ABSTRACT
Diabetic rats were daily fed with a high-cholesterol diet containing 1% or 3% freeze-dried whole submerged G. lucidum culture or its mycelia for 5 weeks. Body weight, adipose tissue weight and plasma triglyceride levels were reduced, while high-density lipoprotein-cholesterol levels were elevated in rats fed with G. lucidum powder supplement diets. Notably, G. lucidum supplements downregulated the activities of hepatic acetyl-CoA carboxylase, fatty acid synthase and lipoprotein lipase, but upregulated the activity of hormone-sensitive lipase in the perirenal adipose tissues. Moreover, G. lucidum supplements increased the faecal triglyceride excretion. Therefore, daily supplementation of submerged G. lucidum culture, especially mycelia, can ameliorate dyslipidemia and reduce visceral fat accumulation in diabetic rats fed with a high-fat diet, which is closely related to the modulation of lipid synthesis, metabolism, and excretion.
Introduction
Type 2 diabetes is a chronic metabolic disease with hyperglycaemia and insulin resistance. It is mainly caused by obesity and accounts for 90% of diabetes cases. (Wu et al. Citation2014). The symptoms of type 2 diabetes usually develop slowly. As a result, complications such as cardiovascular disease have often been associated with the diagnosis of diabetes (Grundy et al. Citation1999). Increasing evidence suggests that lipid metabolism and glucose metabolism are equally important in the development of diabetes. Visceral fat is thought to be the culprit in the development of insulin resistance, which plays an important role in the pathogenesis of dyslipidemia in type 2 diabetes (Patel and Abate Citation2013). Dyslipidemia is a well-recognised and modifiable risk factor and should be detected early to prevent cardiovascular diseases (Nelson Citation2013). Type 2 diabetes is associated with abnormal plasma lipids and lipoproteins, including decreased high-density lipoprotein cholesterol (HDL-C), predominant low-density lipoprotein cholesterol (LDL-C), and elevated triglyceride (TG) levels. These dyslipidemia characteristics increase the risk of cardiovascular disease (Nelson Citation2013). Increased liver secretion of very low-density lipoprotein (VLDL) and impaired clearance, leading to its conversion to LDL, are crucial in the pathophysiology of dyslipidemia (Krauss Citation2004). Although proper diet, exercise, and weight management may improve diabetic dyslipidemia and body fat accumulation, pharmacological treatment is still needed (Krauss Citation2004).
Ganoderma lucidum is a well-known medicinal mushroom that has been used as a health supplement (Galor et al. Citation2011). G. lucidum is widely consumed because it has various medicinal properties including antitumor, immune regulation, liver protection, antioxidant, anti-ageing, hypoglycaemic and hypolipidemic effects (Galor et al. Citation2011). In recent decades, G. lucidum has attracted great interest from scientists due to its potential as an alternative adjuvant therapy for diabetes. Both carbohydrate and lipid metabolism are targets of G. lucidum for improving the outcome of diabetes. For example, 26-oxysterols isolated from G. lucidum fruiting bodies showed an inhibitory effect on cholesterol synthesis (Hajjaj et al. Citation2005). Lanostane triterpenes harvested from G. lucidum fruiting bodies suppressed 3T3-L1 adipocytes differentiation (Lee et al. Citation2010). Genetic resources of Mexican G. lucidum showed cholesterol-lowering properties in mice fed a high-cholesterol diet (Meneses et al. Citation2016). The extracts of G. lucidum fruiting bodies, spores, and a proteoglycan PTP1B inhibitor isolated from G. lucidum also showed hypolipidemic activities in diabetic rodents (Wang et al. Citation2012, Citation2015; Bach et al. Citation2018). Moreover, water extracts of mycelia of G. lucidum have been reported to reduce the weight of the body, liver, and adipose tissue of mice fed a high-fat diet (Chang et al. Citation2015). At the same time, the results of clinical studies showed that G. lucidum could improve the occurrence of diabetic dyslipidemia (Chu et al. Citation2012). Therefore, it has been considered as a potential therapeutic candidate for lipogenic diseases.
Ganoderma fungus is composed of fruiting body (the basidiocarp), mycelium and spores (Galor et al. Citation2011). Compared with the cultivation of fruiting bodies and spores, the submerged G. lucidum culture has the advantages of lower production and time cost, less space requirements, easy-to-control culture conditions, and higher yield, purity and regeneration ability. (Galor et al. Citation2011). However, the components and their relative proportions in G. lucidum from basidiocarp cultivation are different from those in submerged culture (Galor et al. Citation2011).
