ABSTRACT
Nematode-trapping fungi (NTF) are the majority of carnivorous microbes to capture nematodes through diverse and sophisticated trapping organs derived from hyphae. They can adopt carnivorous lifestyles in addition to saprophytism to obtain extra-nutrition from nematodes. As a special group of fungi, the NTF are not only excellent model organism for studying lifestyle transition of fungi but also natural resources of exploring biological control of nematodes. However, the carnivorous mechanism of NTF remains poorly understood. Nowadays, the omics studies of NTF have provided numerous genes and pathways that are associated with the phenotypes of carnivorous traits, which need molecular tools to verify. Here, we review the development and progress of gene manipulation tools in NTF, including methodology and strategy of transformation, random gene mutagenesis methods and target gene mutagenesis methods. The principle and practical approach for each method was summarized and discussed, and the basic operational flow for each tool was described. This paper offers a clear reference and instruction for researchers who work on NTF as well as other group of fungi.
1. Introduction
Nematode-trapping fungi (NTF) are a kind of fascinating filamentous fungi capable of capturing free-living nematodes by forming sophisticated trapping structures (Yang et al. Citation2012). NTF live mainly as saprobes in soil. When sensing the presence of living nematodes, they adopt carnivorous lifestyle in addition to saprophytism by developing delicate trapping structures to capture free-living nematodes for extra nutrients. The special lifestyle transition of NTF makes them attractive and appealing to biologists who are interested in fungal ecology and evolution (Yang et al. Citation2007). They also receive considerable attentions as biocontrol agents against parasitic nematodes of crops and livestock that can cause devastating economic losses. Nowadays, attempt to improve the virulence of NTF against nematode pests are conducting and biological nematicides containing NTF have been commercialized or are under developing (Abawi and Widmer et al. Citation2000; Dong and Zhang Citation2006; Khan et al. Citation2006; Singh et al. Citation2012; Li et al. Citation2015).
The species of NTF affiliate in different lineages including Zoopagomycota, Ascomycota and Basidiomycota but more than 90% of the species are Ascomycetes. NTF produce various trapping devices which can be divided into 2-dimensional adhesive traps (adhesive knobs, adhesive columns, non-constricting ring), 3-dimensional adhesive trap (adhesive nets) and mechanical trap (constricting rings). Generally, the process of NTF attacking nematodes includes attraction and trap inducing, adhesion and recognition, penetration and nematode consuming (Dijksterhuis et al. Citation1994). In order to understand the mechanisms of lifestyle transition, predation process and virulence of NTF, multiple omics studies have been conducted (), including genomics (Yang et al. Citation2011; Meerupati et al. Citation2013; Liu et al. Citation2014; Youssar et al. Citation2019; Fan et al. Citation2021), transcriptomics (Fekete et al. Citation2008; Andersson et al. Citation2014; Ramesh et al. Citation2015; Pandit et al. Citation2017; Zhang et al. Citation2020), proteomics (Andersson et al. Citation2013; Liang et al. Citation2019) and metabolomics (Wang et al. Citation2018; Kuo et al. Citation2020). Those studies have revealed numerous functional genes, gene families, pathways, proteins and metabolites that involve in growth, sporulation, evolution and predatism. Comparative omics studies are also under progress to uncover the common and specific nature of lifestyle transition as well as the mechanism of predatory process in NTF (Pandit et al. Citation2017; Kuo et al. Citation2020). Thus, molecular tools are prerequisite to verify those gene functions in NTF.
Table 1. Omics studies in NTF.
Compared with bacteria and yeasts, the development of molecular tools in filamentous fungi is confined due to the cell complexity, such as multicellular morphology, thick chitinous cell wall and multinuclear cell. Great effort to establish multiple molecular tools in NTF has been carried out and the versatile CRISPR system has been established in adhesive net-forming NTF Duddingtonia flagrans (Youssar et al. Citation2019) and Arthrobotrys oligospora (Chen et al. Citation2021). Therefore, we summarize the molecular tools that have been established in NTF. This review covers the chassis of genetic manipulation and methodology of transformation, random gene mutagenesis methods and target gene mutagenesis methods, and aims to offer comprehensive understanding and practical strategy for NTF researches.
2. The chassis of genetic manipulation
Genetic manipulation serves the purpose of providing a better understanding of gene function as well as constructing novel mutant. It is achieved by selective breeding traditionally but directly DNA manipulation nowadays. The methods to modify DNA and introduce DNA to the host are two prerequisites. Before that, promoters, selection markers, and fluorescence indicators are fundamental elements needed to be considered for further genetic manipulation.
2.1. Promoters
The choice of promoter is a critical step in initiating gene transcription and optimizing the efficiency and stability of recombinant protein production (Adnan et al. Citation2022). There are three types of promoters in filamentous fungi, e.g. the RNA polymerase I, II, and III promoters. Among them, the RNA polymerase II promoters contain constitutive and inducible types, and are extensively applied in genetic manipulation. Up to now, the constitutive promoter used in NTF contains heterologous ones and endogenous ones.
Heterologous promoters used in NTF include glyceraldehyde 3-phosphate dehydrogenase (gpdA) promoter, oliC promoter and tryptophan biosynthesis gene (trpC) promoter from A. nidulans, cpc-1 promoter from Neurospora crassa (Tunlid et al. Citation1999), o2tef promoter from Ustilago maydis (Youssar et al. Citation2019). Among them, the trpC promoter from A. nidulans was most commonly used in NTF. ().
Table 2. Promoters used in NTF.
The published cases about endogenous promoters used in NTF were only reported in D. flagrans. The first case is that the native promoters of tubA, sipC and H2B from D. flagrans were used to express fusion fluorescent proteins endogenously to observe the location and expression levels of the target proteins (Youssar et al. Citation2019). For example, the natural promoter of H2B was amplified together with its coding sequence by PCR using D. flagrans genomic DNA as template, producing a 1.6 kb fragment including the ORF of DFL_000203 (0.6 kb without stop codon), + 1 kb of the 5’ region and a 1 kb fragment 3’ downstream of the locus as reported in Youssar et al.’ s study. Another case is also operated in D. flagrans, the three promoters (artA promoter: 1,407 bp, artB promoter: 1,141 bp, artC promoter: 1,567 bp) were fused with the H2B gene and mCherry respectively to monitor the expression of the artA-C genes in subcellular spatiotemporal dynamics (Yu et al. Citation2021). The endogenous promoters are preferred choice if researchers want to observe the expression levels of endogenous genes by expressing fusion fluorescent proteins.
Both heterologous promoters and endogenous promoters can be good choices if they drive the expression of target genes efficiently in host cells. By now, there is lack of studies in NTF about the inducible promoters which can be used for temporally adjustable expression of target genes and RNA polymerase III promoters which can be used for fine-tuned expression of small RNAs to regulate cellular conditions. Although the constitutive promoter can meet the demand in most cases, new kinds of promoters should be explored and applied for the NTF studies in future.
2.2. Selection markers
After introducing constructed DNA into host cells, selection markers help select the positive transformants effectively. The commonly used selection markers in filamentous fungi can be divided into the antibiotic resistance markers (e.g. hygromycin-B, benomyl and oligomycin), the auxotrophic markers (e.g. uracil auxotrophic markers and lysine auxotrophic markers), and the visual distinction markers (e.g. Gus and LacZ) (Wang et al. Citation2017). Among them, the antibiotic resistance makers including hygromycin B and geneticin (G418) have been established in the NTF ().
Table 3. Selection markers used in NTF.
