide a rational and critical discussion on the available knowledge about the antimicrobial properties of F. officinalis in the light of the secondary metabolites characterisation.
2. Secondary metabolites in Fomitopsis officinalis showing antimicrobial activity
A complete review of secondary metabolites from F. officinalis and other medicinal polypores until 2013 was provided by (Grienke et al. Citation2014); an updated list is reported in . Although a plethora of molecules has been isolated and chemically characterised from the structural point of view, only a minority was tested as it concerns antimicrobial properties.
Table 1. Secondary metabolites isolated from F. officinalis.
2.1. Chlorinated coumarins
Two new chlorinated coumarins have been isolated from mycelia of F. officinalis by (Hwang et al. Citation2013) resembling a substituted derivative of simple coumarin ().
In their simplest form coumarins are heterocyclic compounds basically constituted by a benzene ring and a pyrone one (benzopyrone). A wide range of derivatives including furan rings and pyran rings has been reported from bacteria, fungi and plants. As it regards fungi, coumarins have been up to now isolated from several species both in Ascomycota and Basidiomycota, including polypores (Costa et al. Citation2016); nevertheless, chlorination has been reported from F. officinalis only, despite chlorinated coumarins are known in plants (Guz et al. Citation2001; Patnam et al. Citation2005).
The biological activity of coumarins has been referred to a wide range of pharmacological properties, including antiviral, antibacterial, antifungal, antiparasitic and antielmintic. Chlorination and bromination (halogen substituents on an aromatic ring) are generally regarded as factors increasing antimicrobial activity as a whole (Basanagouda et al. Citation2009; Asif Citation2015; Al-Majedy et al. Citation2017).
As it namely regards F. officinalis, (Hwang et al. Citation2013) tested both the two naturally occurring coumarins (showing Cl in position 6) and two other synthesised analogues (showing Cl in position 7) (). The molecules were tested versus a broad panel of bacteria (Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Enterococcus faecalis, Pseudomonas aeruginosa), including several species in genus Mycobacterium (M. smegmatis, M. chelonae, M. abscessus, M. marinum, M. kansasii, M. avium, M. bovis and M. tuberculosis) as well as the yeast Candida albicans. Quaintly, only a narrow spectrum of significant antibacterial activity was observed, with lower MICs for Mycobacterium tuberculosis. Structural variations containing an ethyl ester reported higher anti-TB activity when Cl was in position 6 (naturally occurring) instead of 7 (synthesised). On the other hand, lowest MICs were observed in the newly synthetised compound 3 ().
2.2. Lanostane-type triterpenoids (LTR)
Lanostane-type triterpenoids (hereafter LTRs) formally derive from the cyclic terpenoid lanostane (). They have been reported in several plant and fungal species including the basidiomes of F. officinalis (Kim et al. Citation2004; Wu et al. Citation2004; Feng et al. Citation2010; Han et al. 2016a; Isaka et al. Citation2017; Shi et al. Citation2017; Naranmandakh et al. Citation2018) (). As particularly studied in genus Fomitopsis, some LTRs are also referred as fomitopsins ().
Table 2. Fomitopsins isolated from Fomitopsis species.
Figure 2. Naturally occurring coumarins (1–2) and newly synthesised analogues (3–4) from F. officinalis. (1) 6-chloro-4-phenyl-2H-chromen-2-one; (2) ethyl 6-chloro-2-oxo-4-phenyl-2H-chromen-3-carboxylate. From Hwang et al. (Citation2013).
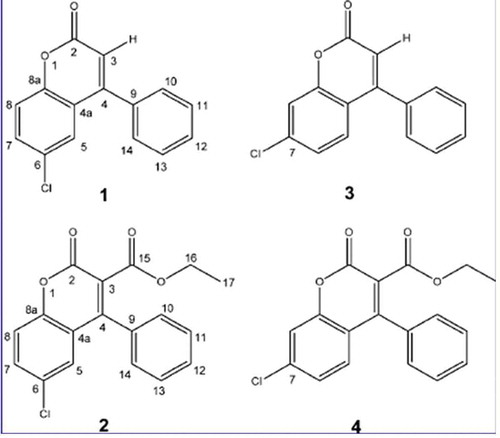
Figure 3. Lanostane or (5S,8R,9S,10R,13R,14S,17R)-4,4,10,13,14-pentamethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,5,6,7,8,9,11,12, 15,16,17-dodecahydro-1H-cyclopenta[a]phenanthrene. From: https://pubchem.ncbi.nlm.nih.gov/compound/Lanostane.