As the effects of submerged G. lucidum culture on blood lipid and visceral fat accumulation are unknown, we used a type 2 diabetic rat model to deepen the understanding of the ameliorating effects of submerged G. lucidum cultures and their mycelia on hyperlipidaemia and visceral fat accumulation. Streptozotocin (STZ)-induced diabetic rats were daily fed with a high-cholesterol diet containing freeze-dried whole submerged G. lucidum cultures or mycelia for 5 weeks. Body weight and adipose tissue weight as well as TG, total cholesterol (TC), HDL-C, LDL-C, and VLDL-C levels were measured. Since dyslipidemia and visceral fat accumulation are involved in lipid synthesis, metabolism and excretion, the activities of key enzymes and faecal excretion of TG and TC were studied to clarify the underlying mechanism behind the beneficial effects of submerged G. lucidum cultures.
Materials and methods
Culture, chemicals and reagents
G. lucidum BCRC 36123 was obtained from Bioresources Collection and Research Centre (Hsinchu, Taiwan). All chemicals and reagents were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) unless otherwise stated. AIN-93 vitamin mixture and AIN-93 mineral mixture were purchased from MP Biomedicals LLC (Santa Ana, CA, USA). The rat insulin enzyme-linked immunosorbent assay kit was purchased from Randox Laboratories, Ltd. (Crumlin, UK), and the glucose detection kit was purchased from Audit Diagnostics (Cork, Ireland).
Submerged culture of G. lucidum and sample preparation
Pieces of mycelium pad (0.5 x 0.5 cm2) of G. lucidum BCRC 36123 grown on the Gano medium (2.4 g glucose, 0.6 g yeast extract in 100 mL deionised water) agar plate at 30°C for 7 days were put into 1 mL frozen preserving medium (10 mL glycerol, 2 g dextran, 2 g trehalose dehydrate in 90 mL deionised water) for storage at −80°C. The frozen pieces of G. lucidum mycelium pad inoculated on Gano medium agar plates were incubated at 30°C for 7 days and two mycelial pieces (1 x 1 cm2) from the culture plate were added into a flask containing 250 mL Gano medium and incubated at 30°C, 110 rpm for 7 days. Based on the method described by Chang et al. (Citation2006), the extracellular polysaccharides and mycelia in cultures were separated and measured. Whole submerged cultures and collected mycelia were freeze-dried and stored at −20°C for further experiments.
Animals and experimental design
Male Sprague Dawley rats (8 weeks old) were purchased from BioLASCO Taiwan Co., Ltd (Taipei, Taiwan). The rats were housed in stainless steel cages with diet and water provided ad libitum in a temperature- and humidity-controlled animal room. All animal experiments were approved by the Institutional Animal Care and Use Committee of the National Taiwan Ocean University, and the rats were handled and euthanised in accordance with its guidelines.
On arrival, rats were fed with normal diet for 1 week and then randomly divided into six groups (n = 8): normal control (NC), diabetic control (DC), and four experimental groups including diabetic rats fed with 1% or 3% freeze-dried whole submerged culture of G. lucidum powder supplement diet (1G or 3G), and diabetic rats fed with 1% or 3% freeze-dried submerged culture of G. lucidum mycelia powder supplement diets (1M or 3M). The rats for diabetic control and experimental groups were subcutaneously injected with nicotinamide (230 mg/kg B.W.) and STZ (65 mg/kg B.W.) for the induction of type 2 diabetes (Masiello et al. Citation1998). One week later, oral glucose tolerance test was performed to confirm the successful induction of diabetes (Figure S1), and the rats were then daily fed with a high-cholesterol diet containing 1G, 3G, 1M, or 3M for 5 weeks. The composition of employed diets is shown in . Body weight and adipose tissue weights were measured, and samples of blood, faeces, liver, and perirenal and epididymal adipose tissues were harvested after sacrifice for further analysis ().
Table 1. Composition of experimental diets
Figure 1. Protocols of type 2 diabetes induction and G. lucidum supplement diets treatment
Determination of triglyceride (TG), total cholesterol (TC), and lipoprotein-cholesterol levels in the plasma
Blood samples were collected at sacrifice for the isolation of plasma by centrifugation (1,570 × g at 4°C for 20 min). Plasma concentrations of TG and TC were measured using enzymatic kits (Randox Laboratories Limited, UK) according to the manufacturer’s instructions.
A modified phosphotungstic acid/MgCI2 precipitation procedure was employed to isolate VLDL-C, LDL-C, and HDL-C (Assmann et al. Citation1983). The concentrations of cholesterol in the HDL-C-containing supernatants and VLDL-C- and LDL-C-containing precipitations were measured using enzymatic kits (Randox Laboratories Limited, UK) according to the manufacturer’s instructions to evaluate the plasma levels of HDL-C and LDL-C.