The principle of hygromycin-B as selection marker is to inhibit protein synthesis by interfering with 70S ribosome translocation and inducing misreading of mRNA templates (Borovinskaya et al. Citation2008). Likewise, the geneticin (G418) also inhibits protein synthesis through binding with 80S ribosome (Prokhorova et al. Citation2017). Optimal concentration of antibiotics is important for transformant screening. Low concentrations lead to high false positive rate and labour-consuming process, while high concentration results in reagent waste and longer selection process. The optimal concentration of antibiotics is not only species-dependent, but also strain-dependent. For example, hygromycin-B concentration was 200 µg/ml for A. oligospora ATCC 24927 (Zhen et al. Citation2018), while 100 μg/mL for A. oligospora TWF154 (Yang et al. Citation2020). Thus, optimal antibiotics concentrations should be determined when new strain is applied. To do such experiment, the professional way is to transfer hypha fragments and/or spores (not fungus culture discs) without substrate or medium by sterilized toothpicks to the agar plate (24 well tissue culture plate is recommended) containing a series of concentrations of the antibiotics.
Gus from E. coli, a visible report marker, was also applied in A. oligospora under the control of gpdA promoter (Tunlid et al. Citation1999). Adding the substrate X-Gluc (5-bromo-4-chloro-3-indyol-β-glucuronide) at a concentration of 50 μg/mL into the selection medium, the positive transformants show blue appearance on agar plate (Tunlid et al. Citation1999). The difference of visible appearance between the wide type and transformants significantly shortened the selection process. However, further genetic confirmation should be conducted.
Some fungal strains have selection difficulties using antibiotic resistance markers because loss of inhibition after continuous culture or the extremely high resistance to the antibiotics. The auxotrophic selection markers can be an alternative. However, it seems that the two kinds of commonly used auxotrophic markers are inapplicable in NTF since urea metabolism and amino acid metabolism are essential for their lifestyle transition (Dijksterhuis et al. Citation1994; Wang et al. Citation2014). New auxotrophic markers that are capable of selecting transformants effectively without interfering the predation process and lifestyle transition in NTF should be explored in the future. Besides, new and various selection markers also provide possible choice for multiple gene deletions in NTF.
2.3. Fluorescence indicators
To illustrate a protein function, monitoring its subcellular spatiotemporal localization is required. The fluorescence indicators provide powerful tools to link to the target genes which can directly exhibit the location of target proteins during the biological process under a fluorescence microscope.
Fluorescence indicators including GFP (green fluorescent protein), RFP (red fluorescent protein), YFP (yellow fluorescent protein) and mCherry have been widely applied in filamentous fungi. provides information about fluorescence indicators used in NTF. The first report to apply the fluorescent proteins in NTF is for adhesive network-forming D. flagrans and genes encoding GFP and mCherry were tested with gpdA promoter from A. nidulans (Youssar et al. Citation2019). Although genes encoding GFP and mCherry have been extensively applied in N. crassa, A. nidulans, U. maydis, C. albicans, and Botrytis cinerea, only that applied in B. cinerea was successfully expressed in D. flagrans (Youssar et al. Citation2019). Both mCherry and GFP genes were successfully fused to the target gene histone H2B at C-terminus to visualize nuclei in living cells (Youssar et al. Citation2019) and the mCherry gene was tagged with the target gene TubA at N-terminus in D. flagrans (Wernet et al. Citation2022). Thus, either C or N terminus of target genes can be tagged with the fluorescence indicators to effectively monitor the location and expression levels of target proteins. Obviously, application of fluorescence indicators to visualize the expression of target proteins in vivo without disrupting cells is a good choice since there was no fluorescence background detected in NTF so far.
3. Transformation methods
Transformation method helps deliver or introduce foreign nucleic acid or proteins into hosts. Several transformation methods have been applied in filamentous fungi, including protoplast-mediated transformation, Agrobacterium tumefaciens-mediated transformation (ATMT), electroporation, biolistic bombardment, and shock wave-mediated transformation (Li et al. Citation2017). Up to now, only protoplast transformation method and ATMT method have been established in NTF.
3.1. Polyethylene glycol (PEG) mediated protoplast transformation method
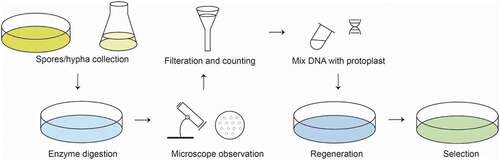
PEG mediated protoplast transformation method can deliver exogenous nucleic acids into protoplast cells due to the change of cell membrane permeability with help of PEG (De Filippis et al. Citation2000). Protoplast transformation method was first applied in Saccharomyces cerevisiae (Hutchison et al. Citation1967), then expanded rapidly to other filamentous fungi, such as Neurospora crassa (Case et al. Citation1979) and A. nidulans (Tilburn et al. Citation1983). The protoplast transformation with CaCl2/PEG treatment was first developed in A. oligospora in 1999 (Tunlid et al. Citation1999), then gradually established in other hematophagous fungus including Monacrosporium sphaeroides (Jin et al. Citation2005) and Monacrosporium haptotylum (Zhen et al. Citation2018). The brief procedure of PEG-mediated protoplast transformation for NTF is summarized as following. ().
Procedure
Spores/hypha culture and collection
Fungus is incubated on agar plates for conidia production (normally 1 week – 4 weeks) depending on the conidiation ability of different fungi. Conidia and hypha fragments are washed from agar plates.
Hypha harvest and enzyme digestion
Circa 108 conidia were then incubated in Gamborg’s B-5 basal medium (Sigma) in an Eppendorf tube on a rotary shaker (130 r/min –180 r/min) at 24 °C – 28 °C for 36 h – 48 h. The fresh mycelia are harvested on membrane filters (hydrophilic Durapore filters, Millipore) and washed with MM solution, then suspended into enzymes solution (0.5 mL – 10 mL) containing 5 mg/mL snailase, 5 mg/mL cellulase or 15 mg/mL of Glucanex for cell wall digestion to release protoplasts on shaker at 28 °C for 3 h – 12 h.
Filtration and counting
The protoplasts are passed through a plug of glass wool fitted in a funnel to remove undigested mycelium, then collected by centrifugation at 6,000 r/min for 10 min at 4 °C. After washed with 500 μL KTC or MTC buffer, the protoplasts are resuspended in 100 μL KTC and used for transformation immediately.
Mix with DNA
Circa 107 protoplasts (ca. 100 μL – 500 μL) are mixed with 10 µg of vector DNA and incubated on ice for 30 min.
Regeneration
Equal or double volumes of PTC are added and mixed gently. The mixture is incubated at 28 °C for 10 min – 60 min for the regeneration of protoplasts.
Selection
200 µL – 300 µL of the mixture is then added to 10 mL OCM medium (2% agar), and poured into Petri dishes. The plates are incubated overnight at room temperature, after which they were overlaid with 10 mL OCM medium (1% agar) containing antibiotics to select for the growth of transformants.
Reagents and media
MM medium
20 mmol/L 2-(N-morpholino) ethanol-sulfonic acid (MES), pH 5.8, 1 mol/L MgSO4.
KTC buffer
1.2 mol/L KCl, 10 mmol/L Tris–HCl, pH 7.5, 50 mmol/L CaCl2.
MTC medium
10 mmol/L Tris-HCl, pH 7.5, 10 mmol/LCaCl2, 1 mol/L MgSO4.
PTC buffer
10 mmol/L Tris–HCl, pH 7.5, 50 mmol/L CaC12, 50% w/v PEG6000 (10 mmol/L CaC12, 20% w/v PEG4000 was used in another version).
OCM medium (/L)
1 g yeast extract, 1 g mycological peptone; 1 g casein hydrolysate, 10 g glucose, 273.8 g sucrose, 0.5 g KCl, 0.5 g MgSO4•7H2O, 1.5 g KH2PO4, 0.8 mg MnSO4•4H2O, 0.8 mg FeCl2•4H2O, 8.0 mg ZnSO4•7H2O, 0.8 mg Na2MoO4•2H2O, 0.4 mg CuSO4•5H2O, 0.04 mg sodium tetraborate, 0.10 mg nicotinic acid, 0.05 mg thiamine, 0.25 mg pyridoxine, 0.40 mg myoinositol, 0.40 mg p-aminobenzoic acid, 0.20 mg calcium pantothenate, 0.10 mg riboflavin, 1.40 mg choline chloride, 0.01 mg biotin. The pH was set to 6.5.
