ABSTRACT
Holocarpic oomycetes infecting freshwater diatoms are obligate endobiotic parasites reported from a wide range of habitats. So far, the taxonomy of and phylogeny of most species remains unresolved, since most have not been reported throughout the past decades and sequence data are available for only the four species, Aphanomycopsis bacillariacearum, Diatomophthora gillii, Ectrogella bacillariacearum, and the recently-discovered species Miracula moenusica. In the current study, a new freshwater diatom parasite resembling Ectrogella bacillariacearum in the sense of Scherffel was discovered from pennate diatoms (Ulnaria acus, Ulnaria ulna) collected from the small stream Einbúalækur on Víkurskarð, North Iceland and investigated for its life cycle and phylogenetic placement. In contrast to the original description, Scherffel reports an achlya-like spore discharge for Ectrogella bacillariacearum. The phylogenetic reconstruction and morphological characterisation in this study revealed that Scherffel’s E. bacillariacearum is largely unrelated to the epitype of the species and is a member of the early-diverging genus Miracula. Consequently, the new species is described as M. einbuarlaekurica in the present study. This adds a second freshwater member to the genus, demonstrating the high ecological adaptability of the genus, which thrives in both freshwater and marine ecosystems.
Introduction
Holocarpic oomycete endoparasitoids of diatoms are diverse and widespread in freshwater habitats (Dick Citation2001; Karling Citation1942; Sparrow Citation1960). They infect a wide range of pennate (e.g. Synedra, Pinnularia) and centric diatoms (e.g. Cyclotella, Pleurosira), with most of the known species isolated from temperate regions (Buaya and Thines 2019 c; Scherffel Citation1925; Sparrow Citation1933; Zopf Citation1884). In addition to the recently-described species Miracula moenusica (Buaya and Thines Citation2019b), only three other freshwater species (Aphanomycopsis bacillariacearum, Diatomophthora gillii, Ectrogella bacillariacearum) have been investigated for their molecular phylogeny (Buaya et al. Citation2019a, Citation2021; Buaya and Thines Citation2020a), leaving the majority of known species unplaced. Freshwater diatom parasitoids were classified in the genera Aphanomycopsis (Scherffel Citation1925), Ectrogella (Zopf Citation1884), Lagenidium (Zopf Citation1878), Miracula (Buaya et al. Citation2017), and Olpidiopsis (Cornu Citation1872). Of these genera, Ectrogella contains the largest assemblage of species infecting freshwater diatoms (E. bacillariacearum, E. monostoma, E. gomphonematis, E. eunotiae, E. brachystoma, E. cyclotellae), but also includes some species infecting marine diatoms (E. licmophorae, E. eurychasmoides), macro-algae (E. marina, E. lauderia), as well as a hyperparasite (E. besseyi) (Friedmann Citation1952; Feldmann and Feldmann Citation1955; Scherffel Citation1925; Sparrow and Ellison Citation1949; Zopf Citation1884). The genus Ectrogella has been subjected to various interpretations in the past (Karling Citation1942; Sparrow Citation1960), and Dick (Citation2001) further broadened the genus concept by including almost all diatom-infecting oomycetes regardless of their life cycle traits, e.g. zoospore formation and release. To date, only two Ectrogella species have been rediscovered, sequenced, and epitypified – the type species E. bacillariacearum (Buaya and Thines Citation2020a) and the marine species E. perforans (Buaya et al., Citation2020b). The former species was speculated to belong to the early-diverging oomycete lineages (e.g. Olpidiopsis, Haptoglossa) (Hanic et al. Citation2009; Garvetto et al. Citation2018) but was found to be embedded in the Leptomitales of the Saprolegniomycetes, in line to its classical taxonomic placement (Karling Citation1942; Sparrow Citation1960; Dick Citation2001). The marine, plurivorous species E. perforans was subsequently reclassified into the early-diverging genus Diatomophthora, adding to D. drebesii and D. gillii (Buaya et al. Citation2017, Citation2020b; Buaya and Thines Citation2020a). Thus, only the type species of Ectrogella has so far been confirmed as a member of the genus. Interestingly, Scherffel (Citation1925) depicted an achlyoid zoospore release for E. bacillariacearum in Figure 6, although he stated in the figure legend that this behaviour was exceptional. In addition, he also did not draw the thickened exit tubes with a Spreizapparat that Zopf considered typical for E. bacillariacearum, casting doubts on the identification of the specimen. While screening for freshwater diatom parasitoids from phytoplankton and riverbed biofilm samples from the brook Einbúalækur on Víkurskarð pass, North Iceland, in the autumn of 2019, a diatom parasitoid matching to Figure 6 of Scherffel (Citation1925) was found in the pennate diatom Ulnaria acus. It was the aim of this study to document its life cycle and to clarify whether it is conspecific with E. bacillariacearum.