Determination of TG and TC levels in the liver and faeces
Rat liver tissues were mixed with chloroform/methanol solution (2:1; v/v) and homogenised (Hong Sheng, SA-50 Max. 3000, Taipei, Taiwan) for 1 min. Supernatants were harvested by centrifugation (1,570 × g for 10 min) for lipid acquisition (Folch et al. Citation1957) and mixed with Triton X-100 followed by solvent removal using vacuum evaporation (SC 110; Savant Instruments, Holbrook, NY, USA.). TG and TC levels were measured using enzymatic kits (Randox Laboratories Limited, UK) according to the manufacturer’s instructions.
Faecal samples were dried following the method described in the Association of Official Analytical Chemists and ground into powder. The procedures of lipid extraction from dried faecal powder and determination of TG and TC levels were performed using the same method as that for the liver tissues.
Evaluation of the activities of acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) in the liver
Activities of ACC and FAS were measured and calculated as described previously (Ahmad et al. Citation1981). Briefly, liver samples were homogenised in citrate buffer/tri-sodium citrate solution. After centrifugation (10,600 × g at 4°C for 30 min), ACC activity was measured by mixing the supernatant with Tri-HCl buffer, MgCl2, monohydrate potassium citrate, glutathione, KHCO3, bovine serum albumin, acetyl-CoA, and ATP, followed by NADPH, and incubation for 5–6 s. Absorbance was measured at 340 nm under 37°C in 30-s intervals for 5 min using a UV–vis spectrophotometer (UV-7800, JASCO Co., Tokyo, Japan) to calculate the activity of ACC. FAS activity was measured by mixing the supernatant with K2HPO4, dithiothreitol, acetyl-CoA, EDTA‧2Na, and malonyl-CoA, followed by NADPH, and incubation for 5–6 s. Absorbance was measured at 340 nm under 37°C in 30-s intervals for 5 min using a UV–vis spectrophotometer. The optical density values were then used to calculate the specific activities of ACC and FAS according to the formula [V1 ÷ (ε × d × FP × V2)] × (ΔA/Δt) × 1000, where V1 represents the total reaction volume (2.9 mL), ε represents the molecular absorption coefficient (6.22 mL/μmol‧cm), d represents the optical path (1 cm), FP represents the protein concentration of the enzyme (mg protein/mL), V2 represents the volume of the enzyme (0.1 mL), and ΔA/Δt represents the change in absorbance of the enzyme reaction solution per unit time.
Evaluation of hormone-sensitive lipase (HSL) and lipoprotein lipase (LPL) activity in perirenal adipose tissues
HSL activity was evaluated using methods based on the study of Morimoto et al. with minor modifications (Morimoto et al. Citation2001). Briefly, isolated perirenal adipose tissues were washed with 0.9% saline, cut up, and mixed with TES buffer followed by incubation at 37°C and 50 rpm in a shaking incubator. After incubation for 1, 2, and 3 h, samples were transferred into Eppendorf Microcentrifuge tubes and further incubated in a 70°C water bath for 10 min followed by centrifugation (100 × g at 25°C for 30 s). Lipolysis rate of the supernatant was measured using glycerol assay kits (Randox Laboratories Limited, UK) according to the manufacturer’s instructions.
LPL activity was evaluated using methods based on previous studies with minor modifications (Murase et al. Citation1981; Quinn et al. Citation1982; Shirai and Jackson Citation1982). Briefly, isolated perirenal adipose tissues were washed with 0.9% saline, cut up, and then mixed with Krebs-Ringer bicarbonate buffer, followed by incubation at 37°C and 50 rpm in a shaking incubator. After incubation for 1 h, the incubated samples were mixed with enzyme reaction solution and substrate solution. After incubation at 37°C for 10 min, the incubated solution was mixed with methanol/chloroform/heptane (10:9:7, v/v/v) and incubated at 42°C for 3 min followed by centrifugation (1,500 × g for 5 min). Absorbance of the supernatant was measured at 400 nm, and the optical density value was used for the calculation of LPL activity according to the Beer–Lambert law.
Statistical analysis
All data were expressed as the mean ± standard error of the mean for each treatment group. Independent sample t-test was used to assess the statistical difference between the treatment groups and the DC control. All p-values <0.05 were considered statistically significant.