Note: Normally, protoplasts should be placed on ice and the transformation process should be carried out at low temperature. Protoplast transformation method is common and effective for filamentous fungi; however, the process of protoplast transformation method involves many critical reagents. Enzymes used for digesting cell walls and regeneration conditions are needed to be optimized.
3.2. Agrobacterium tumefaciens mediated transformation (ATMT)
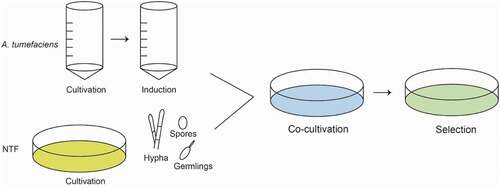
Foreign DNA was delivered into the genome of host cells through the binary plasmid of A. tumefaciens in ATMT. Naturally, A. tumefaciens can infect the root of injured plants through the tumor-inducing plasmid (Ti plasmid). (Van Larebeke et al. Citation1974) Furtherly, scientists optimize the Ti plasmid to two plasmids, one plasmid conserving the tumor-inducing ability, the other plasmid carrying the T-DNA insertion fragment between the left and right borders. (Gordon et al. Citation2014) Like PEG-mediated protoplast transformation, the ATMT method was also first applied in S. cerevisiae (Bundock et al. Citation1995), and then in many other filamentous fungi (de Groot et al. Citation1998; Gouka et al. Citation1999; Campoy et al. Citation2003). Nowadays, three NTF species e.g. Arthrobotrys conoides, A. oligospora (Nourani et al. Citation2018) and Drechslerella dactyloides (Fan et al. Citation2021) were reported to be successfully established with ATMT. The brief procedure of ATMT for NTF is summarized as follows. ().
Procedure
NTF preparation
Conidia or hyphae are inoculated on agar plates or in liquid medium and incubated for 2 weeks –3 weeks. The conidia are washed from agar plates and then resuspended in an Eppendorf tube. The mycelia in liquid culture are harvested through membrane filters (Miracloth) and washed with sterile water, then homogenized in 1.5 mL centrifuge tube with tissue grinder.
A. tumefaciens cultivation
The A. tumefaciens is inoculated on agar plates of YEP containing selection pressure and incubated at 28 °C for 24 h. The individual colony is transferred to 15 mL – 50 mL centrifuge tube or flask with minimal medium (MM) containing selection pressure and incubated at 28 °C for 24 h – 48 h under constant shaking at 100 r/min.
A. tumefaciens induction
One ml of the above culture mixture is transferred to induction medium (IM) containing selection pressure and acetosyringone and kept at 28 °C on shaker (120 r/min) for 3 h – 6 h to reach OD600 to 0.2 – 0.4.
Co-cultivation
The collected conidia or homogenized mycelia of fungus and the A. tumefaciens culture in the IM are mixed. The mixture is inoculated and smeared with sterilized glass balls on nitrocellulose membrane pre-placed on IM plates for co-cultivation at 26 °C.
Selection
The nitrocellulose membrane with co-cultures of fungus and A. tumefaciens is transferred onto PDA plate containing selection pressures and co-cultured at 26 °C for two weeks. Putative transformants (colonies) are transferred to fresh selective agar plates for further PCR verification and phenotype assay.
Reagents and media
YEP medium
Yeast extract peptone media (Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
MM medium
10 mmol/L K2HPO4, 10 mmol/L KH2PO4, 2.5 mmol/L NaCl, 2 mmol/L MgSO4 · 7H2O, 0.7 mmol/L CaCl2, 9 μmol/L FeSO4 · 7H2O, 4 mmol/L (NH4)2SO4, 10 mmol/L glucose, pH 7.0.
IM medium
MM containing 0.5% (wt/vol) glycerol, 200 μmol/L acetosyringone, 40 mmol/L 2-(N-morpholino)-ethanesulfonic acid (MES), pH 5.3.
PDA
Potato dextrose agar (Difco, Detroit, Mich).
Mitotic stability test
A piece of mycelial from the colony edges of transformants are serially transferred five times at one-week intervals on PDA plate with hygromycin-B. Then the colony diameters are measured at the day 4 and 10 respectively.
Note: The ATMT is a stable transformation method and can transform diverse samples, such as spores and mycelial fragments. Since the T-DNA fragment is randomly integrate into the genome of the host cells, the ATMT is also used as a random gene mutagenesis method. According to previous studies, ectopic integration of single-copy T-DNA into non-coding region is usually observed. Factors influencing the ATMT efficiency include the type of fungal material (spores, hypha or germlings) and A. tumefaciens strains, the concentration of acetosyringone, the ratio of fungi to A. tumeaciens and the co-cultivation conditions. The A. tumefaciens strains and binary plasmids used in NTF were summarized in . Other influence factors need to be considered and optimized when ATMT efficiency is not high.
Table 4. Agrobacterium tumefaciens strains and binary plasmids used in NTF.
4. Random gene mutagenesis methods
Random gene mutagenesis offers a good choice to screen genes related to certain phenotypic properties. The mutants are generated randomly by insertion a foreign DNA or by the movement of mobile genetic elements. The commonly used methods for random gene mutagenesis in filamentous fungi include ATMT, restriction enzyme-mediated integration (REMI) and transposon-arrayed gene knockout (TAGKO) (Wang et al. Citation2017). The REMI was first established in S. cerevisiae (Schiestl et al. Citation1991) and also successfully applied in NTF (Tunlid et al. Citation1999). The REMI method originates from protoplast transformation, but it increases transformation frequency by delivering enzymes that can cut genomic DNA to provide integration site for the linear DNA fragment that is digested with the same enzymes.
The REMI method for random gene mutation applied in NTF was established in A. oligospora in 1999 (Tunlid et al. Citation1999) and 13 transformants were achieved and 7 of them were single copy integrated. Subsequently, this method was applied in M. sphaeroides and five mitotic stable mutants were obtained, three of which exhibited integration at a single location (Jin et al. Citation2005). Recently, this method has been applied in A. oligospora to identify novel genes related to trap formation, conidiation and virulence, 37 random-insertional mutants were screened (Jiang et al. Citation2017). Here, the brief procedures of REMI for NTF were summarized as follows.
Procedure
Protoplast preparation
Protoplast suspension can be prepared following the previous description and ca. 1 × 107 – 108 protoplasts/mL can be generated and used.
Enzyme digestion of plasmid
Recombinant plasmid such as pBChygro containing selection marker gene (hph) and restriction enzyme (including XbaI, KpnI, SmaI, XmaI, SacI, SalI and HindIII) sites is digested with the selected enzymes (HindIII). The enzyme digestion system is prepared according to the manufacturers’ instructions.
Protoplast transformation
The rate of endonuclease-induced damage to protoplasts is determined for suitable concentration (normally 30 U). For transformation, 100 μL protoplasts (ca. 8.0 × 107) is mixed with the restriction enzyme (HindIII), then10 μg of the above linear vector DNA is added into the mixture. Incubate the whole transformation mixture for 30 min on ice. Then 250 μL of PTC are added and mixed gently.
Transformants selection
After incubation at 28 oC for 20 min – 30 min, 100 μL of the protoplast mixture is added to 10 mL of PDSSA medium containing 100 μg/mL ~ 400 μg/mL of hygromycin-B (previously kept at 48 °C), then poured onto Petri dishes. The plates are incubated at 28 oC for transformants growth.
PDSSA medium
PDA supplying with 0.6 mol/L sucrose, 0.3 g/L yeast extract, 0.3 g/L tryptone, 0.3 g/L peptone.
Note: The transformation frequency of the REMI is affected by the concentration of restriction enzyme and PEG. The highest transformation frequency of 175 transformants/μg of DNA was obtained (the concentration of PEG3350 was 20%) when 30 units HindIII per transformation reaction was used (Xu et al. Citation2005). There was no obvious difference of transformation frequency when PEG3350, PEG4000 or PEG6000 were applied (Xu et al. Citation2005). Another advantage of REMI is that the disrupted genes can be obtained by plasmid rescue in E. coli, which is more efficient for subsequent function studies.