Material and methods
Isolation, characterisation and establishment of the host and parasite culture
Diatom samples were collected from the upper watercourse of Einbúalækur on Víkurskarð pass, North Iceland (65°48ʹ20.3” N; 17°57ʹ38.0” W), on the 12th of September 2019, using 20 µm mesh plankton nets (Hydro-Bios, Kiel, Germany) and by directly scraping biofilms from the riverbed sediments with the mouth of 1 L plastic bottles, which were subsequently filled with freshwater of the streamlet. About 10 mL of phytoplankton concentrate was poured into each of several 9 cm Petri-dishes with the addition of sterile freshwater and screened for oomycete-infected diatoms using an inverted compound light microscope (Type AE31, Motic, Xiamen). Infected diatoms were individually picked using a 10-µL micropipette (Brandt, Germany), rinsed multiple times by transfer through sterile deionised water and transferred to 2-mL tubes containing 0.5 mL RNAlater solution (Invitrogen, Thermo Fisher, Lithuania) or 70% ethanol (VWR, France) for subsequent DNA extraction. Approximately 60–70 infected cells of the diatom Ulnaria acus were collected this way for DNA extraction. Samples collected and preserved in 70% ethanol in the same manner were deposited in the herbarium collection of the Senckenberg Museum of Natural History (Herbarium Senckenbergianum, FR), Cryptogams Section, Frankfurt am Main (accessions: FR-0046131 for isolate 1, FR-0046132 for isolate 2, both isolated from the same raw sample). Morphological characterisation of the parasite was done as described earlier (Buaya et al. Citation2021) using a compound light microscope (Imager2, Carl Zeiss Göttingen, Germany) with DIC, and photographs were taken using a Zeiss Axiocam MRc5 (Carl Zeiss, Göttingen, Germany). In addition, cellulose presence in the thallus wall of the diatom parasitoid was tested using zinc iodine chloride solution (Carl Roth GmbH, Germany). The identity of the diatom host was determined by light microscopy and 18S nrSSU gene sequence barcoding as outlined below.
Axenic host and parasite dual culture of the isolated diatom pathogen were attempted in 9 cm petri dishes (Sarstedt, Nümbrecht, Germany) using Guillard’s f/2 enrichment medium (Guillard and Ryther Citation1962). For this, infected diatoms were collected as described above and incubated in a climate chamber (Conviron, CMP 6010, Canada) at 16 °C and 12 °C, for 14 h and 10 h, respectively, and with 18 µM/sm2 light during the 14 h lighting period (Narva, bio-vital, Germany). Uninfected U. acus individuals were isolated for the establishment of host and parasite dual culture, and serial subculturing for achieving pure cultures was done as described previously (Buaya et al. Citation2019d, Citation2020b). After every 2 weeks, 6–8 infected diatoms containing a single parasite thallus were inoculated into healthy 2-week-old cultures of U. acus to maintain the cultures. The parasite inoculum originated from a single thallus of an infected U. acus cell, but an epibiotic chytrid parasite was also carried along. This parasite persisted in the cultures even after more than a year of various attempts to remove it, as the spores of the chytrid are also present, even if sometimes in low quantities, on diatoms infected by the oomycete. Thus, the isolation of chytrid-free cells with the oomycete parasitoid was greatly obstructed. As the present study was focussed on the oomycete parasitoid, the chytrid epiparasite was not further characterised.