Results
Daily supplementation with a submerged culture of G. lucidum ameliorated hyperlipidaemia and visceral fat accumulation in diabetic rats fed with a high-cholesterol diet
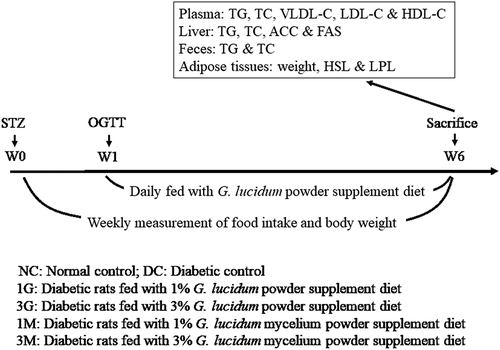
Plasma concentrations of TC and TG were measured to verify the hypolipidemic activities of G. lucidum supplement diets. Plasma TG level was significantly (p < 0.05) elevated in diabetic rats fed with a high-cholesterol diet compared with that in the NC group ()). However, supplementation of the high-cholesterol diet with submerged culture of G. lucidum or mycelia significantly (p < 0.05) decreased the plasma TG concentration ()). Although the plasma concentrations of TC and LDL-C+ VLDL-C were comparable between each group ( (b,c)), supplementation with 3% whole submerged G. lucidum culture significantly (p < 0.05) reversed the high-cholesterol diet-induced HDL-C reduction in diabetic rats ()). Based on the above data, daily supplementation with submerged G. lucidum cultures exhibited preventive effects against high-cholesterol diet-induced plasma TG elevation and HDL-C reduction in rats with type 2 diabetes.
Figure 2. The concentrations of (a) triglyceride (TG), (b) total cholesterol (TC), (c) low density lipoprotein-cholesterol (LDL-C) and very low density lipoprotein-cholesterol (VLDL-C) and (d) high density lipoprotein-cholesterol (HDL-C) in the plasma of diabetic rats fed with G. lucidum supplement high-cholesterol diets
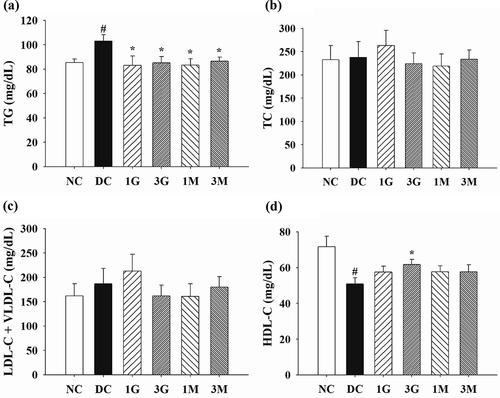
In order to explore the effect of G. lucidum supplement diets on the attenuation of body fat accumulation, the amount of food intake as well as body weight (B.W.) gain and adipose tissue weight were measured. Throughout the animal experiment, the amount of food intake was comparable between each group ()). Nonetheless, B.W. gain was significantly (p < 0.05) reduced in the 1M and 3M groups compared with those in the DC group ()). Consistent with these findings, decreasing weights of perirenal and epididymal adipose tissues were observed in rats in the 1M and 3M groups ( (c,d)). These results indicate that daily dietary supplementation with mycelia harvested from submerged G. lucidum cultures attenuated B.W. gain and visceral fat accumulation in diabetic rats fed with a high-cholesterol diet.
Figure 3. The amount of (a) food intake, (b) gain of body weight and weights of (c) perirenal and (d) epididymal adipose tissues of diabetic rats fed with G. lucidum supplement high-cholesterol diets
Daily dietary supplementation with the submerged culture of G. lucidum modulated lipid synthesis/metabolism enzyme activity and lipid excretion in diabetic rats fed with a high-cholesterol diet
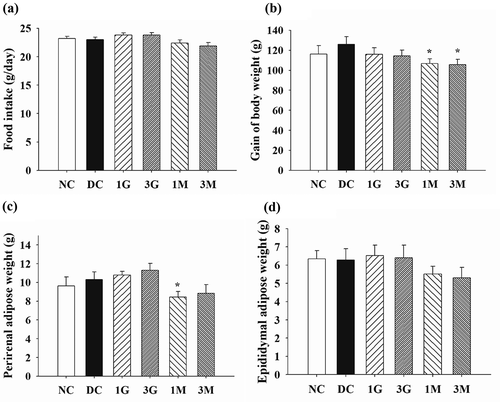
In order to elucidate the mechanism behind G. lucidum-induced hypolipidemic effects and prevention of visceral fat accumulation, we further measured the activities of lipid synthesis/metabolism enzymes and the level of lipid excretion. First, TG and TC concentrations and the activities of lipid synthesis enzymes in the liver were investigated. Although the concentrations of TG and TC were comparable between each group ( (a,b)), the activities of ACC and FAS were significantly elevated in diabetic control rats ( (c,d)). Notably, dietary supplementation with G. lucidum downregulated the activities of ACC and FAS ( (c,d)), especially the ACC activity being marked reduced compared to that of DC group, indicating the suppressive effect of G. lucidum on lipid synthesis.