![Figure 3. Lanostane or (5S,8R,9S,10R,13R,14S,17R)-4,4,10,13,14-pentamethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,5,6,7,8,9,11,12, 15,16,17-dodecahydro-1H-cyclopenta[a]phenanthrene. From: https://pubchem.ncbi.nlm.nih.gov/compound/Lanostane.](/cms/asset/f348bdb4-f4e4-44fd-9412-24cd8787848a/tmyc_a_1536680_f0003_oc.jpg)
Figure 4. Lanostane-type triterpenoids isolated from F. officinalis. (1) 3-[2-(carboxyacetyl)oxy]-12-hydroxy24-methyl-lanost-8,24-dien-26,23-lactone named fomitopsin G; (2) 3-[2-(carboxyacetyl)oxy]-18,23-epoxy-12-hydroxy-24- methyl-lanost-8-en-26,23-lactone named fomitopsin H; (3) 18,23-epoxy-3,12,-dihydroxy-24-methyl-lanost-8-en-26,23-lactone named demalonyl fomitopsin H; (4) ethyl ester derivative of fomitopsin D; (5) fomitopsin F; (6) (25S)-(+)-12α-hydroxy-3αmalonyloxy-24-methyllanosta-8,24(31)-dien-26-oic acid; (7) fomeofficinic acid G; (8) 15α-hydroxy-3-oxo-24-methylenelanosta-7,9(11)-dien-21- oic; (9) fomitopsin C; (10) officimalonic acid A; (11) (3R, 12R, 23S)-3-carboxyacetyloxy-12-hydroxy-24-methyl-7-oxo-lanost-8,24-dien-26,23-lactone, named officimalonic acid B; (12) officimalonic acid C; (13) officimalonic acid D; (14) officimalonic acid E; (15) officimalonic acid F; (16) officimalonic acid G; (17) officimalonic acid H. Modified from Quang et al. (Citation2005), Han et al. (2016) and Naranmandakh et al. (Citation2018).
![Figure 4. Lanostane-type triterpenoids isolated from F. officinalis. (1) 3-[2-(carboxyacetyl)oxy]-12-hydroxy24-methyl-lanost-8,24-dien-26,23-lactone named fomitopsin G; (2) 3-[2-(carboxyacetyl)oxy]-18,23-epoxy-12-hydroxy-24- methyl-lanost-8-en-26,23-lactone named fomitopsin H; (3) 18,23-epoxy-3,12,-dihydroxy-24-methyl-lanost-8-en-26,23-lactone named demalonyl fomitopsin H; (4) ethyl ester derivative of fomitopsin D; (5) fomitopsin F; (6) (25S)-(+)-12α-hydroxy-3αmalonyloxy-24-methyllanosta-8,24(31)-dien-26-oic acid; (7) fomeofficinic acid G; (8) 15α-hydroxy-3-oxo-24-methylenelanosta-7,9(11)-dien-21- oic; (9) fomitopsin C; (10) officimalonic acid A; (11) (3R, 12R, 23S)-3-carboxyacetyloxy-12-hydroxy-24-methyl-7-oxo-lanost-8,24-dien-26,23-lactone, named officimalonic acid B; (12) officimalonic acid C; (13) officimalonic acid D; (14) officimalonic acid E; (15) officimalonic acid F; (16) officimalonic acid G; (17) officimalonic acid H. Modified from Quang et al. (Citation2005), Han et al. (2016) and Naranmandakh et al. (Citation2018).](/cms/asset/166080e7-d9a6-46f6-9376-5ab53e78d8db/tmyc_a_1536680_f0004_b.gif)
Lanostane triterpenoids have been mostly investigated as it regards cytotoxicity and antitumor properties (Wu et al. Citation2013; Zhang et al. Citation2014), nevertheless antimicrobial activity has been reported too. Namely, fomitopsin D showed activity against herpes simplex virus type 1 (HSV-1) with an IC50 value of 17 μg/mL, whereas fomitopsins E and F resulted in inhibition of Bacillus cereus with MIC values of 6.25 μg/mL (Isaka et al. Citation2017). Moderate trypanocidal activity was reported versus Trypanosoma congolense by a panel of compounds including fomitopsin F (26 μM, IC50) and H (27.1 μM), a demalonyl derivative of fomitopsin H (12.5 μM), an ethyl ester derivative of fomitopsin D (15.0 μM) and especially the compound named 15α-hydroxy-3-oxo-24-methylenelanosta-7,9(11)-dien-21-oic acid (7 μM) (Naranmandakh et al. Citation2018). Trypanocidal activity by fomitopsin G was found negligible by the same authors. Antibacterial activity versus S. aureus has been reported also by lanostane derivatives in F. rosea, despite the same compounds have not been to date recognised in F. officinalis (Popova et al. Citation2009).