DNA extraction, PCR amplification, and phylogenetic analyses
DNA extraction was performed using an innuPREP Plant DNA extraction Kit (Analytik Jena, Germany), as previously described (Buaya et al. Citation2017). The PCR amplification of the nuclear encoded small ribosomal subunit gene (nrSSU) was performed as described in Buaya et al. (Citation2019a) using Mango-Taq DNA Polymerase (Bioline, UK) but with the 18S primer pair EUK422–445 and EUK1422–1440_R (Wang et al. Citation2014). Amplicons were sent for sequencing to the laboratory centre of the Senckenberg Biodiversity and Climate Research Centre, Frankfurt am Main (SBiK-F, Frankfurt, Germany), using the primer pairs used in PCR. In addition, direct single-cell PCRs were also done as described in Buaya et al. (Citation2019a). Some PCR amplicons were cloned as previously described (Buaya et al. Citation2020b). The resulting sequences were edited and assembled using Geneious (version 5.6, Biomatters, Auckland, New Zealand), and the assembled consensus sequences were added to the dataset of Buaya et al. (Citation2020b), supplemented with additional sequences from Buaya et al. (Citation2021). Subsequently, alignments were done using mafft version 7 as implemented on the mafft webserver (Katoh et al. Citation2019), with the Q-INS-i algorithm and a gap opening penalty of 2.4 to account for the high conservation of the nrSSU. Phylogenetic analyses were done using MEGA7 (Kumar et al. Citation2016) for Minimum Evolution analysis with 1000 bootstrap replicates, and Maximum Parsimony analysis with 500 bootstrap replicates, with all parameters set to default, except for using the Tamura-Nei substitution model and pairwise deletion. Maximum Likelihood inference was performed on the TrEase webserver (http://thines-lab.senckenberg.de/trease/), using RAxML (Stamatakis Citation2014), with 1000 bootstrap replicates applying the GTRGAMMA algorithm. Developayella elegans and Hyphochytrium catenoides were used as outgroup. The partial 18S (nrSSU) sequences obtained in this study were deposited in GenBank (accession numbers MW671553 for isolate 1 and MW671554 for isolate 2).
Results
Parasite detection and culture establishment
In phytoplankton collected from the freshwater stream Einbúalækur near the old Þjóðvegur on Víkurskarð, North Iceland, Ulnaria acus was observed to contain sporadic infections of a holocarpic oomycete. About 10% of these diatoms screened were infected. After further incubation for 2–4 weeks with supplementation of f/2 enrichment medium infections were observed to increase quickly in parallel to the abundance of the host diatom, but then declined after consuming almost all U. acus cells. Few cells of U. ulna were also observed to be parasitised at a very low frequency, but as no high-quality sequences could be obtained from these isolations, the identity of the pathogen could not be ascertained. However, infection trials revealed that both host species are susceptible to the parasitoid described in this study. Pinnularia viridis, Nitzschia sigmoidea, Gyrosigma acuminatum and several Ulnaria species, which were reported to be host of other holocarpic oomycetes (e.g. Aphanomycopsis, Diatomophthora, Ectrogella, “Olpidiopsis”) (Karling Citation1942; Sparrow Citation1960), were co-occurring with the infected individuals of U. acus, but no individuals of these species were observed to be parasitised throughout the examination period. Establishment of a stable host and parasite axenic dual culture of the pathogen isolates was attempted using Guillard’s f/2 medium (Guillard and Ryther Citation1962). These attempts were successful apart from the fact that a single epibiotic chytrid contaminant was present in all cultures. The parasitoid cultures were maintained by periodic subculturing in a controlled climate chamber environment (Buaya et al. Citation2019c). The fungal contaminant was observed to spread more quickly on fresh U. acus cultures than the holocarpic oomycete, and attempts to remove the contaminant by multiple serial transfers, single-cell isolation using glass needles, and additions of antifungal agents (e.g. Benomyl, Hymexazol) were unsuccessful due to the high abundance of the chytrid infections and the resulting zoospores. After prolonged (approximately 3–4 months) serial subculturing, the four originally isolated susceptible single-cell strains of U. acus gradually became resistant to the infections after undergoing sexual reproduction. Five new strains of U. acus and five new strains of U. ulna were isolated from the river Main in Frankfurt am Main to replace the increasingly resistant old host strains. However, even though initially susceptible, after several inoculations, most of the newly isolated strains became resistant to the pathogens, except for two strains of U. ulna (s2, s5), which are currently used as host diatoms for the long-term culture of the oomycete isolate.