Except molecular tools, gene mutagenesis can be induced by physical mutagens or chemical treatment. The ethyl methane sulfonate and UV as the mutagens were applied in NTF to identify potential genes and pathways involved in trap morphogenesis and predation in A. oligospora, and 15 mutants with strong defects in trap morphogenesis were identified from 5,552 randomly mutagenized A. oligospora mutants (Huang et al. Citation2021). Although the process of screening is labor-consuming and tedious, physical or chemical mutagens are easily to apply and screen multiple potential genes for certain physiological features without conducting genetic manipulation.
5. Target gene mutagenesis methods
5.1. Homologous recombination (HR)
HR is a common strategy for deleting target genes in genetic manipulation. The principle is based on the HR pathway of DNA double strands breaks repairing (DSBR) by delivering foreign DNA sequences flanked by homologous fragments (Jasin and Haber Citation2016). A number of target genes in NTF have been knocked out through HR approach. The homologous sequence length used in NTF ranges from 1.3k bps to 2.5k bps, but the most frequently used length is around 2k bps ().
Table 5. Gene deletion in NTF through HR.
Usually, expression cassette of the gene sequence encoding selection marker locates between the forward and downstream homologous fragments. In a special case, the author not only insert the selection marker expression cassette between the homologous fragments, but also smartly altered the start codon ATG to a stop codon TAG in the forward fragment sequence using Gene Editor mutagenesis kit (Balogh et al. Citation2003). Total 15 transformants were screened through PCR assay from two hundred mutants and 5 mutants were identified to contain the target integrated fragments by Southern blot analysis (Balogh et al. Citation2003). This strategy provides double insurance for target gene knockout.
HR strategy is a good choice for target gene deletion, but the deletion rate is depending on the HR rate of the host. In mammalian cells, non-homologous end joining (NHEJ) pathway is dominant over the HR-pathway when DNA double strands breaks happen (Jasin and Haber Citation2016). Thus, in order to improve the HR rate to further increase the gene deletion efficiency, researchers usually delete genes essential in NHEJ pathway, e.g. ku70 and ku80. It is reported that the HR rate in A. oligospora was extremely low (~3%) (Kuo et al. Citation2020). A NHEJ deficient A. oligospora mutant generated by deleting ku70 showed no phenotypic difference from the wide type but improved HR rate (Kuo et al. Citation2020). The HR strategy is still the first choice for gene manipulation in NTF although it is difficult to delete multiple genes.
5.2. CRISPR technique
The CRISPR is abbreviation of the clustered regularly interspaced short palindromic repeats. The type II CRISPR/CRISPR-associated gene (Cas) system is the most used genome-editing tool currently (Lanigan et al. Citation2020). The principle to disrupt target genes through CRISPR technique is using RNA-guided nuclease to nick DNA double strand breaks at target gene composed of a 20 bp sequence matching the guide RNA (gRNA) and an adjacent downstream 5’-NGG nucleotide sequence (protospacer-adjacent motif (PAM) (Nødvig et al. Citation2015). The CRISPR technique is originated from an immune defence system for foreign DNA invasion in bacteria and archaebacteria. In filamentous fungi, it was first established in Trichoderma reesei (Liu et al. Citation2015).
CRISPR technique was applied in NTF since 2019 in Duddingtonia flagrans (Youssar et al. Citation2019). After attempt was failed with the plasmid-based approach, the Cas9 ribonucleoproteins (RNPs) was applied with tested simultaneous transformation of the linear resistance gene hph and the repair template. Total four transformants were obtained and the resistance gene hph was successfully inserted into the cleavage site of three of the four transformants. Then, the RNP form of CRISPR system was also applied in A. oligospora (Chen et al. Citation2021). The basic procedures for RNP form of CRISPR in NTF are as follows.
Procedure
sgRNA design
The sgRNA was designed using online website (http://crispr.mit.edu). To avoid off-target cleavage, the uniqueness of the protospacer region and the protospacer adjacent motif (PAM) sequence should be analyzed in the host genome using BLASTN.
sgRNA synthesis
The sgRNA for the target gene mutation can be synthesized using the EnGen sgRNA Synthesis Kit (New England Biolabs, Frankfurt).
Ribonucleoprotein formation
RNP formation is carried out at 37 °C for 20 min with 4 μL sgRNA, 10 μL Cas9 (200 nmol/L), 5 μL 10x Cas9 Nuclease Reaction Buffer and DEPC-treated water in 50 μL total volume.
Linear resistance gene amplification
The linear resistance gene is amplified by PCR, subsequently purified using FastGene Gel/PCR Extraction kit (Nippon Genetics) and lastly about 2 μg purified PCR-product is added to the transformation-mix.
Protoplast preparation
The protoplast of NTF is prepared as described above (Xu et al. Citation2005).
Protoplast transformation
100 μL protoplasts (ca. 5 × 106) are mixed with 5 μg – 8 μg of DNA and incubated for 2 min on ice. Then 1 mL of PTC is added and incubated at room temperature for 20 min. 10 mL PDSSA medium is added to the transformation mix and poured onto PDA plates supplemented with corresponding antibiotics and the plates incubate at 28 °C for 4 – 7 days.
The mentioned reagents and media in the procedure are covered in previous description (Youssar et al. Citation2019).
CRISPR technology is a powerful and versatile tool of gene editing and can manipulate multiple genes at the same time. However, off target effect and cell damage effect in CRISPR limit its application to some extent (Haapaniemi et al. Citation2018; Ihry et al. Citation2018; Manghwar et al. Citation2020). RNP and plasmid are the two kinds of strategies for delivering the CRISPR system. RNP strategy extremely depends on the protoplast transformation method, while the plasmid strategy is still not established in NTF yet. The sgRNA array construction strategies and Cas proteins choice are two factors to be considered. Currently, CRISPR is only used for target gene mutagenesis in NTF and extensive application of CRISPR technique should be exploited in future.
When gene deletion is vital to host cells, RNA interference (RNAi) technology is a pivotal alternative to investigate the function of target gene. Instead of changing DNA sequence to eliminate or impair the function of target genes, RNAi repress the transcription of target genes by triggering gene-silencing mechanism in the host to degrade the target mRNA. Although it has been successfully applied in many filamentous fungi (Zheng et al. Citation1998; Ngiam et al. Citation2000; Kadotani et al. Citation2003; Janus et al. Citation2007), but still not be extensively applied in NTF. RNAi approach is just established for a knob-forming NTF in our lab. Knockdown of the transcription of DhFIG_2, a component of NTF-specific low-affinity calcium uptake system in knob-forming Dactylellina haptotyla by hairpin RNA forming binary plasmid together with ATMT significantly decreased its conidiation and knob formation (Zhao et al. Citation2023). RNAi will offer a good genetic manipulation option in NTF when target genes are indispensable.
6. Summaries and perspectives
Nematode-trapping fungi are the important enemies to regulate nematode dynamics in nature. As fascinating creatures with dual lifestyles of saprophytism and carnivorism and sophisticated trapping devices, NTF is attracting researchers to study the mystery of their lifestyle, trapping mechanisms and morphogenesis. Genetic manipulation is an essential and basic tool for NTF studies. Here we summarized the current development and application of gene manipulation tools and basic procedures in NTF, which should provide crucial methodological reference to promote the NTF studies. However, other molecular tools should be established in NTF in the near future, such as inducible promoters, auxotrophic markers, the transposon-arrayed gene knockout, the plasmid form CRISPR, as well as the RNA interference strategies. We believe those tools are under establishing by mycologists who work on NTF.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Abawi GS, Widmer TL. 2000. Impact of soil health management practices on soilborne pathogens, nematodes and root diseases of vegetable crops. Appl Soil Ecol. 15(1):37–47. doi:10.1016/S0929-1393(00)00070-6.
- Adnan M, Ma X, Olsson S, Wang J, Liu G. 2022. Promoter regulation and genetic engineering strategies for enhanced cellulase expression in Trichoderma reesei. Microbiol Res. 259:127011. doi:10.1016/j.micres.2022.127011.