3. Antimicrobial properties of crude extracts
As the most bioactive metabolites in fungi show oxygenated groups, they can be easily recovered by ethanol extraction. Apart from pure hot water extraction, high graduation ethanol-water mixtures have been also the base of traditional medical use in several cultures, thus literature dealing with similar extraction methods provide a useful comparison term.
Hleba et al. (Citation2016) tested 1-year-old crude ethanolic extracts from basidiomes of four medicinal species versus Gram-positive (Bacillus thuringiensis, Staphylococcus aureus) and Gram-negative (Klebsiella pneumoniae, Enterobacter aerogenes) bacteria by both MIC and disc diffusion methodology. Results showed that F. officinalis was the only species able to inhibit all the bacterial strains whereas MICs are very high. This is consistent with the previously mentioned (Hwang et al. Citation2013), yet target pathogens are different except for S. aureus.
Despite the attention paid by Russian and Ukrainian authors to F. officinalis, several full-texts are apparently not available to the international scientific community as unrecoverable on search engines. This problem has been partially overcome by the abundant reviewing production in the same Countries (Zaichenko et al. Citation2017). The work by Sidorenko and Buzoleva (Citation2012) has its background in a patent for mycelia-based preparations against pseudotuberculosis thanks to the inhibitory effect towards Yersinia pseudotuberculosis (Sidorenko Citation2009a, Citation2009b). The same authors did not report instead any significant inhibition towards E. coli, Pseudomonas putida, P. fluorescens, S. aureus, Listeria monocytogenes, Salmonella typhimurium. Analogously, still unclear is the action of F. officinalis against the Gram-positive bacteria Bacillus subtilis and B. anthracis. Bacteriolytic effect towards Vibrio sp. has been related instead to the immunotropic activity shown by F. officinalis extracts, that is another topic producing an increasing literature (Yui et al. Citation2009; Kalinkevich et al. Citation2014; Vedenicheva et al. Citation2016).
By using an analogous methodological approach, Mithöfer and Boland (Citation2012) is consistent with Coletto and Striano (Citation2000), who observed inhibition of bacterial strains under examination, belonging to B. cereus, B. subtilis, S. aureus, A. tumefaciens, E. coli, S. typhimurium; weak inhibition of the yeast C. albicans was observed too.
Difference between efficiency of ethanolic and aqueous extracts was observed by Parkash and Sharma (Citation2016) when testing F. officinalis against phytopathogenic microfungi (Alternaria solani, Curvularia lunata, Aspergillus terreus and Fusarium oxysporum) and bacteria (E. coli and B. subtilis). Pure ethanolic extract of F. officinalis was able to completely inhibit the growth of A. solani and A. terreus, whereas C. lunata and F. oxysporum were completely inhibited even when applying 1:4 diluted extract. Yet the same result was obtained when applying aqueous extract, C. lunata quaintly reported highest inhibition when applying 1:4 dilution. As it regards the antibacterial activity, highest inhibition was reached by applying the pure extract for E. coli and 1:4 dilution for B. subtilis. Aqueous extract only reported inhibition towards E. coli by 1:4 dilution. Despite basically consistent with literature on the existence of antimicrobial activity in F. officinalis, results by Parkash and Sharma (Citation2016) are questionable both as concerning the methodology and significance of results themselves. As it regards the preparation of extracts authors in fact report the extremely low ratio of 0.01 g/10 mL between powdered basidiome and distilled water or ethanol (ethanol purity is not specified). Inhibition radius is investigated much below the millimetre after 3 days and the accuracy is therefore minimal.