Morphology and life cycle traits
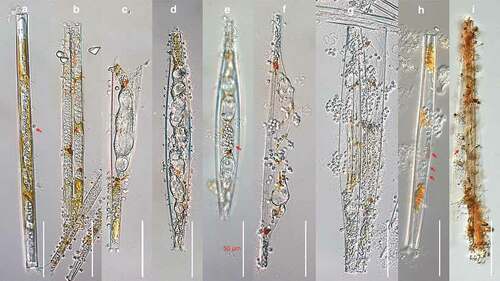
The development of the parasitoid isolated in this study, from the start of infection until zoospore release was documented using several specimens of infected U. ulna and U. acus ()). The life cycle of this parasite started when an encysted spore attached on the outer surface of the host frustule, germinated (), red arrow), and developed a very fine, needle-like penetration tube that grew through the girdle bands and extended into the host cytoplasm. The thalli usually settled in the central area of the frustule near the nucleus and were naked at this stage. Once established, the thallus enlarged quickly )), elongating into an unbranched, sausage-like amorphous thallus containing dense refractive granules, surrounded by very thin, colourless, smooth wall, and causing gradual degradation of the host phaeoplasts. At the late stage, the phaeoplasts were disintegrated into orange-golden brown to chestnut-coloured residues deposited at the apices of the thalli (). At this stage, the parasite thalli began to swell at various locations, producing one to several large central vacuoles, forcing the valves of the host apart (). After some time, zoospore initials began to form at the periphery of the vacuoles, progressing into small refractive globules until the entire thallus developed into granular, irregularly shaped droplets of zoospores initials (). Usually, a single host cell contained one unbranched thallus, but occasionally several thalli were present in one host cell, sometimes more than 10. The amorphous silvery thalli were highly variable in size at maturity, usually 150–200 µm long by 5–20 µm in diameter when single, and 20–60 µm long by 5–15 µm in diameter in case of multiple infections per host cell. As the thalli further matured, 1–8 or sometimes more very short tubular discharge tube were formed per thallus, each with a slightly thickened base, normally with 2–6 µm in diameter (), red arrows). After some time, mature, non-flagellated primary spores of 3–5 µm in diameter (), red arrow), slowly emerged from the orifice of the discharge tube, and subsequently formed dense clusters in an achlya-like fashion (). The spores then developed into grape seed like biflagellate secondary zoospores with a ventral furrow, of 2–4 µm long by 2–3 µm in diameter, swimming away with a zigzag motion pattern for a few minutes before coming to a rest. After this, the zoospores started to develop into spherical hyaline spores of 2–5 µm in diameter, with a smooth and slightly thickened colourless wall. Germination of these potential resting stages and the sexuality of the organism were not observed. The entire sporangium of the parasitoid, especially the thickened portion of the exit tubes tested positive for the presence of cellulose as evidenced by a strong violet to blueish tint after staining with a solution of zinc iodine chloride ()).