Figure 4. The concentrations of (a) triglyceride (TG) and (b) total cholesterol (TC) and the activities of (C) acetyl-CoA carboxylase (ACC) and (d) fatty acid synthase (FAS) in the liver of rats fed with G. lucidum supplement high-cholesterol diets
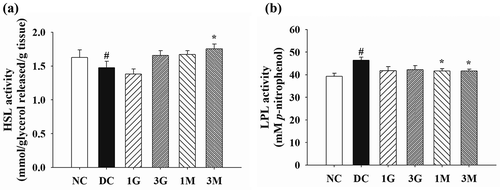
On the other hand, the impact of G. lucidum on lipid metabolism in perirenal adipose tissues was elaborated by investigating the activities of HSL and LPL. A markedly reduced HSL activity and a significantly elevated LPL activity were observed in the perirenal adipose tissues of rats in the DC group compared with those in the NC group (). Noticeably, HSL activity was significantly upregulated in the 3M group, whereas LPL activity was significantly downregulated in the 1M and 3M groups compared with those in the DC group ( (a,b)). These findings suggest that dietary supplementation with mycelia harvested from submerged G. lucidum cultures potentially induced intracellular TG hydrolysis and prevented TG storage in adipose tissues by modulating HSL and LPL activities, respectively.
Figure 5. The activity of (a) hormone sensitive lipase (HSL) and (b) lipoprotein lipase (LPL) in the perirenal adipose tissues of diabetic rats fed with G. lucidum supplement high-cholesterol diets
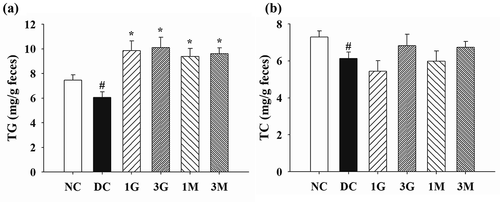
Finally, the faecal concentrations of TG and TC were investigated to understand the effect of G. lucidum on lipid excretion. We observed that the faecal concentrations of TG and TC were markedly reduced in diabetic rats compared with those of rats in the NC group (). Dietary supplementation with G. lucidum significantly increased the faecal concentration of TG compared with that in the DC group ()). Consistently, rats in the 3G and 3M groups had higher faecal concentrations of TC than those in the DC group, although no statistical difference was observed between the treatment groups and the DC group ()). Our findings suggest that daily dietary supplementation with G. lucidum could reverse diabetes-induced reduction of lipid excretion, particularly that of TG, via the faecal route.
Figure 6. The levels of (a) triglyceride (TG) and (b) total cholesterol (TC) in the faeces of rats fed with G. lucidum supplement high-cholesterol diets
Discussion
Although beneficial effects of G. lucidum fruiting bodies on obesity and dyslipidemia have been reported, limited information is available pertaining to the impact of submerged G. lucidum cultures on abnormal lipid metabolism. In the present study, we demonstrated the ameliorating effect of submerged G. lucidum cultures on dyslipidemia and visceral fat accumulation in diabetic rats fed with a high-cholesterol diet. This notion has been substantiated by several lines of evidence. Firstly, dietary supplementation with whole submerged culture of G. lucidum or its mycelia for 5 weeks downregulated the plasma TG levels and upregulated the plasma HDL-C levels. Secondly, B.W. and adipose tissue weight gains were decreased in rats fed with a mycelia supplement diet. Moreover, the potential mechanism of action behind the effects of submerged G. lucidum cultures was elucidated and supported by reduced activities of ACC and FAS in liver, reduced activities of LPL and enhanced activities of HSL in adipose tissue, as well as increased faecal excretion of TG. Our data clearly substantiate the beneficial effects of submerged G. lucidum cultures and their mycelia on high-cholesterol-induced abnormal lipid metabolism in rats with type 2 diabetes and provide critical insights into its underlying mechanisms of action.
Several lipid metabolising enzymes with different expression and activity have been identified between healthy individuals and patients with diabetes. These enzymes are also considered to be key factors that cause dyslipidemia and visceral fat accumulation. For example, ACC catalyzes the first step in the carboxylation of acetyl CoA to form malonyl CoA, which is then catalysed by FAS to synthesise fatty acids. Increasing evidence suggests that reducing ACC activity is necessary to reduce lipid accumulation and improve insulin action (Mao et al. Citation2006; Choi et al. Citation2007; Abu-Elheiga et al. Citation2012; Cordonier et al. Citation2016). (2007). Consistent with our results, Abu-Elheiga et al. (Citation2012) and Choi et al. (Citation2007) observed reductions in fat, lean body mass, and serum non-esterified fatty acids level, and prevention from diet-induced obesity in the ACC2 gene deleted (ACC2−/-) mice. In addition, ACC2−/- mice were protected from fat-induced systemic and hepatic insulin resistance. It has also been reported that treatment of murine 3T3-L1 preadipocytes with soraphen A, ACC1 and ACC2 inhibitors prevents the accumulation of fat in adipocytes (Cordonier et al. Citation2016). Therefore, inhibition of ACC activity is a viable therapeutic target for the treatment of obesity (Moraski et al. Citation2013). Moreover, FAS also plays an important role in lipogenic pathway, which is related to the causal relationship between the consequences of excessive energy intake and the development of obesity and type 2 diabetes (Berndt et al. Citation2007). Our data show that in diabetic rats supplemented with Ganoderma diet, ACC activity was significantly reduced, while FAS activity was slightly reduced, suggesting that inhibition of lipid synthesis, especially ACC activity, is one of the potential mechanisms of beneficial effects of G. lucidum on dyslipidemia and visceral fat accumulation.