A broad range of concrete applications relying on antimicrobial properties has been suggested by Stamets in distinct deposited patents exploiting the mycelial stage. Although including a great number of fungal species, the author always remarks the peculiarity of F. officinalis. In (Stamets Citation2011), dealing with “antiviral activity from medicinal mushrooms,” direct antiviral activity in HFF cells is reported by F. officinalis versus Cowpox and Vaccinia virus. Interestingly, the extract is reported to lack of agaric acid, whose bioactivity therefore remains unclear. In (Stamets Citation2014), dealing with “antiviral and antibacterial activity from medicinal mushrooms,” decrease in CFU is shown in E. coli and S. aureus. By relying on the antiviral activity of ethanol/water extracts from mycelia submitted to previous projects, Stamets (Citation2018) deposited a patent concerning “antiviral activity of medicinal mushrooms and their active constituents.” Yet dealing with a plethora of species, the patent text remarks that 1–2% extract from F. officinalis is able to inhibit virus-induced cellular damage by 50% (EC50). 1:106 diluted crude extract was reported as still effective against pathologically relevant virus of influenza A, influenza B and herpes. Selectivity index indicated extremely high activity. Significant inhibition is reported against Mycobacterium tuberculosis as well.
Further on, (Stamets Citation2016) deals with “integrative fungal solutions for protecting bees,” the target being the etiological agents of the colony collapse disorder (CCD). Yet showing not negligible activity in decreasing the total viral burden by 2 weeks, values of F. officinalis are low in comparison to several other polyporoid species under examination. More remarkable are the values reported as it regards the average percent improvement in longevity of bees even at 0.1% extract. As a whole, the LV Index (=the average percent improvement in bee longevity multiplied by the average percent decrease in total viral burden) indicates a competitive species for this purpose.
Antiviral activity against bird influenza (H5N1) and human influenza (H3N2) is also reported by Teplyakova et al. (Citation2012) by applying F. officinalis aqueous extract (1 g basidiome biomass/5 mL water). Neutralisation index (ID50 ctrl-ID50 exp, logarithmic scale), respectively resulted 3.0 and 1.5, that is competitive yet not the highest in the test panel.
4. Conclusion
A rich literature consistently confirms the existence of antimicrobial properties by F. officinalis, namely as antiviral, antibacterial and trypanocidal. Such an activity has been specifically recognised in a few molecules, e.g. coumarines as antibacterial and lanostane triterpenoids as trypanocidal, although most metabolites have not been singularly tested yet. The different range of metabolites in basidiome and mycelium is of major concern in assessing the antimicrobial potential as well as to manage the possible cultivation or harvest.
The relative efficiency of F. officinalis in comparison to other medicinal species requires further investigation. Although few species can display contingently higher values in inhibition, F. officinalis may be more competitive when applied on very specific targets such as M. tuberculosis or Y. pseudotuberculosis.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Airapetova A, Gromovykh T. 2013. Выделение и идентификация агарициновой кислоты из мицелия Fomitopsis officinalis (Vill. Fr.:) Bond. et Sing. [Selection and identification of agaricine acid from micelia of Fomitopsis officinalis (Vill. Fr.:) Bond. et Sin]. Химия Растительного Сырья [Chem Plant Raw Mater]. 2:101–106.
- Airapetova AY, Gavrilin MV, Dmitriev AB, Mezenova TD. 2010. Examination of the structure of agaricinic acid using 1 H and 13 C NMR spectroscopy. Pharm Chem J. 44(9):510–513.
- Al-Majedy YK, Kadhum AAH, Al-Amiery AA, Mohamad AB. 2017. Coumarins: the antimicrobial agents. Sys Rev Pharm. 8(1):62.
- Anderson C, Epstein W. 1971. Metabolic intermediates in the biological oxidation of lanosterol to eburicoic acid. Phytochemistry. 10:2713–2717.
- Anderson CG, Vanlear G, Epstein WW. 1972. Minor triterpenes of Fomes officinalis. Phytochemistry. 11:2847–2852.
- Asif M. 2015. Pharmacologically potentials of different substituted coumarin derivatives. Chem Int. 1(1):1–11.
- Basanagouda M, Kulkarni MV, Sharma D, Gupta VK, Sandhyarani P, Rasal VP. 2009. Synthesis of some new 4-aryloxmethylcoumarins and examination of their antibacterial and antifungal activities. J Chem Sci. 121(4):485–495.
- Bernicchia A. 2005. Polyporaceae sl. Alassio (Savona): Candusso; p. 222–224.
- Blanchette RA, Compton BD, Turner NJ, Gilbertson RL. 1992. Nineteenth century shaman grave guardians are carved Fomitopsis officinalis sporophores. Mycologia. 84(1):119–124.
- Coletto MAB, Striano B. 2000. Antibiotic activity in Basidiomycetes. XIII. Antibiotic activity of mycelia and cultural filtrates. Allionia. 37:253–255.