Figure 1. DIC light micrographs of Miracula einbuarlaekurica at different lifecycle stages in the freshwater pennate diatoms Ulnaria acus and Synedra ulna. (a) Early infection, with developing naked endobiotic holocarpic thalli, with remnants of a single encysted spore attached at the central area of the frustule (red arrow); (b) elongating unbranched thallus in an intermediate stage of development, causing gradual degradation of the host chloroplast into Orange-golden brown to chestnut-coloured residues; (c–f) late developmental stages, in diatoms containing a single (c) and multiple (d) thalli, with swellings forcing apart the host valves (c) or causing hyperthrophy (d); (e) fully formed mass of primary aplanospores within the sporangium (red arrow); (g, h) clusters of immotile primary spores at the orifice of the discharge tubes (h, red arrows); (i) zinc iodine stained empty thallus of M. einbuarlaekurica with a Spreizapparat at the base of each discharge tube (red arrows).
Molecular phylogeny
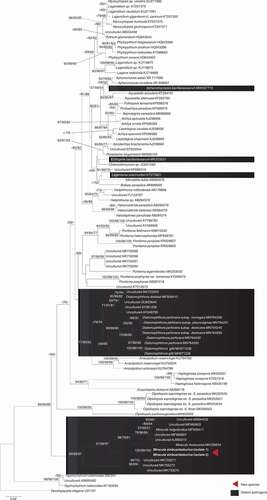
In the phylogenetic tree () based on the partial nrSSU (18S) sequences the oomycete isolates from U. acus grouped within the genus Miracula with strong support in all analyses, together with two diatom-infecting holocarpic species described previously (M. helgolandica, M. moenusica), and several environmental sequences. Notably, the isolates obtained in the current study were sister to all confirmed lineages of Miracula, and the second freshwater species, M. moenusica, was the sister lineage to M. helgolandica and some marine environmental sequences. In line with previous studies, the other diatom-infecting species were scattered throughout the phylogeny of oomycetes, with Diatomophthora as a sister-group to Anisolpidium in the early diverging lineages of the Oomycota. Two genera were placed among the Leptomitales (Ectrogella, Lagenisma), while Aphanomycopsis was located in the Saprolegniales. In line with previous studies, the relationships of the various early diverging lineages to each other could not be resolved, and the placement of Miracula as the earliest-diverging lineage did not receive significant support.
Figure 2. Molecular phylogeny based on minimum evolution analysis inferred of partial nrssu (18s) sequences. numbers on branches denote bootstrap values from maximum likelihood, minimum evolution, and maximum parsimony analyses, in the respective order. A dash “-“ indicates less than 60% bootstrap support or a conflicting topology.
Taxonomy
Based on life cycle traits and phylogenetic placement of the diatom parasitoid isolated in this study, a new species of Miracula is introduced here.
Miracula einbuarlaekurica A.T. Buaya and Thines, sp. nov., Mycobank number MB838935
Etymology: Named after Einbúalækur, the stream in Iceland from which the species was isolated.
Description: Thallus holocarpic, endobiotic, sausage-like, usually one, sometimes more than 10 in individuals of the diatom genus Ulnaria, naked at early developmental stages, later surrounded by a thin, colourless, smooth wall, developing swellings with large central vacuoles during maturation, forcing the valves of the host apart, ellipsoidal to fusiform in shape, usually 150–200 µm long by 5–20 µm in diameter when single, and 20–60 µm long by 5–15 µm in diameter in case of multiple infections. Discharge tubes single or, more frequenty, multiple per thallus, short, 3–6 µm wide, with a slightly thickened base, forming where the thallus wall is exposed to the outside medium at the girdle band. Zoospores formed within the thallus non-motile, after discharge resting in clusters at the orifice of the discharge tubes until developing into grape seed shaped, biflagellate zoospores, 2–4 µm long and 2–3 µm broad, with subapically inserted flagellum, swimming in random directions for a few minutes before coming to a rest and encysting. Sexual reproduction not observed.
Type: Iceland, Northern Iceland, Víkurskarð, stream Einbúalækur near the old Þjóðvegur (65°48ʹ20.3” N; 17°57ʹ38.0” W), 12 September 2019, collected by Anthony T. Buaya and Marco Thines, isolated by Marco Thines and Anthony T. Buaya, holotype specimen deposited in the Herbarium Senckenbergianum (FR) under the accession number FR-0046131. Ex-type partial nrSSU sequence MW671553.