- Andersson KM, Kumar D, Bentzer J, Friman E, Ahrén D, Tunlid A. 2014. Interspecific and host-related gene expression patterns in nematode-trapping fungi. BMC Genomics. 15(1):968. doi:10.1186/1471-2164-15-968.
- Andersson KM, Meerupati T, Levander F, Friman E, Ahren D, Tunlid A. 2013. Proteome of the nematode-trapping cells of the fungus Monacrosporium haptotylum. Appl Environ Microbiol. 79(16):4993–5004. doi:10.1128/AEM.01390-13.
- Bai N, Zhang GS, Wang WJ, Feng HH, Yang XW, Zheng YQ, Yang L, Xie MH, Zhang KQ, Yang JK. 2021. Ric8 acts as a regulator of G-protein signalling required for nematode-trapping lifecycle of Arthrobotrys oligospor. Environ Microbiol. 24(4):1714–1730. doi:10.1111/1462-2920.15735.
- Balogh J, Tunlid A, Rosén S. 2003. Deletion of a lectin gene does not affect the phenotype of the nematode-trapping fungus Arthrobotrys oligospora. Fungal Genet Biol. 39(2):135. doi:10.1016/s1087-1845(03)00023-9.
- Borovinskaya MA, Shoji S, Fredrick K, Cate JH. 2008. Structural basis for hygromycin B inhibition of protein biosynthesis. RNA. 14(8):1590–1599. doi:10.1261/rna.1076908.
- Bundock P, den Dulk-Ras A, Beijersbergen A, Hooykaas PJ. 1995. Trans-kingdom T-DNA transfer from Agrobacterium tumefaciens to Saccharomyces cerevisiae. EMBO J. 14(13):3206–3214. doi:10.1002/j.1460-2075.1995.tb07323.x.
- Campoy S, Pérez F, Martín JF, Gutiérrez S, Liras P. 2003. Stable transformants of the azaphilone pigment-producing Monascus purpureus obtained by protoplast transformation and Agrobacterium-mediated DNA transfer. Curr Genet. 43(6):447–452. doi:10.1007/s00294-003-0417-0.
- Case ME, Schweizer M, Kushner SR, Giles NH. 1979. Efficient transformation of Neurospora crassa by utilizing hybrid plasmid DNA. Proc Natl Acad Sci U S A. 76(10):5259–5263. doi:10.1073/pnas.76.10.5259.
- Chen YL, Gao Y, Zhang KQ, Zou GG. 2013. Autophagy is required for trap formation in the nematode-trapping fungus Arthrobotrys oligospora. Env Microbiol Rep. 5(4):511–517. doi:10.1111/1758-2229.12054.
- Chen SA, Lin HC, Schroeder FC, Hsueh YP. 2021. Prey sensing and response in a nematode-trapping fungus is governed by the MAPK pheromone response pathway. Genetics. 217(2):iyaa008. doi:10.1093/genetics/iyaa008.
- Chen YH, Liu X, Dai R, Ou X, Xu ZF, Zhang KQ, Niu XM. 2020. Novel polyketide-terpenoid hybrid metabolites and increased fungal nematocidal ability by disruption of genes 277 and 279 in nematode-trapping fungus Arthrobotrys oligospora. J Agric Food Chem. 68(30):7870–7879. doi:10.1021/acs.jafc.0c01720.
- De Filippis LF, Hampp R, Ziegler H. 2000. Membrane permeability changes and ultrastructural abnormalities observed during protoplast fusion. J Plant Physiol. 156(5–6):628–634. doi:10.1016/s0176-1617(00)80223-0.
- de Groot MJ, Bundock P, Hooykaas PJ, Beijersbergen AG. 1998. Agrobacterium tumefaciens-mediated transformation of filamentous fungi. Nat Biotechnol. 16(9):839–842. doi:10.1038/nbt0998-839.
- Dijksterhuis J, Veenhuis M, Harder W, Nordbring-Hertz B. 1994. Nematophagous fungi: physiological aspects and structure-function relationships. Adv Microb Physiol. 36: 111–143. Record ID: 865208.
- Dong LQ, Zhang KQ. 2006. Microbial control of plant-parasitic nematodes: a five-party interaction. Plant Soil. 288(1–2):31–45. doi:10.1007/s11104-006-9009-3.
- Fan YN, Zhang WW, Chen Y, Xiang MC, Liu XZ. 2021. DdaSTE12 is involved in trap formation, ring inflation, conidiation, and vegetative growth in the nematode-trapping fungus Drechslerella dactyloides. Appl Microbiol Biotechnol. 105(19):7379–7393. doi:10.1007/s00253-021-11455-z.
- Fekete C, Tholander M, Rajashekar B, Ahrén D, Friman E, Johansson T, Tunlid A. 2008. Paralysis of nematodes: shifts in the transcriptome of the nematode-trapping fungus Monacrosporium haptotylum during infection of Caenorhabditis elegans. Environ Microbiol. 10(2):364–375. doi:10.1111/j.1462-2920.2007.01457.x.
- Gordon JE, Christie PJ. 2014. The Agrobacterium Ti Plasmids. Microbiol Spectr. 2(6):6. doi:10.1128/microbiolspec.
- Gouka RJ, Gerk C, Hooykaas PJ, Bundock P, Musters W, Verrips CT, de Groot MJ. 1999. Transformation of Aspergillus awamori by Agrobacterium tumefaciens-mediated homologous recombination. Nat Biotechnol. 17(6):598–601. doi:10.1038/9915.
- Haapaniemi E, Botla S, Persson J, Schmierer B, Taipale J. 2018. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat Med. 24(7):927–930. doi:10.1038/s41591-018-0049-z.
- Huang TY, Lee YY, Vidal-Diez de Ulzurrun G, Hsueh YP. 2021. Forward genetic screens identified mutants with defects in trap morphogenesis in the nematode-trapping fungus Arthrobotrys oligospora. G3-Genes Genom Genet. 11(2):jkaa022. doi:10.1093/g3journal/jkaa022.
- Hutchison HT, Hartwell LH. 1967. Macromolecule synthesis in yeast spheroplasts. J Bacteriol. 94(5):1697–1705. doi:10.1128/jb.94.5.1697-1705.1967.
- Ihry RJ, Worringer KA, Salick MR, Frias E, Ho D, Theriault K, Kommineni S, Chen J, Sondey M, Ye C, et al. 2018. p53 inhibits CRISPR-Cas9 engineering in human pluripotent stem cells. Nat Med. 24(7):939–946. doi:10.1038/s41591-018-0050-6.
- Janus D, Hoff B, Hofmann E, Kück U. 2007. An efficient fungal RNA-silencing system using the DsRed reporter gene. Appl Environ Microbiol. 73(3):962–970. doi:10.1128/AEM.02127-06.
- Jasin M, Haber JE. 2016. The democratization of gene editing: insights from site-specific cleavage and double-strand break repair. DNA Repair (Amst). 44:6–16. doi:10.1016/j.dnarep.2016.05.001
- Jiang KX, Liu QQ, Bai N, Zhu MC, Zhang KQ, Yang JK. 2022. Aossk1, a response regulator required for mycelial growth and development, stress responses, trap formation, and the secondary metabolism in Arthrobotrys oligospora. J Fungi. 8(3):260. doi:10.3390/jof8030260.
- Jiang DW, Zhou J, Bai GZ, Xing XJ, Tang LY, Yang XW, Li J, Zhang KQ, Yang JK. 2017. Random mutagenesis analysis and identification of a novel C2H2-type transcription factor from the nematode-trapping fungus Arthrobotrys oligospora. Sci Rep. 7(1):5640. doi:10.1038/s41598-017-06075-5.
- Ji XL, Li H, Zhang WH, Wang JA, Liang LM, Zou CG, Yu ZF, Liu SQ, Zhang KQ. 2020. The lifestyle transition of Arthrobotrys oligospora is mediated by microRNA-like RNAs. Sci China-Life Sci. 63(4):543–551. doi:10.1007/s11427-018-9437-7.