- Costa TM, Tavares LBB, de Oliveira D. 2016. Fungi as a source of natural coumarins production. Appl Microbiol Biotechnol. 100(15):6571–6584.
- Dioscorides Pedanios. I century A.D. De Materia Medica. Aboca Edizioni (2013)
- Epstein WW, Sweat FW, Van Lear G, Lovell FM, Gabe EJ. 1979. Structure and stereochemistry of officinalic acid, a novel triterpene from Fomes officinalis. J Am Chem Soc. 101:2748–2750.
- Epstein WW, Van Lear G. 1966. Metabolites of Fomes officinalis. J Org Chem. 31:3434–3435.
- Erb B, Borschberg HJ, Arigoni D. 2000. The structure of laricinolic acid and its biomimetic transformation into officinalic acid. J Chem Soc Perkin Trans. 1(15):2307–2309.
- Feng W, Yang J, Xu X, Liu Q. 2010. Quantitative determination of lanostane triterpenes in Fomes officinalis and their fragmentation study by HPLC‐ESI. Phytochem Anal. 21(6):531–538.
- Gargano ML, van Griensven LJ, Isikhuemhen OS, Lindequist U, Venturella G, Wasser SP, Zervakis GI. 2017. Medicinal mushrooms: valuable biological resources of high exploitation potential. Plant Biosyst. 151(3):548–565.
- Grienke U, Zöll M, Peintner U, Rollinger JM. 2014. European medicinal polypores–A modern view on traditional uses. J Ethnopharmacol. 154(3):564–583.
- Guz NR, Lorenz P, Stermitz FR. 2001. New coumarins from Harbouria trachypleura: isolation and synthesis. Tetrahedron Lett. 42(37):6491–6494.
- Han J, Li L, Zhong J, Tohtaton Z, Ren Q, Han L, Huang X, Yuan T. 2016a. Officimalonic acids A−H, lanostane triterpenes from the fruiting bodies of Fomes officinalis. Phytochemistry. 130::193–200.
- Han ML, Chen YY, Shen LL, Song J, Vlasák J, Dai YC, Cui BK. 2016b. Taxonomy and phylogeny of the brown-rot fungi: Fomitopsis and its related genera. Fungal Divers. 80(1):343–373.
- Hleba L, Kompas M, Hutková J, Rajtar M, Petrová J, Cubon J, Kántor A, Kacániová M. 2016. Antimicrobial activity of crude ethanolic extracts from some medicinal mushrooms. J Microbiol Biotechnol Food Sci. 5:60.
- Hwang CH, Jaki BU, Klein LL, Lankin DC, McAlpine JB, Napolitano JG, Fryling NA, Franzblau SG, Cho SH, Stamets PE, et al. 2013. Chlorinated coumarins from the polypore mushroom Fomitopsis officinalis and their activity against Mycobacterium tuberculosis. J Nat Prod. 76(10):1916–1922.
- Isaka M, Chinthanom P, Srichomthong K, Thummarukcharoen T. 2017. Lanostane triterpenoids from fruiting bodies of the bracket fungus Fomitopsis feei. Tetrahedron Lett. 58(18):1758–1761.
- Kalinkevich K, Karandashov VE, Ptitsyn LR. 2014. In vitro study of the anti-inflammatory activity of some medicinal and edible plants growing in Russia. Russ J Bioorgan Chem. 40(7):752–761.
- Kim HJ, Choi EH, Lee IS. 2004. Two lanostane triterpenoids from Abies koreana. Phytochemistry. 65(18):2545–2549.
- Mithöfer A, Boland W. 2012. Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol. 63:431–450.
- Naranmandakh S, Murata T, Odonbayar B, Suganuma K, Batkhuu J, Sasaki K. 2018. Lanostane triterpenoids from Fomitopsis officinalis and their trypanocidal activity. J Nat Med. 72(2):523–529.
- Parkash V, Sharma A. 2016. In vitro efficacy of bracket fungi for their potential antimicrobial activity. J Microbiol Biotechnol Food Sci. 6(2):818.
- Patnam R, Kadali SS, Koumaglo KH, Roy R. 2005. A chlorinated coumarinolignan from the African medicinal plant, Mondia whitei. Phytochemistry. 66(6):683–686.
- Popova M, Trusheva B, Gyosheva M, Tsvetkova I, Bankova V. 2009. Antibacterial triterpenes from the threatened wood-decay fungus Fomitopsis rosea. Fitoterapia. 80(5):263–266.