Related to lipid metabolism, HSL plays a key role in the hydrolysis of TG in various tissues, including adipose tissue. It has been reported that HSL activity is reduced in adipose tissue of patients with type 2 diabetes, and HSL protein expression is reduced in obese insulin-resistant states (Watt et al. Citation2005; Jocken et al. Citation2007). On the other hand, LPL, a well-known enzyme in TG-rich granules and HDL metabolism, is a determinant of plasma TG and HDL concentrations (Taskinen Citation1987). As our results indicate the modulation of HDL-C levels and HSL and LPL activity, the acceleration of lipid metabolism is considered to be another potential mechanism behind the beneficial effects of supplementing the Ganoderma diet. Furthermore, increased faecal TG levels were observed in diabetic rats fed with a G. lucidum supplement diet. Accordingly, we suggest that increased TG excretion into the faeces is another mechanism of action behind the beneficial effects of G. lucidum. Taken together, the ameliorating effects of submerged G. lucidum cultures and their mycelia on dyslipidemia and visceral fat accumulation are closely associated with their modulatory effects on lipid synthesis, metabolism, and excretion.
Except for 26-oxysterol, lanostane triterpenes and proteoglycan PTP1B inhibitors, there is limited information on the role of G. lucidum in regulating lipid metabolism (Hajjaj et al. Citation2005; Lee et al. Citation2010; Wang et al. Citation2012). In this study, compared to submerged Ganoderma culture, Ganoderma mycelium had higher efficacy in improvement of body weight and adipose tissue weight and HSL and LPL activity, while the whole submerged Ganoderma culture had a better effect on regulating plasma HDL-C levels. Both Ganoderma culture and its mycelia had similar efficacy in regulating plasma TG levels, ACC activity, and faecal TG excretion. These findings indicate that not only mycelium but also other active ingredients, such as extracellular polysaccharides, can also help improve the ameliorating effects of submerged G. lucidum cultures on abnormal lipid metabolism. Although many studies have shown that medical mushrooms have multiple pharmacological activities, these literature use crude extracts, including undetermined metabolite mixtures, whose efficacy in vitro is far from being standardised due to widely differing extraction and treatment methods (Chen et al. Citation2018; Girometta Citation2019). For submerged Ganoderma sp., in vivo experiments and pharmacological studies of the identified compounds are also very limited. Therefore, there is an urgent need to develop in vivo and pharmacological analysis methods for known compounds used in medical mushrooms. In order to clarify possible adverse reactions and mechanisms of action, it is necessary to have a better understanding of the bioactive compounds in Ganoderma sp., which will be beneficial for clinical applications (Basnet et al. Citation2017). Recently, Ogbole et al. (Citation2019) have identified and authenticated a puffball mushroom using molecular tools and investigated its anti-diabetic properties of crude extract and partitioned fractions in vitro and in vivo. By using a similar strategy, further research is needed to identify the active ingredients that contribute to the regulation of lipid metabolism by submerged G. lucidum cultures.
Conclusion
Our findings provide the first evidence that the improvement of dyslipidemia and visceral fat accumulation in type 2 diabetic rats fed with high-cholesterol diets by submerged G. lucidum culture and its mycelium in the diet may be through regulating lipid synthetic/metabolic enzyme activities and lipid excretion. Since submerged cultures have advantages over basidiocarp cultivation and have promising activity against abnormal lipid metabolism, we recommend using submerged G. lucidum culture and its mycelium as functional foods for controlling type 2 diabetes, especially for diabetes with complications of lipid metabolism disorder.
Author’s Contribution
G. J. Tsai conceived, designed and supervised this study. W. K. Lin performed the experiments. C. H. Huang and S. H. Chang analyzed the data and wrote the paper. G. J. Tsai revised the manuscript.
Supplemental Material
Download MS Word (23.4 KB)Disclosure Statement
There are no conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Supplementary Material
Supplemental data for this article can be accessed here.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Additional information
Funding
References
- Abu-Elheiga L, Wu H, Gu Z, Bressler R, Wakil SJ. 2012. Acetyl-CoA carboxylase 2-/- mutant mice are protected against fatty liver under high-fat, high-carbohydrate dietary and de novo lipogenic conditions. J Biol Chem. 287(15):12578–12588. doi:https://doi.org/10.1074/jbc.M111.309559.