- Jin X, Mo MH, Wei Z, Huang XW, Zhang KQ. 2005. Transformation and mutagenesis of the nematode-trapping fungus Monacrosporium sphaeroides by restriction enzyme-mediated integration (REMI). J Microbiol. 43(5):417–423. PMID: 16273033.
- Kadotani N, Nakayashiki H, Tosa Y, Mayama S. 2003. RNA silencing in the phytopathogenic fungus Magnaporthe oryzae. Mol Plant Microbe Interact. 16(9):769–776. doi:10.1094/MPMI.2003.16.9.769.
- Khan A, Williams KL, Nevalainen HKM. 2006. Control of plant-parasitic nematodes by Paecilomyces lilacinus and Monacrosporium lysipagum in pot trials. Biocontrol. 51(5):643–658. doi:10.1007/s10526-005-4241-y.
- Kuo CY, Chen SA, Hsueh YP. 2020. The high osmolarity glycerol (HOG) pathway functions in osmosensing, trap morphogenesis and conidiation of the nematode-trapping fungus Arthrobotrys oligospora. J Fungi. 6(4):191. doi:10.3390/jof6040191.
- Kuo TH, Yang CT, Chang HY, Hsueh YP, Hsu CC. 2020. Nematode-trapping fungi produce diverse metabolites during predator-prey interaction. Metabolites. 10(3):117. doi:10.3390/metabo10030117.
- Lanigan TM, Kopera HC, Saunders TL. 2020. Principles of genetic engineering. Genes (Basel). 11(3):291. doi:10.3390/genes11030291.
- Liang M, Du S, Dong WI, Fu JT, Li ZH, Qiao YD, Yin XJ, Nie FG, Yang XY, Wang R. 2019. iTRAQ-based quantitative proteomic analysis of mycelium in different predation periods in nematode trapping fungus Duddingtonia flagrans. Biol Control. 134:63–71. doi:10.1016/j.biocontrol.2019.04.005
- Liang LM, Gao H, Li JZ, Liu L, Liu ZH, Zhang KQ. 2017. The woronin body in the nematophagous fungus Arthrobotrys oligospora is essential for trap formation and efficient pathogenesis. Fungal Biol. 121(1):11–20. doi:10.1016/j.funbio.2016.08.010.
- Liang LM, Liu ZH, Liu L, Li JZ, Gao H, Yang JK, Zhang KQ. 2016. The nitrate assimilation pathway is involved in the trap formation of Arthrobotrys oligospora, a nematode-trapping fungus. Fungal Genet Biol. 92:33–39. doi:10.1016/j.fgb.2016.05.003
- Liang LM, Shen RF, Mo YY, Yang JK, Ji XL, Zhang KQ. 2015. A proposed adhesin AoMad1 helps nematode-trapping fungus Arthrobotrys oligospora recognizing host signals for life-style switching. Fungal Genet Biol. 81:172–181. doi:10.1016/j.fgb.2015.02.012
- Li X, Kang YQ, Luo YL, Zhang KQ, Zou CG, Liang LM. 2017b. The NADPH oxidase AoNoxA in Arthrobotrys oligospora functions as an initial factor in the infection of Caenorhabditis elegans. J Microbiol. 55(11):885–891. doi:10.1007/s12275-017-7169-x.
- Li DD, Tang Y, Lin J, Cai WW. 2017. Methods for genetic transformation of filamentous fungi. Microb Cell Fact. 16(1):168. doi:10.1186/s12934-017-0785-7.
- Liu R, Chen L, Jiang YP, Zhou ZH, Zou G. 2015. Efficient genome editing in filamentous fungus Trichoderma reesei using the CRISPR/Cas9 system. Cell Discov. 1(1):15007. doi:10.1038/celldisc.2015.7.
- Liu QQ, Li DN, Jiang KX, Zhang KQ, Yang JK. 2022. Aopex1 and Aopex6 are required for mycelial growth, conidiation, stress response, fatty acid utilization, and trap formation in Arthrobotrys oligospora. Microbiol Spectr. 10(2):e0027522. doi:10.1128/spectrum.00275-22.
- Liu KK, Zhang WW, Lai YL, Xiang MC, Wang XN, Zhang XY, Liu XZ. 2014. Drechslerella stenobrocha genome illustrates the mechanism of constricting rings and the origin of nematode predation in fungi. BMC Genom. 15(1):114. doi:10.1186/1471-2164-15-114.
- Li J, Wu RN, Wang M, Borneman J, Yang JK, Zhang KQ. 2019. The pH sensing receptor AopalH plays important roles in the nematophagous fungus Arthrobotrys oligospora. Fungal Biol. 123(7):547–554. doi:10.1016/j.funbio.2019.05.008.
- Li J, Zou CG, Xu JP, Ji XL, Niu XM, Yang JK, Huang XW, Zhang KQ. 2015. Molecular mechanisms of nematode-nematophagous microbe interactions: basis for biological control of plant-parasitic nematodes. Annu Rev Phytopathol. 53(1):67–95. doi:10.1146/annurev-phyto-080614-120336.
- Ma N, Jiang KX, Bai N, Li DN, Zhang KQ, Yang JK. 2022. Functional analysis of two affinity camp phosphodiesterase in the nematode trapping fungus Arthrobotrys oligospora. Pathogens. 11(4):405. doi:10.3390/pathogens11040405.
- Manghwar H, Li B, Ding X, Hussain A, Lindsey K, Zhang XL, Jin SX. 2020. CRISPR/Cas systems in genome editing: methodologies and tools for sgRNA design, off-target evaluation, and strategies to mitigate off-target effects. Adv Sci (Weinh). 7(6):1902312. doi:10.1002/advs.201902312.
- Ma YX, Yang XW, Xie MH, Zhang GS, Yang L, Bai N, Zhao YN, Li DN, Zhang KQ, Yang JK. 2020. The Arf-GAP AoGlo3 regulates conidiation, endocytosis, and pathogenicity in the nematode-trapping fungus Arthrobotrys oligospora. Fungal Genet Biol. 138:103352. doi:10.1016/j.fgb.2020.103352
- Ma N, Zhao YN, Wang YC, Yang L, Li DN, Yang JK, Jiang KX, Zhang KQ, Yang JK. 2021. Functional analysis of seven regulators of G protein signaling (RGSs) in the nematode-trapping fungus Arthrobotrys oligospora. Virulence. 12(1):1825–1840. doi:10.1080/21505594.2021.1948667.
- Meerupati T, Andersson KM, Friman E, Kumar D, Tunlid A, Ahrén D. 2013. Genomic mechanisms accounting for the adaptation to parasitism in nematode-trapping fungi. PLoS Genet. 9(11):e1003909. doi:10.1371/journal.pgen.1003909.
- Ngiam C, Jeenes DJ, Punt PJ, Van Den Hondel CA, Archer DB. 2000. Characterization of a foldase, protein disulfide isomerase A, in the protein secretory pathway of Aspergillus Niger. Appl Environ Microbiol. 66(2):775–782. doi:10.1128/AEM.66.2.775-782.2000.
- Nødvig CS, Nielsen JB, Kogle ME, Mortensen UH. 2015. A CRISPR-Cas9 System for genetic engineering of filamentous fungi. PLoS One. 10(7):e0133085. doi:10.1371/journal.pone.0133085.
- Nourani SL, Goltapeh EM, Safaie N, Javaran MJ, Pourjam E. 2018. Enhancing the pathogenicity of Arthrobotrys conoides and A. oligospora against Meloidogyne javanica J(2) by transferring of protease (Ac1) gene and evaluation of antagonistic capability of transgenic isolates. Biol Control. 122:127–135. doi:10.1016/j.biocontrol.2018.03.017
- Pandit R, Patel R, Patel N, Bhatt V, Joshi C, Singh PK, Kunjadia A. 2017. RNA-Seq reveals the molecular mechanism of trapping and killing of root-knot nematodes by nematode-trapping fungi. World J Microbiol Biotechnol. 33(4):65. doi:10.1007/s11274-017-2232-7.