Known distribution: Iceland.
Discussion
The phylum Oomycota contains several obligate biotrophic parasites of marine and freshwater diatoms (Sparrow Citation1960). The majority of species occurring in freshwater have been classified in the genus Ectrogella (Friedmann Citation1952; Scherffel Citation1925; Zopf Citation1884). Most of these pathogens were isolated from pennate diatoms (e.g. Eunotia, Gomphonema, Gyrosigma, Navicula, Nitzschia, Pinnularia, Synedra) with only two freshwater species reported from centric diatoms Cyclotella and Pleurosira (Buaya and Thines Citation2019b; Karling Citation1942). The knowledge about these endoparasitoids is still fragmentary since many species have been reported only very few times, long-term cultures for freshwater parasitoids were lacking before this study, and only few of the species described have been investigated for their molecular phylogeny (Beakes and Thines Citation2017; Buaya and Thines Citation2020c). Despite their superficial similarity (Dick Citation2001) diatom parasitoids are phylogenetically scattered throughout the phylogeny of oomycetes (Buaya and Thines Citation2020c) and a genus-level classification without the aid of molecular phylogenetics is difficult, as it requires the study of the entire lifecycle.
The superficial resemblance of the species has led to confusion over time, complicated by the possibility of dual infections by unrelated species in a single diatom host cell. An example for this is provided by the two species occurring in Gomphonema (Scherffel Citation1925). In this diatom species, Scherffel (Citation1925) described two different species, Lagenidium brachystomum and E. gomphonematis. The description of L. brachystomum seems to be attributable to a member of the Peronosporales, the situation in the other species is less clear. For E. gomphonematis, Scherffel (Citation1925) reports a clustering of spores at the orifice of the broad tubular exit tubes, similar to Achlya and Aphanomyces. However, he also describes a diplanetism with a movement of the primary spores inside the thallus, which would support a placement in Ectrogella or Diatomophthora. Interestingly, both species can co-exist in a single host cell (Scherffel Citation1925). The species discovered in this study differs from the species Scherffel (Citation1925) assigned to Ectrogella, as no movement of the zoospores inside the thallus could be observed, and the primary spores also did not move once discharged, but formed clusters at the orifice of the short discharge tubes. This is very similar to the situation Scherffel (Citation1925) describes for his E. monostoma. However, he reports the presence of only a single exit tubes and a locally restricted swelling of the mature thallus, which is unlike M. einbuarlaekurica, in which wide parts of the thallus swell and usually multiple exit tubes are formed. Interestingly, it seems possible that Scherffel (Citation1925) had seen the species we describe here, as he reports a similar behaviour for some cases of E. bacillariacearum. For example, he reported that as an exception the spores formed clusters at the orifice of short discharge tubes (Scherffel Citation1925, figure 6), and he also depicts a swelling similar to the one observed in M. einbuarlaekurica (Scherffel Citation1925, figure 4e). Thus, it might be possible that his assessment of E. bacillariacearum was, at least in part, based on M. einbuarlaekurica or a closely related species. While, as discussed above, some aspects of spore discharge are similar between E. monostoma and M. einbuarlaekurica, the former species is reported to produce almost twice as large cysts and secondary zoospores that are on average much bigger than in the latter species with about 8 µm in length (Scherffel Citation1925). Thus, the isolate found in this study and described as M. einbuarlaekurica cannot be attributed to any known holocarpic oomycete species.
With the description of M. einbuarlaekurica, the genus Miracula contains two freshwater species, with M. einbuarlaekurica as the sister lineage of all species currently described. However, sequences derived from marine environmental samples with infected Licmophora and Fragillaria (Garvetto et al. Citation2020) form an even deeper-diverging lineage, suggesting a transition from marine to freshwater environments and back. While other scenarios cannot be ruled out based on the presently available data, it seems that, like in several cultivable lineages (Bennett and Thines Citation2020), holocarpic diatom parasitoids cross the border between marine and freshwater habitats easily. How often this transition happened in Diatomophthora, Miracula and probably also other diatom-infecting genera will need to be clarified in future studies.