- Ahmad F, Ahmad PM, Dickstein R, Greenfield E. 1981. Detection of ligand-induced perturbations affecting the biotinyl group of mammalian acetyl-coenzyme A carboxylase by using biotin-binding antibodies. Biochem J. 197(1):95–104. doi:https://doi.org/10.1042/bj1970095.
- Assmann G, Schriewer H, Schmitz G, Hagele EO. 1983. Quantification of high-density-lipoprotein cholesterol by precipitation with phosphotungstic acid/MgCl2. Clin Chem. 29(12):2026–2030. doi:https://doi.org/10.1093/clinchem/29.12.2026.
- Bach EE, Hi EMB, Martins AMC, Nascimento PAM, Wadt NSY. 2018. Hypoglicemic and hypolipedimic effects of ganoderma lucidum in streptozotocin-induced diabetic rats. Medicines (Basel). 5(3):78.
- Basnet BB, Liu L, Bao L, Liu H. 2017. Current and future perspective on antimicrobial and anti-parasitic activities of Ganoderma sp.: an update. Mycology. 8(2):111–124. doi:https://doi.org/10.1080/21501203.2017.1324529.
- Berndt J, Kovacs P, Ruschke K, Kloting N, Fasshauer M, Schon MR, Korner A, Stumvoll M, Bluher M. 2007. Fatty acid synthase gene expression in human adipose tissue: association with obesity and type 2 diabetes. Diabetologia. 50(7):1472–1480. doi:https://doi.org/10.1007/s00125-007-0689-x.
- Chang CJ, Lin CS, Lu CC, Martel J, Ko YF, Ojcius DM, Tseng SF, Wu TR, Chen YY, Young JD, et al. 2015. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat Commun. 6(1):7489. doi:https://doi.org/10.1038/ncomms8489.
- Chang M-Y, Tsai G-J, Houng J-Y. 2006. Optimization of the medium composition for the submerged culture of Ganoderma lucidum by Taguchi array design and steepest ascent method. Enzyme Microb Technol. 38(3–4):407–414. doi:https://doi.org/10.1016/j.enzmictec.2005.06.011.
- Chen YY, Lo CP, Lin CC, Hsieh YH. 2018. Effects of Taiwanofungus camphoratus on non-specific and specific immune activities in mice. Mycology. 9(2):129–135. doi:https://doi.org/10.1080/21501203.2018.1437837.
- Choi CS, Savage DB, Abu-Elheiga L, Liu ZX, Kim S, Kulkarni A, Distefano A, Hwang YJ, Reznick RM, Codella R, et al. 2007. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci U S A. 104(42):16480–16485. doi:https://doi.org/10.1073/pnas.0706794104.
- Chu TT, Benzie IF, Lam CW, Fok BS, Lee KK, Tomlinson B. 2012. Study of potential cardioprotective effects of Ganoderma lucidum (Lingzhi): results of a controlled human intervention trial. Br J Nutr. 107(7):1017–1027. doi:https://doi.org/10.1017/S0007114511003795.
- Cordonier EL, Jarecke SK, Hollinger FE, Zempleni J. 2016. Inhibition of acetyl-CoA carboxylases by soraphen A prevents lipid accumulation and adipocyte differentiation in 3T3-L1 cells. Eur J Pharmacol. 780:202–208. doi:https://doi.org/10.1016/j.ejphar.2016.03.052.
- Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 226(1):497–509.
- Galor S, Yuen J, Buswell J, Benzie I. 2011. Ganoderma lucidum (Lingzhi or Reishi), a medicinal mushroom in herbal medicine: biomolecular and clinical aspects. 2nd ed. Florida: CRC Press/Taylor and Francis.
- Girometta C. 2019. Antimicrobial properties of fomitopsis officinalis in the light of its bioactive metabolites: a review. Mycology. 10(1):32–39. doi:https://doi.org/10.1080/21501203.2018.1536680.
- Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC Jr., Sowers JR. 1999. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 100(10):1134–1146. doi:https://doi.org/10.1161/01.CIR.100.10.1134.
- Hajjaj H, Mace C, Roberts M, Niederberger P, Fay LB. 2005. Effect of 26-oxygenosterols from Ganoderma lucidum and their activity as cholesterol synthesis inhibitors. Appl Environ Microbiol. 71(7):3653–3658. doi:https://doi.org/10.1128/AEM.71.7.3653-3658.2005.
- Jocken JW, Langin D, Smit E, Saris WH, Valle C, Hul GB, Holm C, Arner P, Blaak EE. 2007. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J Clin Endocrinol Metab. 92(6):2292–2299. doi:https://doi.org/10.1210/jc.2006-1318.
- Krauss RM. 2004. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care. 27(6):1496–1504. doi:https://doi.org/10.2337/diacare.27.6.1496.