- Peng H, Dong XY, Lu HQ, Kong XW, Zha XD, Wang YZ. 2022. A putative F-box-domain-encoding gene AOL_s00076g207 regulates the development and pathogenicity of Arthrobotrys oligospora. J Basic Microbiol. 62(1):74–81. doi:10.1002/jobm.202100388.
- Prokhorova I, Altman RB, Djumagulov M, Shrestha JP, Urzhumtsev A, Ferguson A, Chang CT, Yusupov M, Blanchard SC, Yusupova G. 2017. Aminoglycoside interactions and impacts on the eukaryotic ribosome. Proc Natl Acad Sci U S A. 114(51):E10899–E10908. doi:10.1073/pnas.1715501114.
- Ramesh P, Reena P, Amitbikram M, Chaitanya J, Anju K. 2015. Insight into the transcriptome of Arthrobotrys conoides using high throughput sequencing. J Basic Microb. 55(12):1394–1405. doi:10.1002/jobm.201500237.
- Schiestl RH, Petes TD. 1991. Integration of DNA fragments by illegitimate recombination in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 88(17):7585–7589. doi:10.1073/pnas.88.17.7585.
- Si JL, Dong XY, Zhang GH, Lu HQ, Tang KJ, Zhang L, Kong XW, Sheng KL, Wang JM, Zha XD, et al. 2022. The fucose-specific lectin gene AOL_s00054g276 affects trap formation and nematocidal activity of the nematophagous fungus Arthrobotrys oligospora. FEMS Microbiol Lett. 369(1):fnac013. doi:10.1093/femsle/fnac013.
- Singh UB, SahuSingh RK A, Singh DP, Meena KK, Srivastava JS, RenuManna MC. 2012. Evaluation of biocontrol potential of Arthrobotrys oligospora against Meloidogyne graminicola and Rhizoctonia solani in Rice (Oryza sativa L.). Biol Control. 60(3):262–270. doi:10.1016/j.biocontrol.2011.10.006.
- Song TY, Xu ZF, Chen YH, Ding QY, Sun YR, Miao Y, Zhang KQ, Niu XM. 2017. Potent nematicidal activity and new hybrid metabolite production by disruption of a cytochrome p450 gene involved in the biosynthesis of morphological regulatory arthrosporols in nematode-trapping fungus Arthrobotrys oligospora. J Agr Food Chem. 65(20):4111–4120. doi:10.1021/acs.jafc.7b01290.
- Teng LL, Song TY, Chen YH, Chen YG, Zhang KQ, Li SH, Niu XM. 2020. Novel polyketide-terpenoid hybrid metabolites from a potent nematicidal Arthrobotrys oligospora mutant delta AOL_s00215g278. J Agr Food Chem. 68(41):11449–11458. doi:10.1021/acs.jafc.0c04713.
- Tilburn J, Scazzocchio C, Taylor GG, Zabicky-Zissman JH, Lockington RA, Davies RW. 1983. Transformation by integration in Aspergillus nidulans. Gene. 26(2–3):205–221. doi:10.1016/0378-1119(83)90191-9.
- Tunlid A, Ahman J, Oliver RP. 1999. Transformation of the nematode-trapping fungus Arthrobotrys oligospora. FEMS Microbiol Lett. 173(1):111–116. doi:10.1111/j.1574-6968.1999.tb13491.x.
- Van Larebeke N, Engler G, Holsters M, Van den Elsacker S, Zaenen I, Schilperoort RA, Schell J. 1974. Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature. 252(5479):169–170. doi:10.1038/252169a0.
- Wang BL, Chen YH, He JN, Xue HX, Yan N, Zeng ZJ, Bennett JW, Zhang KQ, Niu XM. 2018. Integrated metabolomics and morphogenesis reveal volatile signaling of the nematode-trapping fungus Arthrobotrys oligospora. Appl Environ Microbiol. 84(9):e02749–1. doi:10.1128/AEM.02749-17.
- Wang SX, Chen HQ, Tang X, Zhang H, Chen W, Chen YQ. 2017. Molecular tools for gene manipulation in filamentous fungi. Appl Microbiol Biotechnol. 101(22):8063–8075. doi:10.1007/s00253-017-8486-z.
- Wang X, Li GH, Zou CG, Ji XL, Liu T, Zhao PJ, Liang LM, Xu JP, An ZQ, Zheng X. 2014. Bacteria can mobilize nematode-trapping fungi to kill nematodes. Nat Commun. 5(1):5776. doi:10.1038/ncomms6776.
- Wernet V, Wäckerle J, Fischer R. 2022. The STRIPAK component SipC is involved in morphology and cell-fate determination in the nematode-trapping fungus Duddingtonia flagrans. Genet. 220(1):iyab153. doi:10.1093/genetics/iyab153.
- Wu QY, Zhu YY, Zou CG, Kang YQ, Liang LM. 2016. GPH1 is involved in glycerol accumulation in the three-dimensional networks of the nematode-trapping fungus Arthrobotrys oligospora. J Microbiol. 54(11):768–773. doi:10.1007/s12275-016-6272-8.
- Xie MH, Bai N, Yang JKL, Jiang KX, Zhou DX, Zhao YN, Li DN, Niu XM, Zhang KQ, Yang JK. 2020. Protein kinase ime2 is required for mycelial growth, conidiation, osmoregulation, and pathogenicity in nematode-trapping fungus Arthrobotrys oligospora. Front Microbiol. 10. doi:10.3389/fmicb.2019.03065.
- Xie MH, Ma N, Bai N, Zhu MC, Zhang KQ, Yang JK. 2022. Phospholipase C (AoPLC2) regulates mycelial development, trap morphogenesis, and pathogenicity of the nematode-trapping fungus Arthrobotrys oligospora. J Appl Microbiol. 132(3):2144–2156. doi:10.1111/jam.15370.
- Xie MH, Wang YC, Tang YL, Yang L, Zhou DX, Li Q, Niu XM, Zhang KQ, Yang JK. 2019. AoStuA, an APSES transcription factor, regulates the conidiation, trap formation, stress resistance and pathogenicity of the nematode-trapping fungus Arthrobotrys oligospora. Environ Microbiol. 21(12):4648–4661. doi:10.1111/1462-2920.14785.
- Xie MH, Yang JKL, Jiang KX, Bai N, Zhu MC, Zhu YM, Zhang KQ, Yang JK. 2021. Aobck1 and Aomkk1 are necessary to maintain cell wall integrity, vegetative growth, conidiation, stress resistance, and pathogenicity in the nematode-trapping fungus Arthrobotrys Oligospora. Front Microbiol. 12:649582. doi:10.3389/fmicb.2021.649582
- Xu ZF, Chen YH, Song TY, Zeng ZJ, Yan N, Zhang KQ, Niu XM. 2016. Nematicidal key precursors for the biosynthesis of morphological regulatory arthrosporols in the nematode-trapping fungus Arthrobotrys oligospora. J Agric Food Chem. 64(42):7949–7956. doi:10.1021/acs.jafc.6b03241.
- Xu J, Mo MH, Huang XW, Zhang KQ. 2005. Improvement on genetic transformation in the nematode-trapping fungus Arthrobotrys oligospora and its quantification on dung samples. Mycopathol. 159(4):533–538. doi:10.1007/s11046-005-4334-2.
- Xu ZF, Wang BL, Sun HK, Yan N, Zeng ZJ, Zhang KQ, Niu XM. 2015. High trap formation and low metabolite production by disruption of the polyketide synthase gene involved in the biosynthesis of arthrosporols from nematode-trapping fungus Arthrobotrys oligospora. J Agric Food Chem. 63(41):9076–9082. doi:10.1021/acs.jafc.5b04244.
- Yang L, Li XM, Bai N, Yang XW, Zhang KQ, Yang JK. 2022a. Transcriptomic analysis reveals that rho gtpases regulate trap development and lifestyle transition of the nematode-trapping fungus Arthrobotrys oligospora. Microbiol Spectr. 10(1). doi:10.1128/spectrum.01759-21.