Acknowledgements
ATB is grateful to Katholischer Akademischer Ausländer Dienst (KAAD) for doctoral fellowship, the Stiftung zur Förderung der internationalen Beziehungen der Goethe-Universität Frankfurt am Main, and the Senckenberg Biodiversity and Climate Research Centre (SBiK-F) for add-on scholarships. MT is supported by the LOEWE initiative of the government of Hessen in the framework of the Centre for Translational Biodiversity Genomics. DAAD is also gratefully acknowledged for a travel grant awarded to MT that led to the present study. As suggested by Thines et al. (Citation2020), all scientific names are set in italics.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Beakes GW, Thines M. 2017. Hyphochytriomycota and Oomycota. In: JM A, AGB S, Ch S, editors. Handbook of the Protists. Heidelberg (Germany): Springer Verlag; p. 435–505.
- Bennett R, Thines M. 2020. An overview on Philippine estuarine oomycetes. Philippine Journal of Systematic Biology. 14(7):1–14.
- Buaya AT, Kraberg A, Thines M. 2019c. Dual culture of the oomycete Lagenisma coscinodisci Drebes and Coscinodiscus diatoms as a model for plankton/parasite interactions. Helgoland Marine Research. 73(1):2. doi:https://doi.org/10.1186/s10152-019-0523-0.
- Buaya AT, Ploch S, Hanic L, Nam B, Nigrelli L, Kraberg A, Thines M. 2017. Phylogeny of Miracula helgolandica gen. et sp. nov. and Olpidiopsis drebesii sp. nov. two basal oomycete parasitoids of marine diatoms, with notes on the taxonomy of Ectrogella-like species. Mycological Progress. 16(11–12):1041–1050. doi:https://doi.org/10.1007/s11557-017-1345-6.
- Buaya AT, Ploch S, Inaba S, Thines M. 2019d. Holocarpic oomycete parasitoids of red algae are not Olpidiopsis. FUSE. 4:21–31.
- Buaya AT, Ploch S, Kraberg A, Thines M. 2020b. Phylogeny and cultivation of the holocarpic oomycete Diatomophthora perforans comb. nov., an endoparasitoid of marine diatoms. Mycological Progress. 19(5):441–454. doi:https://doi.org/10.1007/s11557-020-01569-5.
- Buaya AT, Ploch S, Thines M. 2019a. Rediscovery and phylogenetic placement of Olpidiopsis gillii (de Wildeman) Friedmann, a holocarpic oomycete parasitoid of freshwater diatoms. Mycoscience. 60(3):141–146. doi:https://doi.org/10.1016/j.myc.2019.01.002.
- Buaya AT, Scholz B, Thines M. 2021. Taxonomy and phylogeny of Aphanomycopsis bacillariacearum a holocarpic oomycete parasite of the freshwater diatom genus Pinnularia. Mycological Progress. 20(3):289–298. doi:https://doi.org/10.1007/s11557-021-01668-x.
- Buaya AT, Thines M. 2019b. Miracula Moenusica, a New Member of the Holocarpic Parasitoid Genus from the Invasive Freshwater Diatom Pleurosira Laevis. FUSE. 3(1):19–33. doi:https://doi.org/10.3114/fuse.2019.03.04.
- Buaya AT, Thines M. 2020a. Diatomophthoraceae – a new family of olpidiopsis-like diatom parasitoids largely unrelated to Ectrogella. FUSE. 5(1):113–118. doi:https://doi.org/10.3114/fuse.2020.05.06.
- Buaya AT, Thines M. 2020c. An overview on the biology and phylogeny of the early-diverging oomycetes. Philippine Journal of Systematic Biology. 14(4):1–20.
- Cornu M. 1872. Monographie des Saprolegniees, etude physiologique et systematique. Annales des Sciences Naturelles Botanique. 15:1–198.