- Lee I, Kim H, Youn U, Kim J, Min B, Jung H, Na M, Hattori M, Bae K. 2010. Effect of lanostane triterpenes from the fruiting bodies of Ganoderma lucidum on adipocyte differentiation in 3T3-L1 cells. Planta Med. 76(14):1558–1563. doi:https://doi.org/10.1055/s-0030-1249827.
- Mao J, DeMayo FJ, Li H, Abu-Elheiga L, Gu Z, Shaikenov TE, Kordari P, Chirala SS, Heird WC, Wakil SJ. 2006. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci U S A. 103(22):8552–8557. doi:https://doi.org/10.1073/pnas.0603115103.
- Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, Novelli M, Ribes G. 1998. Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 47(2):224–229. doi:https://doi.org/10.2337/diab.47.2.224.
- Meneses ME, Martinez-Carrera D, Torres N, Sanchez-Tapia M, Aguilar-Lopez M, Morales P, Sobal M, Bernabe T, Escudero H, Granados-Portillo O, et al. 2016. Hypocholesterolemic properties and prebiotic effects of Mexican Ganoderma lucidum in C57BL/6 mice. PLoS One. 11(7):e0159631. doi:https://doi.org/10.1371/journal.pone.0159631.
- Moraski GC, Markley LD, Cramer J, Hipskind PA, Boshoff H, Bailey M, Alling T, Ollinger J, Parish T, Miller MJ. 2013. Advancement of Imidazo[1,2-a]pyridines with improved pharmacokinetics and nanomolar activity against mycobacterium tuberculosis. ACS Med Chem Lett. 4(7):675–679. doi:https://doi.org/10.1021/ml400088y.
- Morimoto C, Kameda K, Tsujita T, Okuda H. 2001. Relationships between lipolysis induced by various lipolytic agents and hormone-sensitive lipase in rat fat cells. J Lipid Res. 42(1):120–127.
- Murase T, Yamada N, Matsuzaki F. 1981. The in vitro effect of growth hormone on adipose tissue lipoprotein lipase in rats. Life Sci. 28(2):199–201. doi:https://doi.org/10.1016/0024-3205(81)90553-1.
- Nelson RH. 2013. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 40(1):195–211. doi:https://doi.org/10.1016/j.pop.2012.11.003.
- Ogbole OO, Nkumah AO, Linus AU, Falade MO. 2019. Molecular identification, in vivo and in vitro activities of Calvatia gigantea (macro-fungus) as an antidiabetic agent. Mycology. 10(3):166–173. doi:https://doi.org/10.1080/21501203.2019.1595204.
- Patel P, Abate N. 2013. Body fat distribution and insulin resistance. Nutrients. 5(6):2019–2027. doi:https://doi.org/10.3390/nu5062019.
- Quinn DM, Shirai K, Jackson RL, Harmony JA. 1982. Lipoprotein lipase catalyzed hydrolysis of water-soluble p-nitrophenyl esters. Inhibition by apolipoprotein C-II. Biochemistry. 21(26):6872–6879. doi:https://doi.org/10.1021/bi00269a038.
- Shirai K, Jackson RL. 1982. Lipoprotein lipase-catalyzed hydrolysis of p-nitrophenyl butyrate. Interfacial activation by phospholipid vesicles. J Biol Chem. 257(3):1253–1258.
- Taskinen MR. 1987. Lipoprotein lipase in diabetes. Diabetes Metab Rev. 3(2):551–570. doi:https://doi.org/10.1002/dmr.5610030208.
- Wang CD, Teng BS, He YM, Wu JS, Pan D, Pan LF, Zhang D, Fan ZH, Yang HJ, Zhou P. 2012. Effect of a novel proteoglycan PTP1B inhibitor from Ganoderma lucidum on the amelioration of hyperglycaemia and dyslipidaemia in db/db mice. Br J Nutr. 108(11):2014–2025. doi:https://doi.org/10.1017/S0007114512000153.
- Wang F, Zhou Z, Ren X, Wang Y, Yang R, Luo J, Strappe P. 2015. Effect of Ganoderma lucidum spores intervention on glucose and lipid metabolism gene expression profiles in type 2 diabetic rats. Lipids Health Dis. 14(1):49. doi:https://doi.org/10.1186/s12944-015-0045-y.
- Watt MJ, Carey AL, Wolsk-Petersen E, Kraemer FB, Pedersen BK, Febbraio MA. 2005. Hormone-sensitive lipase is reduced in the adipose tissue of patients with type 2 diabetes mellitus: influence of IL-6 infusion. Diabetologia. 48(1):105–112. doi:https://doi.org/10.1007/s00125-004-1598-x.
- Wu Y, Ding Y, Tanaka Y, Zhang W. 2014. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci. 11(11):1185–1200. doi:https://doi.org/10.7150/ijms.10001.