- Yang L, Li XM, Ma YX, Zhang KQ, Yang JK. 2022b. The Arf-GAP proteins aogcs1 and aogts1 regulate mycelial development, endocytosis, and pathogenicity in Arthrobotrys oligospora. J Fungi. 8(5):463. doi:10.3390/jof8050463.
- Yang L, Li XM, Xie MH, Bai N, Yang JK, Jiang KX, Zhang KQ, Yang JK. 2021. Pleiotropic roles of Ras GTPases in the nematode-trapping fungus Arthrobotrys oligospora identified through multi-omics analyses. iScience. 24(8):102820. doi:10.1016/j.isci.2021.102820.
- Yang XW, Ma N, Yang L, Zheng YQ, Zhen ZY, Li Q, Xie MH, Li J, Zhang KQ, Yang JK. 2018. Two Rab GTPases play different roles in conidiation, trap formation, stress resistance, and virulence in the nematode-trapping fungus Arthrobotrys oligospora. Appl Microbiol Biotechnol. 102(10):4601–4613. doi:10.1007/s00253-018-8929-1.
- Yang CT, Vidal-Diez de Ulzurrun G, Gonçalves AP, Lin HC, Chang CW, Huang TY, Chen SA, Lai CK, Tsai IJ, Schroeder FC. 2020. Natural diversity in the predatory behavior facilitates the establishment of a robust model strain for nematode-trapping fungi. Proc Natl Acad Sci U S A. 117(12):6762–6770. doi:10.1073/pnas.1919726117.
- Yang JK, Wang L, Ji XL, Feng Y, Li XM, Zou CG, Xu JP, Ren Y, Mi QL, Wu JL, et al. 2011. Genomic and proteomic analyses of the fungus Arthrobotrys oligospora provide insights into nematode-trap formation. Plos Pathog. 7(9):e1002179. doi:10.1371/journal.ppat.1002179.
- Yang EC, Xu LL, Yang Y, Zhang XY, Xiang MC, Wang CS, An ZQ, Liu XZ. 2012. Origin and evolution of carnivorism in the Ascomycota (fungi). Proc Natl Acad Sci U S A. 109(27):10960–10965. doi:10.1073/pnas.1120915109.
- Yang Y, Yang EC, An ZQ, Liu XZ. 2007. Evolution of nematode-trapping cells of predatory fungi of the Orbiliaceae based on. Proc Natl Acad Sci U S A. 104(20):8379–8384. doi:10.1073/pnas.0702770104.
- Youssar L, Wernet V, Hensel N, Yu X, Hildebrand HG, Schreckenberger B, Kriegler M, Hetzer B, Frankino P, Dillin A, et al. 2019. Intercellular communication is required for trap formation in the nematode-trapping fungus Duddingtonia flagrans. PLoS Genet. 15(3):31. doi:10.1371/journal.pgen.1008029.
- Yu X, Hu X, Pop M, Wernet N, Kirschhöfer F, Brenner-Weiß G, Keller J, Bunzel M, Fischer R. 2021. Fatal attraction of Caenorhabditis elegans to predatory fungi through 6-methyl-salicylic acid. Nat Commun. 12(1):5462. doi:10.1038/s41467-021-25535-1.
- Zhang WW, Chen JZ, Fan YN, Hussain M, Liu XZ, Xiang MC. 2021. The E3-ligase AoUBR1 in N-end rule pathway is involved in the vegetative growth, secretome, and trap formation in Arthrobotrys oligospora. Fungal Biol. 125(7):532–540. doi:10.1016/j.funbio.2021.02.003.
- Zhang WW, Hu CC, Hussain M, Chen JZ, Xiang MC, Liu XZ. 2019b. Role of low-affinity calcium system member fig1 homologous proteins in conidiation and trap-formation of nematode-trapping fungus Arthrobotrys oligospora. Sci Rep. 9(1):4440. doi:10.1038/s41598-019-40493-x.
- Zhang W, Liu DD, Yu ZC, Hou B, Fan Y, Li ZH, Shang SJ, Qiao YD, Fu JT, Niu JK. 2020. Comparative genome and transcriptome analysis of the nematode-trapping fungus Duddingtonia flagrans reveals high pathogenicity during nematode infection. Biol Control. 143:104159. doi:10.1016/j.biocontrol.2019.104159
- Zhang GS, Zheng YQ, Ma YX, Yang L, Xie MH, Zhou DX, Niu XM, Zhang KQ, Yang JK. 2019a. The velvet proteins vosa and velb play different roles in conidiation, trap formation, and pathogenicity in the nematode-trapping fungus Arthrobotrys oligospora. Front Microbiol. 10:1917. doi:10.3389/fmicb.2019.01917
- Zhang DH, Zhu X, Sun F, Zhang KQ, Niu SM, Huang X. 2017. The roles of actin cytoskeleton and actin-associated protein Crn1p in trap formation of Arthrobotrys oligospora. Res Microbiol. 168(7):655–663. doi:10.1016/j.resmic.2017.05.001.
- Zhao XZ, Fan YN, Zhang WW, Xiang MC, Kang SC, Wang SX, Liu XZ. 2023. DhFIG_2, a gene of nematode-trapping fungus Dactylellina haptotyla that encodes a component of the low-affinity calcium uptake system, is required for conidiation and knob-trap formation. Fungal Genet Biol.
- Zhao XY, Wang YC, Zhao Y, Huang Y, Zhang KQ, Yang JK. 2014. Malate synthase gene AoMls in the nematode-trapping fungus Arthrobotrys oligospora contributes to conidiation, trap formation, and pathogenicity. Appl Microbiol Biotechnol. 98(6):555–563. doi:10.1007/s00253-013-5432-6.
- Zheng XF, Kobayashi Y, Takeuchi M. 1998. Construction of a low-serine-type-carboxypeptidase-producing mutant of Aspergillus oryzae by the expression of antisense RNA and its use as a host for heterologous protein secretion. Appl Microbiol Biotechnol. 49(1):39–44. doi:10.1007/s002530051134.
- Zhen ZY, Xing XJ, Xie MH, Yang L, Yang XW, Zheng YQ, Chen YL, Ma N, Li Q, Zhang KQ, et al. 2018. MAP kinase Slt2 orthologs play similar roles in conidiation, trap formation, and pathogenicity in two nematode-trapping fungi. Fungal Genet Biol. 116:42–50. doi:10.1016/j.fgb.2018.04.011
- Zhen ZY, Zhang GS, Yang L, Ma N, Li Q, Ma YX, Niu XM, Zhang KQ, Yang JK. 2019. Characterization and functional analysis of calcium/calmodulin-dependent protein kinases (CaMKs) in the nematode-trapping fungus Arthrobotrys oligospora. Appl Microbiol Biotechnol. 103(2):819–832. doi:10.1007/s00253-018-9504-5.
- Zhou DX, Xie MH, Bai N, Yang L, Zhang KQ, Yang JK. 2020. The autophagy-related gene Aolatg4 regulates hyphal growth, sporulation, autophagosome formation, and pathogenicity in Arthrobotrys oligospora. Front Microbiol. 11:592524. doi:10.3389/fmicb.2020.592524
- Zhou DX, Zhu YM, Bai N, Xie MH, Zhang KQ, Yang JK. 2021a. Aolatg1 and Aolatg13 regulate autophagy and play different roles in conidiation, trap formation, and pathogenicity in the nematode-trapping fungus Arthrobotrys oligospora. Front Cell Infect Microbiol. 11:824407. doi:10.3389/fcimb.2021.824407
- Zhou DX, Zhu YM, Bai N, Yang L, Xie MH, Yang JK, Zhu MC, Zhang KQ, Yang JK. 2021b. AoATG5 plays pleiotropic roles in vegetative growth, cell nucleus development, conidiation, and virulence in the nematode-trapping fungus Arthrobotrys oligospora. Sci China Life Sci. 65(2):412–425. doi:10.1007/s11427-020-1913-9.