- Quang DN, Arakawa Y, Hashimoto T, Asakawa Y. 2005. Lanostane triterpenoids from the inedible mushroom Fomitopsis spraguei. Phytochemistry. 66(14):1656–1661.
- Ryvarden L, Melo I. 2014. Poroid fungi of Europe. Oslo: Fungiflora. P.; p. 226–227.
- Shi ZT, Bao HY, Feng S. 2017. Antitumor activity and structure-activity relationship of seven lanostane-type triterpenes from Fomitopsis pinicola and F. officinalis. Zhongguo Zhong Yao Za Zhi= Zhongguo Zhongyao Zazhi [China J Chin Mater Med]. 42(5):915–922. Chinese.
- Sidorenko M 2009a. Strain of basidial fungus Fomitopsis officinalis showing antibacterial activity against bacteria Yersinia pseudotuberculosis. Russia Patent 2375439, 2009 Dec 10. Russian.
- Sidorenko ML. 2009b. Антибактериальные свойства лиственничной губки [White agaric antibacterial properties]. Вестник Красгау [Bulletin of KrasGAU – Krasnodar Agrarian University]. 12:80–85. Russian.
- Sidorenko ML, Buzoleva LS. 2012. Поиск новых видов сырья для получения антибактериальных препаратов [Search for new types of raw materials for antibacterial drugs]. Антибиотики И Химиотерапия [Antibiot Chemiother]. 57(5–6):7–10. Russian.
- Stamets PE 2011. Antiviral activity from medicinal mushrooms. U.S. Patent Application No. 11/728,613.
- Stamets PE. 2014. Antiviral and antibacterial activity from medicinal mushrooms. Washington (DC): U.S. Patent and Trademark Office. U.S. Patent No. 8,765,138.
- Stamets PE. 2016. Integrative fungal solutions for protecting bees. Washington (DC): U.S. Patent and Trademark Office. U.S. Patent No. 9,474,776.
- Stamets PE 2018. Antiviral activity from medicinal mushrooms and their active constituents. U.S. Patent Application No 15/918,082.
- Teplyakova TV, Psurtseva NV, Kosogova TA, Mazurkova NA, Khanin VA, Vlasenko VA. 2012. Antiviral activity of polyporoid mushrooms (higher Basidiomycetes) from Altai Mountains (Russia). Int J Med Mushrooms. 14(1):37–45.
- Vedenicheva NP, Al-Maali GA, Mytropolska NY, Mykhaylova OB, Bisko NA, Kosakivska IV. 2016. Endogenous cytokinins in medicinal Basidiomycetes mycelial biomass. Biotechnol Acta. 9(1):55–63.
- Wasser S. 2014. Medicinal mushroom science: current perspectives, advances, evidences, and challenges. Biomed J. 37(6):345–356.
- Wu GS, Guo JJ, Bao JL, Li XW, Chen XP, Lu JJ, Wang YT. 2013. Anti-cancer properties of triterpenoids isolated from Ganoderma lucidum–a review. Expert Opin Invest Drugs. 22(8):981–992.
- Wu X, Yang JS, Dong YS. 2005. Chemical constituents of Fomes officinalis (Ⅰ). Chin Tradit Herb Drugs. 36:811–814.
- Wu X, Yang JS, Yan M. 2009. Four New Triterpenes from Fungus of Fomes officinalis. Chem Pharm Bull. 57:195–197.
- Wu X, Yang JS, Zhou L, Dong YS. 2004. New lanostane-typetriterpenes from Fomes officinalis. Chem Pharm Bull. 52:1375–1377.
- Yui. et al. 2009. Лекарственные грибы в традиционной китайской медицине и современных биотехнологиях [Medicinal Fungi in Traditional Chinese Medicine and Modern Biotechnology]. Kirov, Russia 320 pp.
- Yui L. et al. 2009. Medicinal fungi in traditional Chinese medicine and modern biotechnology. Kirov (Russia): O-Kratkoye. Russian.
- Zaichenko T, Krupodorova T, Barshteyn V, Dekhtiarenko N. 2017. Антибактеріальні властивості деяких макроміцетів [Antibacterial Properties of Some Macromycetes]. Наукові вісті НТУУ “КПІ” [Scientific news of NTUU “KPI”]. 3:19–28. Russian.
- Zhang W, Men X, Lei P. 2014. Review on anti-tumor effect of triterpene acid compounds. J Cancer Res Ther. 10(5):14.