- Dick MW. 2001. Straminipilous Fungi. Netherlands: Kluwer.
- Feldmann J, Feldmann G. 1955. Observations sur quelques Phycomycetes marins nouveaux ou peu connus. Revue Mycologique. 20:231–251.
- Friedmann I. 1952. Über neue und wenig bekannte auf Diatomeen parasitierende Phycomyceten. Österreichische botanische Zeitschrift. 99(2–3):173–219. doi:https://doi.org/10.1007/BF01292873.
- Garvetto A, Nézan E, Badis Y, Bilien G, Arce P, Bresnan E, Gachon CMM, Siano R. 2018. Novel widespread marine oomycetes parasitising diatoms, including the toxic genus Pseudo-nitzschia: genetic, morphological, and ecological characterisation. Front Microbiol. 9:2918. doi:https://doi.org/10.3389/fmicb.2018.02918.
- Garvetto A, Perrineau MM, Dressler‐Allame M, Bresnan E, Gachon CM. 2020. “Ectrogella” parasitoids of the diatom Licmophora sp. are polyphyletic. Journal of Eukaryotic Microbiology. 67(1):18–27. doi:https://doi.org/10.1111/jeu.12750.
- Guillard RR, Ryther JH. 1962. Studies of marine planktonic diatoms: i. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can J Microbiol. 8(2):229–239. doi:https://doi.org/10.1139/m62-029.
- Hanic LA, Sekimoto S, Bates SS. 2009. Oomycete and chytrid infections of the marine diatom Pseudo-nitzschia pungens (Bacillariophyceae) from Prince Edward Island, Canada. Canadian Journal of Botany. 87(11):1096–1105. doi:https://doi.org/10.1139/B09-070.
- Karling JS. 1942. The simple holocarpic biflagellate Phycomycetes. New York (USA): Published by Karling JS.
- Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 20(4):1160–1166. doi:https://doi.org/10.1093/bib/bbx108.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 33(7):1870–1874. doi:https://doi.org/10.1093/molbev/msw054.
- Scherffel A. 1925. Endophytische Phycomyceten-Parasiten der Bacillariaceen und einige neue Monadinen Ein Beitrag zur Phylogenie der Oomyceten (Schröter). Archiv für Protistenkunde. 52:1–141.
- Sparrow FK. 1933. Inoperculate chytridiaceous organisms collected in the vicinity of Ithaca, N.Y., with notes on other aquatic fungi. Mycologia. 25(6):513–535. doi:https://doi.org/10.2307/3754109.
- Sparrow FK. 1960. Aquatic Phycomycetes. USA: The University of Michigan Press.
- Sparrow FK, Ellison B. 1949. Olpidiopsis schenkiana and Its Hyperparasite Ectrogella besseyi n. Sp Mycologia. 41(1):28–35. doi:https://doi.org/10.2307/3755270.
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30(9):1312–1313. doi:https://doi.org/10.1093/bioinformatics/btu033.
- Thines M, Aoki T, Crous PW, Hyde KD, Lücking R, Malosso E, et al. 2020. Setting scientific names at all taxonomic ranks in italics facilitates their quick recognition in scientific papers. IMA Fungus. 11(1):1–5. doi:https://doi.org/10.1186/s43008-020-00048-6
- Wang Y, Tian RM, Gao ZM, Bougouffa S, Qian PY. 2014. Optimal eukaryotic 18S and universal 16S/18S ribosomal RNA primers and their application in a study of symbiosis. PLoS ONE. 9(3):e90053. doi:https://doi.org/10.1371/journal.pone.0090053.
- Zopf W. 1878. Über einem neuen parasitischen Phycomyceten. Mitt V Prov Brandenburg. 20:77–80.
- Zopf W. 1884. Zur Kenntniss der Phycomyceten I. Zur Morphologie und Biologie der Ancylisteen und Chytridiaceen. Nov Act Acad Caes Leopoldino-Carolinae Germ Nat Cur. 47:143–236.
